Abstract
Parasitic diseases, such as malaria and leishmaniasis, are relevant public health problems worldwide. For both diseases, the alarming number of clinical cases and deaths reported annually has justified the incentives directed to better understanding of host’s factors associated with susceptibility to infection or protection. In this context, over recent years, some studies have given special attention to B lymphocytes with a regulator phenotype, known as Breg cells. Essentially important in the maintenance of immunological tolerance, especially in autoimmune disease models such as rheumatoid arthritis and experimentally induced autoimmune encephalomyelitis, the function of these lymphocytes has so far been poorly explored during the course of diseases caused by parasites. As the activation of Breg cells has been proposed as a possible therapeutic or vaccine strategy against several diseases, here we reviewed studies focused on understanding the relation of parasite and Breg cells in malaria and leishmaniasis, and the possible implications of these strategies in the course of both infections.
Keywords: B regulatory cells, malaria, leishmaniasis, immunity, immunomodulation, protozoan infections, cytokines, immunological tolerance
Introduction
B cells, phenotypically characterized by CD19 expression, are divided into B1 and B2. Subsequently, B1 cells are subdivided into B1a (expressing CD5 on surface) and B1b (not expressing CD5), and B2 cells are subdivided into immature transitional cells (T1, T2, and T3), follicular B cells (FO), and marginal zone (MZ) B cells. While B1 cells originate from fetal liver precursors and are enriched in mucosal tissues and pleural and peritoneal cavities, B2 cells originate from bone marrow-derived precursors and are enriched in secondary lymphoid organs [1,2]. B1 and MZ B are considered important components of innate immunity because of their ability to respond rapidly to inflammatory and non-proteic antigenic stimulus besides differentiating into short-lived extra follicular plasma cells. FO B cells are a major component of adaptive immune response and exhibit the ability to differentiate into short-lived plasma cells or enter into the germinal center where it may undergo class switch and affinity maturation. FO B cells that exit the germinal center are named long-lived or memory B cells [1,3].
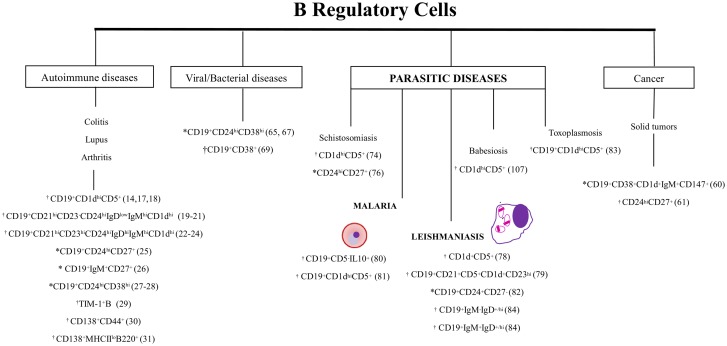
In addition to their function as antibody producing and antigen presenting cells [4−8], B cells have been described as being able to perform other immune functions such as modulation of cytokine production and maintenance of immunological tolerance [9,10]. Specifically, the maintenance of immunological tolerance has been attributed to a heterogeneous B cells subset termed regulatory B cells (Breg), which were originally characterized in autoimmunity and inflammation models [11−16]. Thus far, B cells subsets identified into the B1a (B10 cells-CD19+CD5+CD1dhi) [14,17,18], MZ (CD19+CD21hiCD23-CD24hiIgDloIgMhiCD1dhi) [19−21], and MZP B (T2-MZP-CD19+CD21hiCD23hiCD24hiIgDhiIgMhiCD1dhi) [22−24] lineages have been described in mice as being able to exert regulator/suppressive functions. In humans, Breg cells have been characterized into memory B subsets that exhibit CD19+CD24hiCD27+ (B10 cells related) and CD19+IgM+CD27+ phenotypes [25,26] and into immature transitional B cells (CD19+CD24hiCD38hi) [27,28]. In addition, plasma cells (CD138+MHCIIloB220+) and Tim1+ B cells (Tim1+CD19+) isolated from mice and plasmablasts (CD138+CD44+ and CD19+CD24+CD27+) isolated from both mice and humans are still described as being able to exercise immunoregulatory functions [29−31]. Thus, it is probable that many B cell types can differentiate into a Breg cell through mechanisms such as inflammatory signals and receptor-antigen binding interaction [16]. Although Breg cells express several surface markers, which can be commonly shared by distinct cell subpopulations during the course of various diseases (Figure 1), [6,21,22,25] a precise transcription factor able to define this B cells subset remains unknown. Several studies conducted in mice and humans have, therefore, attributed their suppressive role to the capacity to produce IL-10 [6,22,32−36], but there is evidence that TGF-β and IL-35–producing murine B cells also exercise regulatory functions [6,31,37−40].
