Abstract
Mechanisms that ensure and maintain the stability of genetic information are fundamentally important for organismal function and can have a large impact on disease, aging, and life span. While a multi-layered cellular apparatus exists to detect and respond to DNA damage, various insults from environmental and endogenous sources continuously affect DNA integrity. Over time this can lead to the accumulation of somatic mutations, which is thought to be one of the major causes of aging. We have previously found that overexpression of the essential human DNA repair and splicing factor SNEV, also called PRP19 or hPso4, extends replicative life span of cultured human endothelial cells and impedes accumulation of DNA damage. Here, we show that adult-specific overexpression of dPrp19, the D. melanogaster ortholog of human SNEV/PRP19/hPso4, robustly extends life span in female fruit flies. This increase in life span is accompanied by reduced levels of DNA damage and improved resistance to oxidative and genotoxic stress. Our findings suggest that dPrp19 plays an evolutionarily conserved role in aging, life span modulation and stress resistance, and support the notion that superior DNA maintenance is key to longevity.
Aging: Living longer by improving DNA repair
Increasing levels of DNA repair factor Prp19 in fruit flies extends their life span and protects against stress. Prp19 is a protein that is present in a wide range of organisms and enables human endothelial cells to live longer in vitro. In this article, an international team of scientists from Austria, Germany and Switzerland found that higher Prp19 levels also prolong the life span of a whole organism in fruit flies, reduce DNA damage and increase survival when exposed to DNA damaging compounds. In contrast to female flies, males were unaffected. Their findings support the long-held view that repair of DNA damage, one of the hallmarks of aging, is key to longevity. They also provide an intriguing but poorly understood connection between cellular aging and the survival of whole organisms.
Introduction
Aging is characterized by a time-progressive decline of physiological function at the level of cells, tissues, organs, and ultimately affects the whole organism. According to the “disposable soma” hypothesis of aging, this functional decline results from the accumulation of stochastic damage, for example, due to somatic mutations, and is counteracted by investment into somatic maintenance and repair.1 Accumulation of DNA damage due to decreased repair can accelerate aging, as is observed in segmental progeroid syndromes including the Werner or Hutchinson-Gilford syndromes in humans2 and mouse models.3 Similarly, increased exposure to DNA damaging agents, for instance during chemotherapy, can lead to a phenotype of acquired premature progeroid syndrome.4 Accelerated accumulation of DNA damage and premature aging phenotypes are typically well correlated, but whether improved DNA damage repair (DDR) can extend organismal life span remains largely unclear.
In the fruit fly (Drosophila melanogaster), a well-studied model for dissecting the mechanisms of aging, spontaneous somatic mutations accumulate with age, and defective DNA repair is associated with reduced life span.5, 6 However, overexpression of DNA repair factors in the fly seems to have highly variable, sometimes contradictory effects that depend on sex, developmental stage, and the tissue of intervention. For instance, poly(ADP-ribose) polymerase-1 (PARP-1) modifies histones, transcription factors and repair enzymes in response to DNA breaks, and its endogenous activity is well correlated with life span in several mammalian species.7 In Drosophila, overexpression of PARP-1 prolongs life span in both sexes, yet only when restricted to the adult nervous system.8 Similarly, overexpression of Gadd45 (growth arrest and DNA damage 45) (ref. 9), a regulator of DNA repair and cellular stress responses, in the nervous system increases fly life span but ubiquitous expression is lethal.10, 11 Indeed, a recent study by Shaposhnikov et al. 12 has found that DNA repair factors can affect Drosophila life span and stress resistance either positively or negatively, depending on the sex and on whether overexpression is ubiquitous or limited to the nervous system. Interestingly, all repair factors that were expressed throughout the adult fly body were found to shorten life span. In another study, Barclay and colleagues examined the effects of overexpression of several known D. melanogaster homologs of human DNA repair genes on Drosophila life span in a genetic model of spinocerebral ataxia (SCA), aiming to identify repair pathways that might be relevant for SCA pathology. They found that an extension of life span and improvement of SCA symptoms could not be attributed to a single repair pathway; instead, each pathway included factors that had either detrimental, beneficial, or no effects on life span.13 Yet, these results—obtained in a diseased mutant background—do not necessarily reflect possible life span effects of DNA repair factors in healthy wild-type flies. Thus, to date, the relationship between DNA damage, repair and organismal aging still remains poorly understood.
Here, we examine the role of adult-specific overexpression of the DNA repair factor Prp19 (pre-messenger RNA (mRNA) processing factor 19) in affecting life span, stress resistance, and DNA damage in Drosophila. PRP19 (also called senescence evasion factor, SNEV, or hPso4) was first characterized in a yeast mutant exhibiting increased sensitivity to DNA interstrand crosslinking induced by treatment with psoralen and ultraviolet (UV) radiation.14 Biochemically, PRP19 acts as an E3 ubiquitin ligase15, 16 and interacts with multiple players in the DNA repair pathways, including each of the two core kinases, ATM (ataxia telangiectasia mutated) and ATR (ataxia telangiectasia related).17, 18 Apart from its role in the DNA damage response, an intriguing aspect of PRP19 function is its concomitant and essential involvement in co-transcriptional splicing, where the PRP19 complex regulates the rearrangement of the spliceosome to a catalytically active state through ubiquitination of several factors.19–21 The dual role of PRP19 in DNA repair and transcriptional control is further exemplified by its association with transcription-coupled repair, which is activated when DNA damage blocks elongation,22 but which has not yet been characterized as a DNA repair mechanism in Drosophila.23
In support of a major role of PRP19/SNEV/hPSO4 in the aging process, it has previously been shown that decreased levels of PRP19 accelerate the induction of cellular senescence in mouse embryonic fibroblasts,24 reduce self renewal of mouse hematopoietic stem cells,25 increase psoralen/UV-A-induced skin aging in mice26 and decrease differentiation of human adipose-derived stromal cells.27 Conversely, increased levels of PRP19 extend the replicative potential and total life span of cultured human endothelial cells.17, 28 However, the role of PRP19 in organismal life span is unknown. Here, we show that ubiquitous overexpression of the Drosophila ortholog of PRP19, dPrp19 (http://flybase.org/reports/FBgn0261119.html), reduces DNA damage and extends organismal life span of adult female flies. Our results suggest that PRP19 plays an evolutionarily conserved role in DDR, aging, and stress resistance.