Figure 1.
Phenotypic profile of Breg cells described in autoimmune, viral/bacterial, parasitic diseases and cancer.
Note: Breg population described in: *humans; †experimental model; *†humans and experimental model.
The factors/mediators driving the induction of Breg cells remain to be completely clarified. However, in murine model, B10 cells appear to be induced in vivo and in vitro by B cell-activating factor (BAFF), an important member of tumor necrosis factor (TNF) family cytokines and a regulator for B cell maturation and survival [41]. In fact, paradoxical effects have been attributed to BAFF on mouse B cells: expanding Breg but also sustaining the production of antibodies able to exercise pathogenic function. During multiple sclerosis (MS), BAFF expression is strongly upregulated in the brain where enrichment of B cells subsets and/or follicles have been noted [42,43], which possibly support the production of pathogenic antibodies [44]. However, clinical trials have shown that BAFF blocking worsens the disease prognosis possibly due to inhibition of Breg induction [45]. In a similar manner, during collagen-induced arthritis (CIA), BAFF–induced Breg cells seem to be essential to avoid disease development and progression by IL-10 production [41]. On the other hand, the blocking of BAFF appeared to ameliorate disease symptoms in some cases of systemic lupus erythematosus (SLE) [46] and rheumatoid arthritis (RA) [47,48].
The mechanisms by which B cells are activated to exercise their regulatory effects may occur through distinct stimulus and mediators, some of them perhaps still unknown [49]. In mice and humans, the efficient function of Breg cells appears to be significantly influenced by B cell receptor (BCR), CD40–CD40L interaction, and TLR (Toll Like Receptors) activation besides interaction between others costimulatory molecules such as CD80/CD86–CD152 [21,22,50]. In this context, the production of IL-10, reflecting the activation of human B10 cells, substantially increases following CD40–CD40L interaction and activation of TLR by microbial components [51], whereas the binding of antigens to BCR reduces the production of this cytokine [49]. In mice, the activation of TLR4 and TLR9 is described as an important event able to efficiently suppress the progression of diabetes, EAE (experimental autoimmune encephalomyelitis), and arthritis [22]. However, this effect appears to require still a coordinate interaction among others costimulatory molecules because B cells restrict CD40 deficiency are associated with development of EAE [13,52]. Interestingly, in this same autoimmune disease model, the Breg cell activation still requires signalization through BCR since in the absence of CD19 (co-receptor that optimizes BCR signal) the animals develop severe clinical condition [17,53]. Since Breg cells are activated for distinct signals including TLR, it is important to consider that distinct compounds/products may trigger different B cell targets [54] and, thus, differently modulate their immune regulatory capacity; for example, while TLR4 (expressed on murine B1, MZ, and memory B cells but absent on majority of human B cells) is triggered by lipopolysaccharides (LPS) [54, 55], TLR1/6, TLR2, TLR7, and TLR9, present in murine and humans B cells, are activated by bacterial lipopeptides, peptidoglycans, CpG DNA motifs, and single-stranded RNA, respectively [56]. Furthermore, is notable that sensitivity to TLR activation and expression levels of TLR 6, 7, and 9 is more elevated in memory B cells in comparison with circulating naïve B cells [55]. Since Breg cells have been associated with prevention or increased disposition to immune system-related diseases, infectious and/or cancerous, they have become appealing targets for therapeutic intervention. Despite the fact that in recent years many compounds have been developed to target TLRs for either stimulating or antagonizing their activity [57], questions like the consequences of induction of Breg cells by TLR agonists or antagonists in the host cells with respect to development of diseases like cancer and bacterial or viral infection first need to be addressed. Furthermore, it remains to be elucidated whether blocking or activation of TLR as a therapy negatively or positively affects essential functions performed by other cells amongst many other issues.
Insights about the role of Breg cells in the course of infectious and non-infectious diseases
Breg cells play a protective role in autoimmune settings such as allergy, RA, SLE, MS, and EAE, where the strong proinflammatory Th1 and/or Th17 profile displays serious deleterious effects in affected individuals [58,59]. However, therapeutic inhibition of Breg cells can have beneficial effects in the control of others diseases such as cancer and viral and bacterial infections. In breast, cervical, and ovarian human carcinoma, Breg cells, phenotypically described as CD19+CD38+CD1d+IgM+CD147+ (or GrB+ Bregs), have been observed within the tumor microenvironment possibly contributing to increase in relative tumor volume [60]. In human chronic lymphocytic leukemia (CLL) CD24hiCD27+ cells appear to contribute to general immune suppression of patients through IL-10 secretion and thereby toward disease progression [8,60−63]. Really, Inoue and collaborators (2006) showed that B–cell–deficient mice are able to control or eliminate the tumor growth through IFN-γ, whereas the cancer evolution may be observed in wild type animals [64].
Deleterious effects of Breg cells are also observed in patients infected with human immunodeficiency virus (HIV) and hepatitis B virus (HBV), where T cell responses are essential for antiviral defense [65−67]. Studies, conducted in vitro and/or in vivo, have demonstrated that IL-10 produced by Breg cells act to promote dysfunction of disease-specific CD8+ T cells that favor a higher viral load [66, 67] and inflammation [65]. In fact, Breg cells depletion in vitro restores HBV–specific CD8 T cells responses, suggesting the important role of Bregs to the development of viral load during infections [65]. Relative to bacterial infections, B cells are activated to produce immunoregulatory cytokines following engagement of bacterial LPS with TLR (TLR2, 4, 5, 7/8, and 9) expressed on cellular surface [68]. In a salmonellosis murine model, deletion of the TLR adaptor molecule myeloid differentiation primary response protein 88 (Myd88), or TLR2 and TLR4 exclusively in B cells leads to decreased secretion of IL-10 by B cells making the mice more resistant to Salmonella typhimurium infection [68,69]. In a similar fashion, reduction in the levels of IL-10 in B cell–deficient mice were associated to an efficient control of infection caused by Brucella abortus [70].
While studies investigating the relation among Breg cells and occurrence of autoimmune and viral diseases are in constant development, the phenotypic description and immunomodulatory/immunoregulatory function of these cells remains poorly investigated during parasitic infections [68]. In helminth infections, the role of Breg cells has been investigated particularly during shistosomiasis [71−76]. CD5+ B1 cells seem to be elevated in the peritoneal cavity during the first 5 weeks of experimental infection with Schistosoma mansoni and are associated with lacto-N-fucopentaose III, a component of shistosoma egg antigen [71, 77]. These cells appear to limit pathological infection as B1 cell-deficient animals clearly display a higher susceptibility to S. mansoni infection, as evidenced through increase in the tissue egg load, granuloma density, and elevated mortality [72]. The elevation of CD24hiCD27+ and CD1dhiCD5+ Breg cells following murine and human schistosomiasis have also been observed, but the consequences of this observation in terms of immunomodulation were measured particularly in allergic disorders. During S. mansoni infection, IL-10 produced by B cells suppressed Th2–mediated severe allergic reactions, namely, anaphylaxis and allergic airway inflammation [73, 74, 76].
Relative to protozoan parasites, still are rare the studies that investigate the role of Breg during the course of infection, but there is evidences that this B cell subtype is critically important in immune homeostasis [78−84]. In this context, Jeong and collaborators demonstrated that IL-10–producing CD1dhiCD5+ Breg cells are induced by products that are secreted by fully replicated tachyzoites and are essential to the chronicity of infection in Toxoplasma gondii murine model [83]. Considering that malaria and leishmaniasis, secular diseases caused by protozoans, remain two important causes of morbidity and/or mortality worldwide, insights into the role of Breg cells could provide the development of new therapeutic and vaccine strategies. Therefore, we reviewed and investigated major advances relative to understanding the role of this subset of B cells in the regulation of immune responses and immunopathology during both malaria and leishmaniasis [85].
Occurrence of Breg cells during malaria
Malaria, a parasitic disease caused by protozoa of the Plasmodium genus, is a public health concern worldwide. In the last year, around 214 million clinical cases and 438,000 deaths occurred in tropical and subtropical regions of the globe, with children under five years old and pregnant women being the main target of infection [86]. In general, deaths are primarily due to important clinical syndromes, including neuronal disorders caused as a result of sequestration of infected red blood cells in brain microvessels induced by parasites [87,88]. However, proinflammatory cytokines and other mediators produced during malaria seem to be critical in its pathology, activating and recruiting cytotoxic cells for the infection site [89, 90]. During these pathologic processes, regulatory cells and antiinflammatory cytokines (such as IL-10) are suggested to be important for preventing clinical evolution of malarial neuropathology [80, 81].
Despite the absence of studies directly investigating the regulatory effects of B cells during human malaria, increased BAFF levels and an increase in circulating CD10+ B cells have been observed in subjects reporting uncomplicated malaria [91−93]. As CD10+ B cells may indirectly include BAFF–induced CD10+ Breg cells, it is possible to suggest that Breg cells may be responsible for establishment of parasite–host equilibrium [41, 94, 95].