Results
dPrp19 is the fly ortholog of human PRP19/SNEV/Pso4 and is regulated in an age-dependent manner
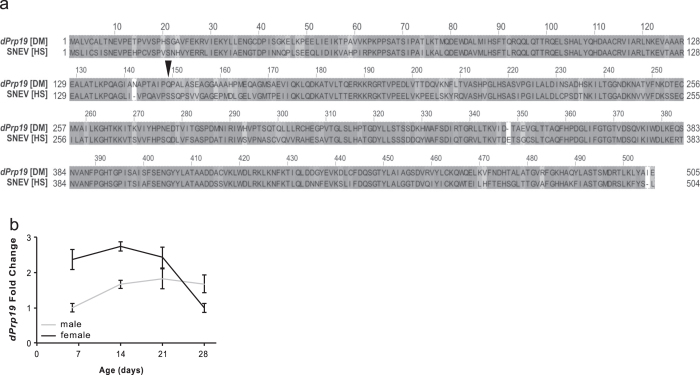
In support of an evolutionarily conserved role of PRP19/SNEV/Pso4 in aging and DNA repair21 in Drosophila, we found a high degree of amino acid identity (66%) and similarity (81%) between human PRP19 and fly dPrp19 (Fig. 1a). To determine whether dPrp19 expression is regulated during the fly’s life span, we performed quantitative reverse transcription PCR (qRT-PCR) in whole-body samples of adult flies and found that dPrp19 transcript levels are rather constant in adult males, whereas they decrease with age in adult females (Fig. 1b). Over the course of 28 days, we observed a ~2.5-fold reduction in dPrp19 mRNA levels in females; in contrast, male expression was lower and largely unaffected by age (Fig. 1b). This observation prompted us to test whether we could—similar to our previous observations in human cells17, 28—extend life span and promote stress resistance in Drosophila by overexpressing dPrp19.
Fig. 1.
dPrp19 is the Drosophila melanogaster ortholog of human PRP19/SNEV/hPso4. a Prp19 is well-conserved from D. melanogaster to humans, as shown in an alignment of the protein sequences of D. melanogaster dPrp19 and human PRP19/SNEV/hPso4. The two orthologs have an amino acid identity of 66% and a sequence similarity of 81%. The human site of ATM phosphorylation, Ser149, is substituted for Gln in Drosophila (indicated by the black arrow). b dPrp19 expression is higher in female D. melanogaster and decreases with age. Comparison of dPrp19 mRNA levels in male (gray) and female (black) wild-type flies at 6, 14, 21, and 28 days of adulthood shows that dPrp19 levels decrease in females between days 21 and 28. Values represent means across three biological replicates (±1 standard deviation), normalized to Gapdh2 and Tubulin and averaged
dPrp19 overexpression extends Drosophila female life span and prevents DNA damage accumulation
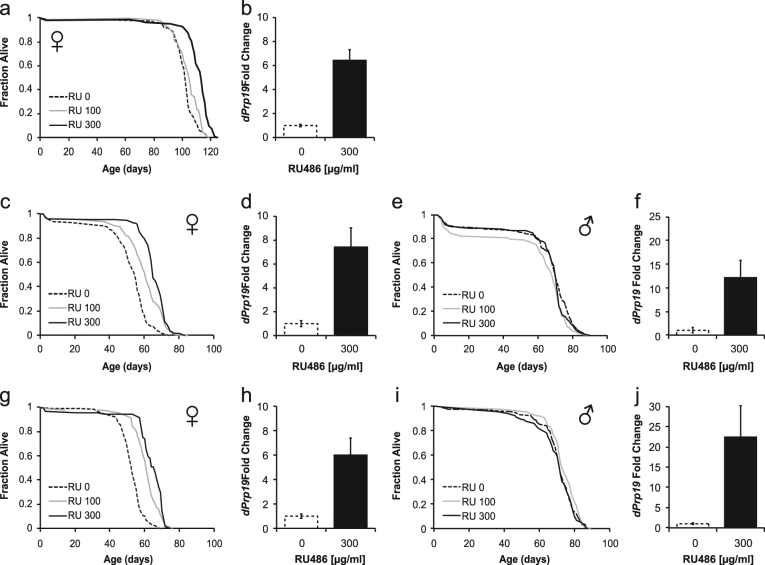
To examine the life span effects of dPrp19 in the fly, we drove adult-specific, ubiquitous overexpression of dPrp19 in three independent transgenic strains carrying a UAS expression cassette (UAS-dPrp19) with the inducible GeneSwitch (GS)-Gal4 driver system.29 By supplementing the fly food medium with the antiprogestin compound RU486 (mifepristone), the GeneSwitch(GS) system can be used to induce overexpression in the F1 of a cross between the UAS responder and the Gal4 driver construct. This allows for spatio-temporal control of candidate gene expression and is commonly used in aging studies.30, 31 To drive overexpression of dPrp19 from the UAS lines, we used two different ubiquitously expressing GS-Gal4 driver lines: a Tubulin–GeneSwitch-Gal4(TubGS-Gal4) line and a daughterless-GeneSwitch-Gal4 (daGS-Gal4) line.32 In females, overexpression of dPrp19 resulted in approximately sevenfold upregulation of mRNA (Fig. 2b, d, and h) and in males in approximately 12- to 22-fold induction (Fig. 2f, j).
Fig. 2.