Specifically, the relationship between IL-10–producing Breg cells and malarial infection has been investigated only in the experimental model of cerebral malaria (ECM) caused by P. berghei ANKA (PbA) [80, 81]. ECM associated with PbA infection involves similar mechanisms to those observed during human cerebral malaria, which is characterized by massive destruction of erythrocytes and release of proinflammatory cytokines and mediators such as TNF-α, lymphotoxin, IL-1, and IL-6, inducing activation and recruitment of natural killer cells (NK) and cytotoxic CD8+ cells in brain blood vessels and upregulation of adhesion molecules in the vascular endothelium. Together, these factors determine leukocyte and infected red blood cell (pRBC) accumulation in microvessels of the brain and lungs, resulting in the death of mice [87, 96−98]. Recently, Liu and colleagues [81] showed that the adoptive transfer of IL-10+ Breg cells, but not Treg cells (CD4+CD25+Foxp3+), to PbA–infected mice was associated with reduced accumulation of NK and CD8+ T cells and hemorrhage in brain tissue, improving the survival rate of the animal. However, parasitemia levels were not altered by the presence or absence of these cells. In addition, the study showed that treatment of Breg cell-recipient mice with anti–IL-10 monoclonal antibodies blocked the protective effect of Breg cells. In summary, Breg cells may selectively exert regulatory effects on the immunopathology of malaria by IL-10 production but appear to have little impact against blood parasitemia development [81]. Using the same model of cerebral malaria, Bao and collaborators [80] showed that Breg cells, characterized by simultaneous expression of CD19+CD5−IL10+ markers, appear to be the main source of plasma IL-10 production and are able to protect the animals from mortality when they are adoptively transferred to naïve mice. Thereby, this study diverges of others, which appoint B1a cells as major sources of IL-10 [14, 15, 17, 18, 99, 100]. Of interest, CD5 is a pan T cell marker being few expressed on B cells. While in mice CD5 expression characterizes B1a lineage [101] into human B cells this marker does not define a specific B cell subset being expressed on B1-like cells, immature B cells, transitional B cells, and pre–naive B cells [102−104]. Importantly, Breg cells have described within both B cell lineages, denoting the importance of CD5 as a possible ‘indicator’ of regulatory activity. The role of CD5 as an immunoregulatory molecule appears to be mediated by its inhibiting capacity of both BCR and TCR activation after binding with these receptors. This bond increases the threshold required to activate a B cell or a T cell and renders it tolerant to its cognate antigens [105]. CD5 may also act by enhancing the production of IL-10 through stimulation of the cytokine gene promoter as demonstrated by Gary-Gouy and colleagues [106]. In this study, the author demonstrated that CD5 transfection for Daudi B cells (CD5−) upregulated several genes, including those belonging to the IL-10 family. However, the ability to produce IL-10 and/or mediate immunoregulatory functions is not restricted to CD5+ cells as noted in previous studies [63, 107].
While the expansion/activation of Breg cells resulting in the production of higher IL-10 levels seems to be essential for preventing cerebral malaria in mice infected with PbA, in the murine infection caused by Babesia microti, a Plasmodium-related apicomplexan parasite, such factors appear to contribute toward enhanced growth and survival of the parasite [80, 81, 108]. In fact, the transfer of IL-10-producing CD1dhiCD5+ Breg cells isolated from B. microti-infected mice allowed the development of high levels of circulating parasitemia on infected recipient mice, whereas B cell–deficient mice were able to control the infection [108]. Thus, it is evident that immune factor–pathogen interactions observed between parasite and host are not easily extrapolated to related species.
Despite two studies highlighting the beneficial role of Breg cells during severe malaria through the inhibition of exacerbated inflammatory responses, it is important to investigate the occurrence and implications of Breg cells during uncomplicated and complicated human malaria and related diseases.
Breg cells and leishmaniasis infection
Human leishmaniasis, usually classified as cutaneous (CL), mucocutaneous (MCL), and visceral leishmaniasis (VL), is caused by over 20 species of the genus Leishmania [109]. Recent estimates indicate that around 14 million people are infected worldwide, with an annual incidence of 1.3 million new cases and 20,000 deaths per year related to VL [110], a systemic disease characterized by parasitism of the spleen, liver, and bone marrow [111, 112]. CL is the most prevalent form, characterized by the development of an ulcerative self-healing skin lesion; MCL is a severe and chronic infection causing diffuse lesions that may even spread to mucosal tissues [113, 114]. These clinical manifestations are, generally, not lethal but result in disfiguration and disability, resulting in societal stigma and marginalization, particularly for women. VL or kala-azar is the most severe leishmaniasis form, characterized by systemic parasitism, and may be fatal if not treated [111, 112].