Overexpression of dPrp19 leads to dose-dependent extension of female but not male life span. Effects of the induction of the dPrp19 UAS cassette in three independent chromosomal insertions of the same transgenic construct on adult survival and dPrp19 mRNA levels: a, b TubGS-Gal4 > UAS-dPrp19-1 (females only), c–f daGS-Gal4 > UAS-dPrp19-2 (females and males), and g–j daGS-Gal4 > UAS-dPrp19-4 (females and males). a, c, e, g, and i show survival curves of experimental flies at three concentrations of the inducer drug RU486; b, d, f, h, and j show quantification of dPrp19 expression levels relative to the Rp49 control after 72 h of exposure to 300 µg/ml RU486. For all three overexpression constructs, we find a significant dose-dependent extension of female life span. Overall, we did not find any life span extension in males (for daGS-Gal4 > UAS-dPrp19-2 males at 100 µg/ml RU486 we observed a slight reduction in survival, possibly due to inadvertent ‘‘setup mortality” that might have occurred when the assay was set up). For details of life span statistics see Supplementary Table 1 (females) and Supplementary Table 2 (males); for experimental details see Materials and Methods
Across independent experiments in our laboratories in Vienna and Lausanne, three different UAS responder constructs, and two different Gal4 drivers we observed significant and robust dose-dependent extension of female life span (Fig. 2, Supplementary Fig. 1, Supplementary Table 1). At the highest level of induction (food supplemented with 300 µg/ml RU486) median female life span was increased by between 9.6 and 25%. In one of these assays, dPrp19 overexpression was carried out on food medium containing 2% yeast (Fig. 2a, b), whereas all other assays were performed on a diet containing 5% yeast (Fig. 2c–j). Although mean and maximum life span of controls were strongly affected by the two dietary conditions, dPrp19 overexpression robustly extended female life span in all cases (Fig. 2a, c, and g, Supplementary Table 1). In contrast, male life span was not consistently affected by dPrp19 overexpression in any of our assays (Fig. 2e, i, Supplementary Table 2), despite higher levels of dPrp19 mRNA than in females.
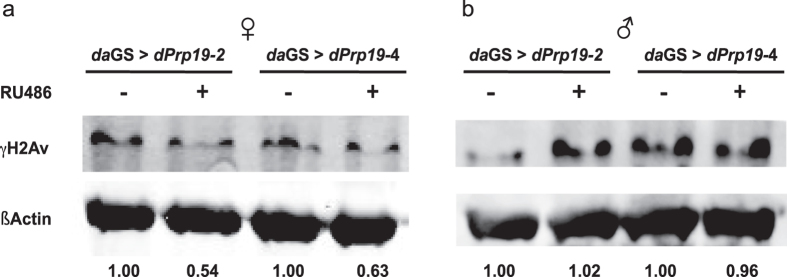
Next, to examine whether ubiquitously increased expression of dPrp19 affects DDR in the fly, we analyzed the abundance of the histone variant γH2Av, a well-established marker for DNA damage.33 Upon dPrp19 overexpression in females, we observed a clear-cut decrease in the levels of γH2Av (Fig. 3a), suggesting a general reduction in the number of DNA double-strand breaks under basal conditions when dPrp19 is upregulated. Interestingly, we did not observe any reduction of γH2Av signal in males (Fig. 3b), indicating that in contrast to females dPrp19 overexpression does not improve DNA repair capacity and probably thereby does not extend life span.
Fig. 3.
Overexpression of dPrp19 decreases γH2Av as marker for DNA double-strand breaks in females but not males. Overexpression of dPrp19 (daGS-Gal4 > UAS-dPrp19-2/4) induced by 300 µg/ml RU486 decreases the accumulation of γH2Av by approximately 40–50% in female flies a as compared to the uninduced controls. In males b, we did not observe an effect of dPrp19 induction on DNA damage levels. Numbers represent protein signals of γH2Av normalized to βActin. The blot shown is representative; qualitatively identical results were obtained with at least two biological and two technical replicates
dPrp19 overexpression increases resistance to DNA damaging compounds
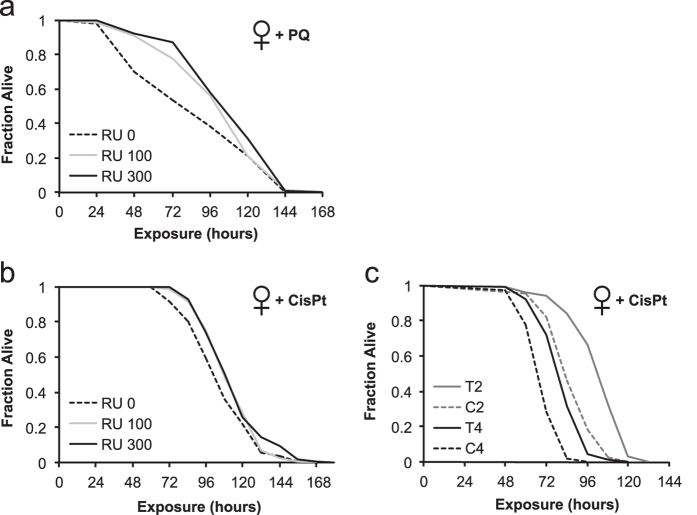
Since human PRP19 is induced by various DNA damaging agents17, 34 and conveys resistance to genotoxic and cytotoxic stress,17, 26–28, 34, 35 we next asked whether dPrp19 overexpression increases resistance against two DNA damaging agents, paraquat (methyl viologen), a herbicide known to induce reactive oxygen species,36 and the genotoxic compound cisplatin, which causes crosslinks between adjacent nucleosomes and which has previously been used to study DNA damage in Drosophila.37 Indeed, overexpression of dPrp19 significantly improved the adult survival of females exposed to both paraquat (Fig. 4a; Supplementary Table 3) and cisplatin (Fig. 4b; Supplementary Table 4).
Fig. 4.