The contribution of B cells in human leishmaniasis pathogenesis is controversial [115, 116]. Various studies have demonstrated that B cells play a negative role in experimental models of leishmaniasis by contributing toward increased susceptibility of infection by producing polyclonal antibodies [117−120] and/or immunosuppressive cytokines such as IL-10 secreted by regulatory or non-regulatory B cells [78, 119−121]. In human infections, a high titer of Leishmania–specific antibodies are observed in patients with a more severe clinical form of the cutaneous disease, diffuse cutaneous leishmaniasis [122, 123], and in patients with VL [117, 124], whereas patients with self-healing localized cutaneous leishmaniasis lack Leishmania-specific antibodies or elicit a very weak response [116]. On the other hand, B cells appear to play a protective role in disease pathogenesis, as observed in high prevalence of seropositive healthy individuals in areas endemic for VL [116, 125] and longer persistence of antibodies against Leishmania (≥15 years) after the infection has been cured [116, 126, 127].
The immunosuppressive role of B cells observed during leishmaniasis appears to be conditioned, at least in part, to generation of Breg cells [78, 79, 82, 84]. In the context of CL, a single study using murine models has demonstrated that Breg cells are required for enhanced susceptibility to infection in several Leishmania species [78]. In a similar manner, Ronet and collaborators [78] reported that IL-10 secreted by CD1d+CD5+ Breg cells is associated with a more severe disease in L. major BALB/c mice as a result of an immune response polarized for Th2 profile. Regarding VL, a recent in vitro study showed that human B cells exposed to L. infantum amastigotes exhibited a regulator phenotype (CD19+CD24+CD27−) and higher capacity to produce IL-10. In addition, the activation, function, and proliferation of CD4+ T cells were inhibited after contact with conditioned media from B cells incubated with L. infantum amastigotes, suggesting regulatory activity of B cells mediated by IL-10 [82]. In mice, using a similar methodology, Bankoti and collaborators [79] identified CD19+CD21+CD5+CD1d+CD23hi B cells, a phenotype that has been associated with Breg cells in hypersensitivity models [17]. These cells were activated to produce high IL-10 levels after stimulation of B cells with L. donovani amastigotes and CpG in a MyD88–dependent manner. Another study conducted in canines infected with VL pointed, recently, to a new Breg cells subset with a regulatory function through IL-10 production. This new Breg cells subset was phenotypically described as CD19+IgM−IgD+/hi or CD19+IgM+IgD+/hi, and levels of these Breg cells increased at least threefold during progressive VL and were critical for suppressing Th1 cell effector function through interaction of B cells and PDL1(programmed death–ligand 1/receptor) [84]. Similar data were obtained in a RA model where pathogenic B cells were observed only when they expressed IgM together with IgD but not when IgM was expressed alone [128]. Since IgM+IgD+ and IgM only, together to the others distinctive marker, represent different subsets (naïve vs. non-switch memory B-cells), they might produce different cytokines and exert distinct regulatory functions. Even expressed simultaneously, IgM and IgD are involved in different surface clusters and signal independently [129]. Of interest, during development in the bone marrow, mIg (membrane immunoglobulin) is restricted to the IgM isotype; however, as soon as the B cell exits the bone marrow to populate peripheral lymphoid organs, it starts to express surface IgD. This event can occur primarily by two distinct mechanisms, alternative splicing and CSR (class switch recombination), but intriguingly, BCR–dependent functions such as activation, receptor desensitization, apoptosis induction, and immunological tolerance are induced by either of the two isotypes or by both isotypes in combination what makes its function still more enigmatic (for more information about B cell class switch we recommended the paper by Geisberger and colleagues) [130].
IL-10 can be produced by innate cells such as macrophages, NK cells, dendritic cells (DCs), and multiple adaptive T and B cells [117, 120]. Importantly, the evidence shown above suggests that this cytokine secreted by B cells, particularly by Breg cells, can play a decisive role in progressive decline of the immune system of human patients with VL and lead to a fatal outcome in untreated cases [82, 131, 132]. Accordingly, the inhibition or neutralization of IL-10 by blocking IL-10 receptor or using anti-IL-10 monoclonal antibodies that allow improved immune response and parasite killing both in mouse and human VL infections may be a good strategy to control infection [117, 133−136].
Since the effects of Breg cells have not been studied in the context of human infection, urgent studies are warranted to determine the clinical relevance of Breg induction by Leishmania parasites [82]. Understanding the role of Breg cells in leishmaniasis may contribute to development of future vaccines or immunotherapies, and new strategies should be developed to manipulate these cells to benefit the host [116, 131, 137].
Concluding remarks
Breg cell depletion therapy or the development of vaccines directed to their generation must be carefully evaluated. The expansion of these lymphocytes in the context of autoimmune and inflammatory diseases and ECM demonstrates a protective role, but in HIV, cancer and leishmaniasis the presence of these cells is associated with increased disease severity. As yet there are few studies focused on Breg cells, especially from human samples; further studies are needed in order to elucidate their role, in the context of different parasitic diseases that can occur simultaneously in a specific area. Future research will help the development of new strategies to combat leishmaniasis and malaria characterized by high rates of morbidity and mortality worldwide.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported by the ‘Fundação de Amparo a Pesquisa do Estado de Minas Gerais’ (FAPEMIG: CBB – APQ-02315-14) and ‘Conselho Nacional de Desenvolvimento Científico e Tecnológico’ (CNPq: 454575/2014-9).