Overexpression of dPrp19 promotes resistance to genotoxic agents in female flies. a Induction of the dPrp19 cassette with either 100 (gray line) or 300 µg/ml RU486 (black, solid line) significantly increases resistance to 20 mM paraquat (methyl viologen) in females (TubGS-Gal4 > UAS-dPrp19-1) as compared to the uninduced control (black, dashed line; see Supplementary Table 3 for statistics). b Resistance of females (daGS-Gal4 > UAS-dPrp19-2) to genotoxic stress induced by treatment with 400 µg/ml cisplatin was improved significantly by dPrp19 induction at 300 µg/ml RU486; the intermediate induction level of 100 µg/ml RU486 was not significant after Bonferroni correction (see Supplementary Table 4 for statistics). c Females with constitutively active dPrp19 expression (T2: Tub-Gal4 > UAS-dPrp19-2 in gray; T4: Tub-Gal4 > UAS-dPrp19-4 in black) showed significantly improved resistance to cisplatin relative to controls (C2: TM6Sb; UAS-dPrp19-2, in dashed gray; C4: TM6Sb; UAS-dPrp19-4, in dashed black line; see Supplementary Table 4 for statistics)
Since in the above experiments dPrp19 overexpression was induced by adding RU486 to the food medium before-but not during-cisplatin exposure, it is possible that transgenic induction of dPrp19 might have decreased during treatment with cisplatin. To rule out this possibility, we examined the adult survival of F1 flies from a cross between a constitutively active (non-inducible) Tub-Gal4 driver and the UAS overexpression lines. Transgenic F1 offspring carrying both the driver and the overexpression cassette had strongly improved survival over sibling F1 controls lacking the driver construct, thus clearly confirming the protective effects of dPrp19 upon exposure to genotoxic stress (Fig. 4c; Supplementary Table 4).
Discussion
Here, we have shown that ubiquitous, adult-specific overexpression of dPrp19, an evolutionarily conserved DNA repair factor, significantly and robustly extends female life span in Drosophila. Overexpression of dPrp19 further reduces DNA damage levels and enhances survival upon oxidative and genotoxic stress. The effects we observed in our experiments are independent of the details of chromosomal insertion position of the UAS-dPrp19 cassette, the Gal4 driver system, and laboratory (food) conditions.
Our data clearly support previous results from cultured human endothelial cells, where PRP19/SNEV/hPso4 overexpression strongly extends replicative life span, lowers basal levels of DNA double-strand breaks, and increases resistance to pro-oxidants as well as to the DNA crosslinker cisplatin.17, 28 In line with these findings, female flies overexpressing dPrp19 exhibited decreased accumulation of DNA double-strand breaks, as indicated by reduced levels of the histone variant γH2Av, the fly homolog of γH2AX. During Drosophila aging increased levels of γH2Av are associated with age-dependent degeneration, for example, in tissues such as the intestine33 and muscle.38
Interestingly, the benefits of dPrp19 overexpression we have observed in terms of increased life span and reduced DNA damage seem to be restricted to females. Sex-specific effects of aging interventions have been widely observed,12, 39 and quantitative genetic analyses indicate that the genetic architecture of life span is very different in female and male Drosophila 40; yet, the reasons for this sex-specificity remain unknown. Further work will be required to understand why males do not benefit from overexpression of dPrp19.
Mechanistically, there are several possible explanations for how dPrp19 might improve DNA repair in females and thereby promote stress survival and longer life span: dPrp19 might either directly recruit other DNA repair factors in order to promote fast and efficient repair, or it might act via the regulation of pre-mRNA processing. Human PRP19, for example, is known to interact with proteins involved in DNA repair such as Werner syndrome helicase (WRN),41 Metnase,42 ATR18 and ATM.17 In response to DNA damage, ATM phosphorylates human PRP19, thereby possibly contributing to an arrest of the cell cycle in order to allow for repair. However, signaling from ATM to PRP19 is not solely responsible for this effect since overexpression of a non-phosphorylatable PRP19 protein still extends replicative potential in human cells, albeit to a lesser extent.17 Since the ATM target phosphorylation site is not conserved in the fly homolog dPrp19, extension of female life span under dPrp19 overexpression could be a consequence of its essential role in pre-mRNA splicing.4, 15, 21
In a large short interfering RNA screen for factors affecting γH2AX levels in human HeLa cells, Paulsen et al. 43 found strong evidence for the importance of mRNA processing factors, such as PRP19, in maintaining genome stability. They observed that the γH2AX signal for double strand breaks was strongly increased upon knockdown of RNA-processing and attributed this at least partly to an increased formation of R-loops. R-loops occur when the transcription machinery is slowed down, for instance by encountering a site of DNA damage, and the transcript folds back on the template duplex DNA strand.44 The resulting DNA–RNA hybrid structure activates non-canonical ATM signaling45 and can become the source of mutation and DNA breaks double strand itself.44
Interestingly, PRP19 has also been described as being a sensor of DNA damage during replication stress: It binds and ubiquitinates replication protein A, a key factor in the response DNA damage which protectively binds single-stranded DNA, which causes recruitment of ATR and thus initiates activation of further DNA repair factors.18 In a Drosophila mutant model of SCA, overexpression of Drosophila RpA had the largest effect on life span of all assayed DNA repair factors,13 indicating a possible joint role in promoting longevity.
In conclusion, overexpression of dPrp19, a protein that functionally interacts with many important DNA repair factors, robustly extends life span of female fruit flies. Importantly, our results demonstrate that this female-specific life span extension is accompanied by decreased DNA damage and by significantly increased resistance to damage caused by paraquat and the DNA crosslinking agent cisplatin. Our data therefore strongly suggest that improved DNA repair capacity can extend the life span of a healthy organism, a view that has so far been almost exclusively based on the study of premature aging phenotypes induced by impaired DNA repair.
Materials and methods
Drosophila stocks and maintenance
A D. melanogaster complementary DNA (cDNA) clone of dPrp19 in pBluescript (clone LD02793, stock 13414) was obtained from the Drosophila Genome Resource Center (DGRC) at Indiana University (Bloomington, IN, USA). The cDNA was cloned into pUAST-C5 and verified by sequencing. We received three independent UAS-dPrp19 insertions on the second chromosome in a w 1118 background (one balanced, two homozygous viable lines) from Genetic Services Inc. (Cambridge, MA, USA). To drive inducible, ubiquitous expression of the UAS constructs we used two different Gal4 constructs: a Tubulin–GeneSwitch-Gal4 (TubGS-Gal4) (courtesy of Scott Pletcher, University of Michigan, USA) and a daughterless-GeneSwitch-GAL4 (daGS-Gal4) line.32 F1 progeny of crosses between the UAS-dPrp19 and either the daGS-Gal4 or the TubGS-Gal4 line were used as experimental flies in stress resistance and life span assays. As controls for potential confounding effects of RU486 we used flies from crosses between daGS-Gal4 with w 1118; these controls were exposed to the same treatment with RU486 as the experimental flies. Constitutive overexpression (for the purpose of continuous dPrp19 expression during cisplatin treatment) was achieved by crossing the UAS-dPrp19 lines to a constitutive balanced Tubulin-Gal4 (Tub-Gal4) driver obtained from the Bloomington Drosophila Stock Center (BDSC, stock #5138). We used the F1 offspring carrying both the driver and dPrp19 expression cassette (Tub-Gal4 > UAS-dPrp19) as experimental flies and F1 flies carrying the balancer and one copy of dPrp19 cassette (UAS-dPrp19; TM6Sb) as controls. Experiments involving the UAS line dPrp19-1 were performed in Vienna on a cornmeal–agar diet containing 2% inactivated yeast (a food level corresponding to life span extending dietary restriction); experiments using the lines UAS-dPrp19-2 and UAS-dPrp19-4 were performed in Lausanne on a cornmeal-sucrose-agar medium with 5% yeast. In both laboratories, flies were maintained and assayed at 25 °C on a 12 h:12 h light:dark cycle at approx. 60–70% humidity.
Sequence alignment
Protein sequences of PRP19/SNEV/Pso4 from Homo sapiens [GenBank ID: NP_055317.1] and of dPrp19 from D. melanogaster [GenBank ID: NP_523783.1] were aligned with T-Coffee.46 We plotted the alignment with the help of Jalview (version 2.7).47 Similarities and identities were derived from a pBLAST alignment of both sequences.
Quantitative reverse transcription-PCR
Ten flies per treatment were suspended in TRI reagent (Sigma-Aldrich, St Louis, USA) and mechanically homogenized by using a pellet pestle (Sigma-Aldrich, St Louis, USA). RNA extraction was performed following the manufacturer’s instructions and reverse-transcribed into cDNA using the DyNAmo cDNA Synthesis Kit (Finnzymes, Vantaa, Finland). We assayed mRNA levels of target genes relative to housekeeping control genes were assayed with SYBR Green-based qRT-PCR on a Rotorgene Q thermal cycler (Qiagen, Hilden, Germany) using the 5× HOT FIREPol EvaGreen® qPCR Mix Plus (Solis BioDyne, Tartu, Estonia) and the following primer pairs:(dPrp19: 5′-GTCATACCGGTCCCATTT-3′, and 5′-TGAATACCTTCAGTTCCTGC-3′;
Gapdh2: 5′-GCGGTAGAATGGGGTGAGAC-3′, and 5′-TGAAGAGCGAAAACAGTAGC-3′; Tubulin: 5′-CGCTCTCTGAGTCAGACCTCGAAA-3′, and 5′-GACACCAGCCTGACCAACATGGA-3′; Rp49: 5′-CCCACCGGATTCAAGAAGTT-3′, and 5′-AATGTGTATTCCGACCACGTTAC-3′). Since Rp49 levels, as compared to the two other “housekeeping” genes, changed with age (data not shown), Gapdh2, and Tubulin were used for normalization of samples from flies of different ages. To test for transgene induction by RU486 (mifepristone; Beta Pharma, Princeton, NJ, USA), we collected a 24 h cohort of flies and kept them on food containing 0, 100, or 300 µg/ml RU486 for 3 days. For each sample, we harvested 10 female or male flies and processed them as described above; data were normalized to Rp49.
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and western blotting
For SDS-PAGE, we used young (3–4 days old) flies that had been exposed to 300 μg/ml RU486 for 3 days. For each sample, we homogenized 5 female or 8 male flies in 150 μl 2× SDS loading dye (1 M Tris-Clat pH 6.8, 4% SDS, 20% glycerol, 0.025% bromophenol blue, 2.5% ß-mercapto-ethanol), heated to 95 °C for 5 min, cooled on ice and spun down. We separated 30 μl of supernatant, corresponding to the protein content of one fly, on a NuPAGE 4–12% Bis/Tris polyacrylamide gel (Invitrogen, Carlsbad, CA, USA) in MOPS buffer at 200 V. Electrophoresis and blotting to polyvinylidene fluoride membrane (Roth, Karlsruhe, Germany) were performed using the Biorad TGX Gel + TransTurbo Blotting system (Biorad, Hercules, CA, USA) in accordance with the manufacturer’s protocol. After incubation with blocking buffer (3% skim milk powder in phosphate-buffered saline (PBS) with 0.1% Tween-20) on an orbital shaker for 1 h at room temperature, membranes were incubated overnight with a mix of both primary antibodies diluted in blocking buffer, followed by 1 h incubation with a mix of the secondary antibodies. Antibody incubations were followed by three washes with PBS with 0.1% Tween-20. Membranes were scanned and signal intensity was quantified using the Odyssey infrared imaging system (LI-COR, Lincoln, NE, USA). We used primary antibodies against Histone H2AvD p137 (rabbit, diluted 1:1000; Rockland, Limerick, PA, USA) and βActin (mouse, diluted 1:2500; Abcam, Cambridge, UK). Secondary antibodies were anti-Rabbit-IR-Dye 800 and anti-Mouse-Alexa 680 (LI-COR, Lincoln, NE, USA), both diluted 1:10,000. Each sample was blotted in at least two biological (lysates from a different set of flies) and two technical (same lysates loaded, blotted and analyzed independently) replicates.