Ethics approval
Ethics approval is not applicable for this review paper.
Acknowledgements
The authors thank the fellowship by ‘Coordenação de Aperfeiçoamento de Pessoal de Nível Superior’ (CAPES) (R.R. Soares and L.M.R. Antinarelli).
References
- [1].Lund FE. Cytokine-producing B lymphocytes-key regulators of immunity. Curr Opin Immunol. 2008;20(3):332–338. 10.1016/j.coi.2008.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Welner RS, Pelayo R, Kincade PW. Evolving views on the genealogy of B cells. Nat Rev Immunol. 2008;8(2):95–106. 10.1038/nri2234 [DOI] [PubMed] [Google Scholar]
- [3].Borhis G, Richard Y.. Subversion of the B-cell compartment during parasitic, bacterial, and viral infections. BMC Immunol. 2015;16(15):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].DiLillo DJ, Hamaguchi Y, Ueda Y, et al. Maintenance of long-lived plasma cells and serological memory despite mature and memory B cell depletion during CD20 immunotherapy in mice. J Immunol. 2008;180(1):361–371. 10.4049/jimmunol.180.1.361 [DOI] [PubMed] [Google Scholar]
- [5].LeBien TW, Tedder TF. B lymphocytes: how they develop and function. Blood. 2008;112(5):1570–1580. 10.1182/blood-2008-02-078071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Han J, Sun L, Fan X, et al. Role of regulatory B cells in neuroimmunologic disorders. J Neurosci Res. 2016;94(8):693–701. 10.1002/jnr.v94.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Liu Y, Wu Y, Ramarathinam L, et al. Gene-targeted B-deficient mice reveal a critical role for B cells in the CD4 T cell response. Int Immunol. 1995;7(8):1353-1362. 10.1093/intimm/7.8.1353 [DOI] [PubMed] [Google Scholar]
- [8].Tedder TF. B10 cells: a functionally defined regulatory B cell subset. J Immunol. 2015;194(4):1395–1401. 10.4049/jimmunol.1401329 [DOI] [PubMed] [Google Scholar]
- [9].Harris DP, Haynes L, Sayles PC, et al. Reciprocal regulation of polarized cytokine production by effector B and T cells. Nat Immunol. 2000;1(6):475–482. 10.1038/82717 [DOI] [PubMed] [Google Scholar]
- [10].Shlomchik MJ, Craft JE, Mamula MJ. From T to B and back again: positive feedback in systemic autoimmune disease. Nat Rev Immunol. 2001;1(2):147–153. 10.1038/35100573 [DOI] [PubMed] [Google Scholar]
- [11].Katz SI, Parker D, Turk JL. B-cell suppression of delayed hypersensitivity reactions. Nature. 1974;251:550–551. 10.1038/251550a0 [DOI] [PubMed] [Google Scholar]
- [12].Neta R, Salvin SB. Specific suppression of delayed hypersensitivity: the possible presence of a suppressor B cell in the regulation of delayed hypersensitivity. J Immunol. 1974;113(6):1716–1725. [PubMed] [Google Scholar]
- [13].Fillatreau S, Sweenie CH, McGeachy MJ, et al. B cells regulate autoimmunity by provision of IL-10. Nat Immunol. 2002;3(10):944–950. 10.1038/ni833 [DOI] [PubMed] [Google Scholar]
- [14].Mizoguchi A, Mizoguchi E, Takedatsu H, et al. Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity. 2002;16(2):219–230. 10.1016/S1074-7613(02)00274-1 [DOI] [PubMed] [Google Scholar]
- [15].Mauri C, Gray D, Mushtaq N, et al. Prevention of arthritis by interleukin 10–producing B cells. J Exp Med. 2003;197(4):489–501. 10.1084/jem.20021293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Rosser EC, Mauri C. Regulatory B cells: origin, phenotype, and function. Immunity. 2015;42(4):607–612. 10.1016/j.immuni.2015.04.005 [DOI] [PubMed] [Google Scholar]
- [17].Yanaba K, Bouaziz JD, Haas KM, et al. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity. 2008;28(5):639–650. 10.1016/j.immuni.2008.03.017 [DOI] [PubMed] [Google Scholar]
- [18].DiLillo DJ, Matsushita T, Tedder TF. B10 cells and regulatory B cells balance immune responses during inflammation, autoimmunity, and cancer. Ann NY Acad Sci. 2010;1183(2010):38–57. 10.1111/j.1749-6632.2009.05137.x [DOI] [PubMed] [Google Scholar]