Life span assays
Adult survival was determined using standard methods. For experiments in Vienna we collected 24-h cohorts of F1 of reciprocal crosses between TubGS-Gal4 and dPrp19-1. In Lausanne, we collected the F1 of reciprocal crosses between daGS-Gal4 and the dPrp19-2 and dPrp19-4 lines as well as w 1118 within 24 h of eclosion. Following sorting of genotypes under mild CO2 anesthesia, we initiated two replicate cages per RU486 concentration (0, 100, or 300 µg/ml food) with 75 females and 75 males for each genotype. Flies were kept in incubators at 25 °C, 60% humidity and a 12:12 light/dark cycle; dead flies were scored every 24 h, blind with respect to genotype identity. We censored flies that escaped or got stuck in the food medium and replaced food vials every second day with fresh ones. To assess pairwise differences in survivorship within each genotype and across RU486 treatments, we used log-rank tests implemented in JMPv.10.0 (SAS Institute Inc., Cary, NC, USA). Effects of dPrp19 overexpression on survival were considered significant if P-values passed a Bonferroni-corrected threshold of P < 0.0167. For details of cohort (sample) sizes see Supplementary Tables 1 and 2.
Paraquat resistance assay
Paraquat resistance assays were performed as previously described,48 with minor modifications. Briefly, newly eclosed flies (TubGS-Gal4 > UAS-dPrp19-1) were allowed to mate in bottles containing 100 µg/ml, 300 µg/ml, or no RU486. After 3 days of mating, flies were sexed and 20 females were flipped to vials containing a solution consisting of 2% agar, 5% glucose, and 20 mM paraquat (Sigma-Aldrich, St Louis, USA). Vials were checked for dead flies every 24 h until the last fly was dead. Significant differences in survival between the different RU486 treatments were tested using log-rank tests implemented in JMPv.10.0 (SAS Institute Inc., Cary, NC, USA). For details of cohort (sample) size see Supplementary Table 3.
Cisplatin resistance assays
Resistance to cisplatin was assayed on F1 cohorts of experimental (daGS-Gal4 > UAS-dPrp19-2) and (no construct) control flies from the same cross (daGS-Gal4; w 1118), collected within 24 h of eclosion on standard food. We selected and counted female flies under mild CO2 exposure and housed them on medium supplemented with different concentrations of RU486 (0, 100, or 300 µg/ml food) for 3 days. For each genotype, we used 20 females per vial (10 replicate vials per genotype-RU486 combination), the vials containing 2 ml of an agar (0.5%)-sucrose (5%) solution supplemented with 400 µg/ml cisplatin (P4394, Sigma-Aldrich, St Louis, USA). Since the cisplatin medium did not contain any RU486, we expected induction of dPrp19 expression to decrease over time. To rule out this possibility, we also included flies from a cross between a constitutively active Tub-Gal4 driver and the overexpression lines to compare experimental flies with an activated dPrp19 cassette (Tub-Gal4 > UAS-dPrp19-2 / UAS-dPrp19-4) to control flies lacking the driver (UAS-dPrp19-2 / UAS-dPrp19-4; TM6Sb). Scoring was performed every 12 h until the last fly died. We tested for pairwise significant differences in survival between different RU486 concentrations or between pairs of experimental flies and their respective controls with log-rank tests in JMPv.10.0 (SAS Institute Inc., Cary, NC, USA). For details of cohort (sample) sizes see Supplementary Table 4.
Electronic supplementary material
Acknowledgements
We wish to thank Gregor Beitel and Alistair P. McGregor for their generous donation of the pUAST-C5 plasmid; the Drosophila Genomics Resource Center (DGRC) at Indiana University (Bloomington, IN, USA) for the dPrp19 cDNA clone; Genetic Services Inc. (Cambridge, MA, USA) for generating the UAS–dPrp19 lines; Scott Pletcher for providing the TubGS-Gal4 line; Veronique Monnier and Herve Tricoire for providing the daGS-Gal4; and Akila Weerasekera, Clemens Heissenberger, and Daria Martynow for technical assistance in the laboratory. This work was supported by the Swiss National Science Foundation (SNF Grant PP00P3 133641 to T.F.) and the Austrian Science Fund (FWF: P24498 and I2514 to J.G.; P21498-B11 to T.F.). Additional support was provided by the Austrian Federal Ministry of Science, Research and Economy, the National Foundation for Research, Technology and Development, the Christian Doppler Research Society, and from Chanel Research and Technology.
Author contributions
K.G., H.D., M.S., T.F., and J.G. designed experiments; K.G., H.D., M.S., and M.G. performed experiments; K.G., H.D., and M.S. designed figures and wrote the manuscript; T.F. and J.G. supervised the writing of the manuscript. All authors read, edited, and approved the final manuscript.
Competing interests
J.G. is a co-founder of Evercyte GmbH. All other authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
A correction to this article has been published and is linked from the HTML version of this article.
Change history
8/15/2017
Correction to: npj Aging and Mechanisms of Disease, advance online publication, 15 March 2017; doi:10.1038/s41514-017-0005-z
Contributor Information
Markus Schosserer, Phone: +43 1 47654-79098, Email: markus.schosserer@boku.ac.at.
Thomas Flatt, Phone: +41 21 692 4203, Email: thomas.flatt@unil.ch.
Electronic supplementary material
Supplementary information accompanies the paper on the npj Aging and Mechanisms of Disease website (doi:10.1038/s41514-017-0005-z).
References
- 1.Kirkwood TBL, Melov S. On the programmed/non-programmed nature of ageing within the life history. Curr. Biol. 2011;21:R701–R707. doi: 10.1016/j.cub.2011.07.020. [DOI] [PubMed] [Google Scholar]
- 2.Martin GM, Oshima J. Lessons from human progeroid syndromes. Nature. 2000;408:263–266. doi: 10.1038/35041705. [DOI] [PubMed] [Google Scholar]
- 3.Schumacher B, Hoeijmakers JH, Garinis GA. Sealing the gap between nuclear DNA damage and longevity. Mol. Cell Endocrinol. 2009;299:112–117. doi: 10.1016/j.mce.2008.10.031. [DOI] [PubMed] [Google Scholar]
- 4.Grillari J, Katinger H, Voglauer R. Contributions of DNA interstrand cross-links to aging of cells and organisms. Nucleic Acids Res. 2007;35:7566–7576. doi: 10.1093/nar/gkm1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garcia AM, et al. Age- and temperature-dependent somatic mutation accumulation in Drosophila melanogaster. PLoS Genet. 2010;6:e1000950. doi: 10.1371/journal.pgen.1000950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whitehead I, Grigliatti TA. A correlation between DNA repair capacity and longevity in adult Drosophila melanogaster. J. Gerontol. 1993;48:B124–B132. doi: 10.1093/geronj/48.4.B124. [DOI] [PubMed] [Google Scholar]
- 7.Bürkle A, Brabeck C, Diefenbach J, Beneke S. The emerging role of poly(ADP-ribose) polymerase-1 in longevity. Int. J. Biochem. Cell Biol. 2005;37:1043–1053. doi: 10.1016/j.biocel.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 8.Shaposhnikov MV, Moskalev AA, Plyusnina EN. Effect of PARP-1 overexpression and pharmacological inhibition of NF-kB on the lifespan of Drosophila melanogaster. Adv. Gerontol. 2011;24:405–419. [PubMed] [Google Scholar]
- 9.Moskalev A, et al. The role of D-GADD45 in oxidative, thermal and genotoxic stress resistance. Cell Cycle. 2012;11:4222–4241. doi: 10.4161/cc.22545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Plyusnina EN, Shaposhnikov MV, Moskalev AA. Increase of Drosophila melanogaster lifespan due to D-GADD45 overexpression in the nervous system. Biogerontology. 2011;12:211–226. doi: 10.1007/s10522-010-9311-6. [DOI] [PubMed] [Google Scholar]
- 11.Peretz G, Bakhrat A, Abdu U. Expression of the Drosophila melanogaster GADD45 homolog (CG11086) affects egg asymmetric development that is mediated by the c-Jun N-terminal kinase pathway. Genetics. 2007;177:1691–1702. doi: 10.1534/genetics.107.079517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shaposhnikov M, Proshkina E, Shilova L, Zhavoronkov A, Moskalev A. Lifespan and stress resistance in drosophila with overexpressed DNA repair genes. Sci. Rep. 2015;5:15299. doi: 10.1038/srep15299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barclay SS, et al. Systems biology analysis of Drosophila in vivo screen data elucidates core networks for DNA damage repair in SCA1. Hum. Mol. Genet. 2014;23:1345–1364. doi: 10.1093/hmg/ddt524. [DOI] [PubMed] [Google Scholar]
- 14.Henriques Pêgas JA, JoséVicente E, Correia Leandro da Silva KV, Guerrini Schenberg AC. PSO4: a novel gene involved in error-prone repair in Saccharomyces cerevisiae. Mutat. Res. Repair. 1989;218:111–124. doi: 10.1016/0921-8777(89)90017-7. [DOI] [PubMed] [Google Scholar]
- 15.Löscher M, et al. Interaction of U-box E3 ligase SNEV with PSMB4, the beta7 subunit of the 20 S proteasome. Biochem. J. 2005;388:593–603. doi: 10.1042/BJ20041517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hatakeyama S, Yada M, Matsumoto M, Ishida N, Nakayama KI. U box proteins as a new family of ubiquitin-protein ligases. J. Biol. Chem. 2001;276:33111–33120. doi: 10.1074/jbc.M102755200. [DOI] [PubMed] [Google Scholar]
- 17.Dellago H, et al. ATM-dependent phosphorylation of SNEVhPrp19/hPso4 is involved in extending cellular life span and suppression of apoptosis. Aging (Albany NY) 2012;4:290–304. doi: 10.18632/aging.100452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maréchal A, et al. PRP19 transforms into a sensor of RPA-ssDNA after DNA damage and drives ATR Activation via a ubiquitin-mediated circuitry. Mol. Cell. 2014;53:235–246. doi: 10.1016/j.molcel.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song EJ, et al. The Prp19 complex and the Usp4Sart3 deubiquitinating enzyme control reversible ubiquitination at the spliceosome. Genes Dev. 2010;24:1434–1447. doi: 10.1101/gad.1925010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chanarat S, Seizl M, Strässer K. The Prp19 complex is a novel transcription elongation factor required for TREX occupancy at transcribed genes. Genes Dev. 2011;25:1147–1158. doi: 10.1101/gad.623411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grillari J, et al. SNEV is an evolutionarily conserved splicing factor whose oligomerization is necessary for spliceosome assembly. Nucleic Acids Res. 2005;33:6868–6883. doi: 10.1093/nar/gki986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuraoka I, et al. Isolation of XAB2 complex involved in pre-mRNA splicing, transcription, and transcription-coupled repair. J. Biol. Chem. 2008;283:940–950. doi: 10.1074/jbc.M706647200. [DOI] [PubMed] [Google Scholar]
- 23.Sekelsky JJ, Brodsky MH, Burtis KC. DNA repair in Drosophila: Insights from the Drosophila genome sequence. J. Cell Biol. 2000;150:31–36. doi: 10.1083/jcb.150.2.F31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fortschegger K, et al. Early embryonic lethality of mice lacking the essential protein SNEV. Mol. Cell. Biol. 2007;27:3123–3130. doi: 10.1128/MCB.01188-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schraml E, et al. Haploinsufficiency of SNEV causes defects of hematopoietic stem cells functions. Stem Cells Dev. 2008;17:355–366. doi: 10.1089/scd.2007.0107. [DOI] [PubMed] [Google Scholar]