- [19].Wei B, Velazquez P, Turovskaya O, et al. 2005. Mesenteric B cells centrally inhibit CD4+ T cell colitis through interaction with regulatory T cell subsets. Proc Natl Acad Sci USA. 2005;102(6):2010-2015. 10.1073/pnas.0409449102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Gray M, Miles K, Salter D, et al. Apoptotic cells protect mice from autoimmune inflammation by the induction of regulatory B cells. Proc Natl Acad Sci USA. 2007;104(35):14080–14085. 10.1073/pnas.0700326104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Mauri C, Blair PA. Regulatory B cells in autoimmunity: developments and controversies. Nat Rev Rheumatol. 2010;6(11):636–643. 10.1038/nrrheum.2010.140 [DOI] [PubMed] [Google Scholar]
- [22].Mauri C, Bosma A. Immune regulatory function of B cells. Annu Rev Immunol. 2012;30:221–241. 10.1146/annurev-immunol-020711-074934 [DOI] [PubMed] [Google Scholar]
- [23].Evans JG, Chavez-Rueda KA, Eddaoudi A, et al. Novel suppressive function of transitional 2 B cells in experimental arthritis. J Immunol. 2007;178(12):7868–7878. 10.4049/jimmunol.178.12.7868 [DOI] [PubMed] [Google Scholar]
- [24].Blair PA, Chavez-Rueda KA, Evans JG, et al. Selective targeting of B cells with agonistic anti-CD40 is an efficacious strategy for the generation of induced regulatory T2-like B cells and for the suppression of lupus in MRL/lpr mice. J Immunol. 2009;182(6):3492–3502. 10.4049/jimmunol.0803052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Iwata Y, Matsushita T, Horikawa M, et al. Characterization of a rare IL-10–competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood. 2011;117(2):530–541. 10.1182/blood-2010-07-294249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Khoder A, Sarvaria A, Alsuliman A, et al. Regulatory B cells are enriched within the IgM memory and transitional subsets in healthy donors but are deficient in chronic GVHD. Blood. 2014;124(13):2034–2045. 10.1182/blood-2014-04-571125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Blair PA, Noreña LY, Flores-Borja F, et al. CD19+CD24hiCD38hi B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic lupus erythematosus patients. Immunity. 2010;32(1):129–140. 10.1016/j.immuni.2009.11.009 [DOI] [PubMed] [Google Scholar]
- [28].Flores-Borja F, Bosma A, Ng D, et al. CD19+CD24hiCD38hi B cells maintain regulatory T cells while limiting TH1 and TH17 differentiation. Sci Transl Med. 2013;5(173):173ra23. 10.1126/scitranslmed.3005407 [DOI] [PubMed] [Google Scholar]
- [29].Xiao S, Brooks CR, Zhu C, et al. Defect in regulatory B-cell function and development of systemic autoimmunity in T-cell Ig mucin 1 (Tim-1) mucin domain-mutant mice. Proc Natl Acad Sci USA. 2012;109(30):12105–12110. 10.1073/pnas.1120914109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Matsumoto M, Baba A, Yokota T, et al. Interleukin-10-producing plasmablasts exert regulatory function in autoimmune inflammation. Immunity. 2014;41(6):1040–1051. 10.1016/j.immuni.2014.10.016 [DOI] [PubMed] [Google Scholar]
- [31].Shen P, Roch T, Lampropoulou V, et al. IL-35-producing B cells are critical regulators of immunity during autoimmune and infectious diseases. Nature. 2014;507(7492):366–370. 10.1038/nature12979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].O’garra A, Chang R, Go N, et al. Ly‐1 B (B‐1) cells are the main source of B cell‐derived interleukin 10. Eur J Immunol. 1992;22(3):711–717. 10.1002/(ISSN)1521-4141 [DOI] [PubMed] [Google Scholar]
- [33].Mizoguchi A, Mizoguchi E, Smith RN, et al. Suppressive role of B cells in chronic colitis of T cell receptor α mutant mice. J Exp Med. 1997;186(10):1749–1756. 10.1084/jem.186.10.1749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Mauri C, Menon M. The expanding family of regulatory B cells. Int Immunol. 2003;27(10):479–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Mizoguchi A, Bhan AK. A case for regulatory B cells. J Immunol. 2006;176(2):705–710. 10.4049/jimmunol.176.2.705 [DOI] [PubMed] [Google Scholar]
- [36].Saraiva M, O’Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol. 2010;10(3):170–181. 10.1038/nri2711 [DOI] [PubMed] [Google Scholar]
- [37].Douglas RS, Woo EY, Capocasale RJ, et al. Altered response to and production of TGF-beta by B cells from autoimmune NZB mice. Cell. Immunol. 1997;179:126–137. 10.1006/cimm.1997.1149 [DOI] [PubMed] [Google Scholar]