- 26.Monteforte R, et al. SNEVPrp19/PSO4 deficiency increases PUVA-induced senescence in mouse skin. Exp. Dermatol. 2016;25:212–217. doi: 10.1111/exd.12910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khan, A. et al. SNEVhPrp19/hPso4 regulates adipogenesis of human adipose stromal cells. Stem Cell Rep. (2016). doi:10.1016/j.stemcr.2016.12.001 [DOI] [PMC free article] [PubMed]
- 28.Voglauer R, et al. SNEV overexpression extends the life span of human endothelial cells. Exp. Cell Res. 2006;312:746–759. doi: 10.1016/j.yexcr.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 29.Osterwalder T, Yoon KS, White BH, Keshishian H. A conditional tissue-specific transgene expression system using inducible GAL4. Proc. Natl Acad. Sci. 2001;98:12596–12601. doi: 10.1073/pnas.221303298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Slack C, Giannakou ME, Foley A, Goss M, Partridge L. dFOXO-independent effects of reduced insulin-like signaling in Drosophila. Aging. Cell. 2011;10:735–748. doi: 10.1111/j.1474-9726.2011.00707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ford D, et al. Alteration of Drosophila life span using conditional, tissue-specific expression of transgenes triggered by doxycyline or RU486/Mifepristone. Exp. Gerontol. 2007;42:483–497. doi: 10.1016/j.exger.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tricoire H, et al. The steroid hormone receptor EcR finely modulates Drosophila lifespan during adulthood in a sex-specific manner. Mech. Ageing Dev. 2009;130:547–552. doi: 10.1016/j.mad.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 33.Park J-S, et al. Age- and oxidative stress-induced DNA damage in Drosophila intestinal stem cells as marked by Gamma-H2AX. Exp. Gerontol. 2012;47:401–405. doi: 10.1016/j.exger.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 34.Mahajan KN, Mitchell BS. Role of human Pso4 in mammalian DNA repair and association with terminal deoxynucleotidyl transferase. Proc. Natl Acad. Sci. U.S.A. 2003;100:10746–10751. doi: 10.1073/pnas.1631060100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu X, Legerski RJ. The Prp19/Pso4 core complex undergoes ubiquitylation and structural alterations in response to DNA damage. Biochem. Biophys. Res. Commun. 2007;354:968–974. doi: 10.1016/j.bbrc.2007.01.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bus JS, Gibson JE. Paraquat: model for oxidant-initiated toxicity. Environ. Health Perspect. 1984;55:37–46. doi: 10.1289/ehp.845537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.García Sar D, et al. Relationships between cisplatin-induced adducts and DNA strand-breaks, mutation and recombination in vivo in somatic cells of Drosophila melanogaster, under different conditions of nucleotide excision repair. Mutat. Res. 2012;741:81–88. doi: 10.1016/j.mrgentox.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 38.Jeon H-J, et al. Age-related change in γH2AX of Drosophila muscle: its significance as a marker for muscle damage and longevity. Biogerontology. 2015;16:503–516. doi: 10.1007/s10522-015-9573-0. [DOI] [PubMed] [Google Scholar]
- 39.Burger JMS, Promislow DEL. Sex-specific effects of interventions that extend fly life span. Sci. Aging Knowl. Environ. 2004;2004:pe30. doi: 10.1126/sageke.2004.28.pe30. [DOI] [PubMed] [Google Scholar]
- 40.Lehtovaara A, Schielzeth H, Flis I, Friberg U. Heritability of life span is largely sex limited in Drosophila. Am. Nat. 2013;182:653–665. doi: 10.1086/673296. [DOI] [PubMed] [Google Scholar]
- 41.Zhang NX, et al. The Pso4 mRNA splicing and DNA repair complex interacts with WRN for processing of DNA interstrand cross-links. J. Biol. Chem. 2005;280:40559–40567. doi: 10.1074/jbc.M508453200. [DOI] [PubMed] [Google Scholar]
- 42.Beck BD, Lee SS, Hromas R, Lee S-H. Regulation of Metnase’s TIR binding activity by its binding partner, Pso4. Arch. Biochem. Biophys. 2010;498:89–94. doi: 10.1016/j.abb.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paulsen RD, et al. A Genome-wide siRNA screen reveals diverse cellular processes and pathways that mediate genome stability. Mol. Cell. 2009;35:228–239. doi: 10.1016/j.molcel.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aguilera A, García-Muse T. R Loops: from transcription byproducts to threats to genome stability. Mol. Cell. 2012;46:115–124. doi: 10.1016/j.molcel.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 45.Tresini M, et al. The core spliceosome as target and effector of non-canonical ATM signalling. Nature. 2015;523:53–58. doi: 10.1038/nature14512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Di Tommaso P, et al. T-Coffee: a web server for the multiple sequence alignment of protein and RNA sequences using structural information and homology extension. Nucleic Acids Res. 2011;39:W13–W17. doi: 10.1093/nar/gkr245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Waterhouse AM, Procter JB, Martin DMA, Clamp M, Barton GJ. Jalview Version 2--a multiple sequence alignment editor and analysis workbench. Bioinformatics. 2009;25:1189–1191. doi: 10.1093/bioinformatics/btp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Minois N, et al. Spermidine promotes stress resistance in Drosophila melanogaster through autophagy-dependent and -independent pathways. Cell Death Dis. 2012;3:e401. doi: 10.1038/cddis.2012.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.