- [38].Wang RX, Yu CR, Dambuza IM, et al. Interleukin-35 induces regulatory B cells that suppress autoimmune disease. Nat Med. 2014;20(6):633–641. 10.1038/nm.3554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Egwuagu CE, Yu CR. Interleukin 35-producing B cells (I35-Breg): a new mediator of regulatory B-cell functions in CNS autoimmune diseases. Crit Rev Immunol. 2015;35(1):49–57. 10.1615/CritRevImmunol.v35.i1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Natarajan P, Singh A, McNamara JT, et al. Regulatory B cells from hilar lymph nodes of tolerant mice in a murine model of allergic airway disease are CD5+, express TGF-beta, and co-localize with CD4+Foxp3+ T cells. Mucosal Immunol. 2012;5(6):691–701. 10.1038/mi.2012.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Yang M, Sun L, Wang S, et al. Novel function of B cell-activating factor in the induction of IL-10–Producing regulatory B cells. J Immunol. 2010;184(7):3321–3325. 10.4049/jimmunol.0902551 [DOI] [PubMed] [Google Scholar]
- [42].Cepok S, Rosche B, Grummel V, et al. Short-lived plasma blasts are the main B cell effector subset during the course of multiple sclerosis. Brain. 2005;128:1667–1676. 10.1093/brain/awh486 [DOI] [PubMed] [Google Scholar]
- [43].Krumbholz M, Theil D, Derfuss T, et al. BAFF is produced by astrocytes and up-regulated in multiple sclerosis lesions and primary central nervous system lymphoma. J Exp Med. 2005;201(2):195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Hartung HP, Kieseier BC. Atacicept: targeting B cells in multiple sclerosis. Ther Adv Neurol Disord. 2010;3(4):205–216. 10.1177/1756285610371146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Magliozzi R, Howell O, Vora A, et al. Meningeal B-cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology. Brain. 2007;130(4):1089–1104. [DOI] [PubMed] [Google Scholar]
- [46].Ramanujam M, Davidson A. BAFF blockade for systemic lupus erythematosus: will the promise be fulfilled? Immunol Rev. 2008;223:156–174. 10.1111/imr.2008.223.issue-1 [DOI] [PubMed] [Google Scholar]
- [47].Gross JA, Dillon SR, Mudri S, et al. TACI-Ig neutralizes molecules critical for B cell development and autoimmune disease impaired B cell maturation in mice lacking BLyS. Immunity. 2001;15:289–302. [DOI] [PubMed] [Google Scholar]
- [48].Wei F, Chang Y, Wei W. The role of BAFF in the progression of rheumatoid arthritis. Cytokine. 2015;76(2):537–544. 10.1016/j.cyto.2015.07.014 [DOI] [PubMed] [Google Scholar]
- [49].Jamin C, Morva A, Lemoine S, et al. Regulatory B lymphocytes in humans: a potential role in autoimmunity. Arthritis Rheum. 2008;58(7):1900–1906. 10.1002/art.v58:7 [DOI] [PubMed] [Google Scholar]
- [50].Noh G, Lee JH. Regulatory B cells and allergic diseases. Allergy Asthma Immunol Res. 2011;3(3):168–177. 10.4168/aair.2011.3.3.168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Berthelot JM, Jamin C, Amrouche K, et al. Regulatory B cells play a key role in immune system balance. Joint Bone Spine. 2013;80(1):18–22. 10.1016/j.jbspin.2012.04.010 [DOI] [PubMed] [Google Scholar]
- [52].Mauri C, Ehrenstein MR. The ‘short’ history of regulatory B cells. Trends Immunol. 2008;29(1):34–40. 10.1016/j.it.2007.10.004 [DOI] [PubMed] [Google Scholar]
- [53].Sato S, Steeber DA, Tedder TF. The CD19 signal transduction molecule is a response regulator of B-lymphocyte differentiation. Proc Natl Acad Sci USA. 1995;92:11558–11562. 10.1073/pnas.92.25.11558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Ma K, Li J, Fang Y, et al. Roles of B cell-intrinsic TLR signals in systemic lupus erythematosus. Int J Mol Sci. 2015;16(6):13084–13105. 10.3390/ijms160613084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Barr TA, Brown S, Ryan G, et al. TLR-mediated stimulation of APC: Distinct cytokine responses of B cells and dendritic cells. Eur J Immunol. 2007;37(11):3040–3053. 10.1002/(ISSN)1521-4141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Chiron D, Bekeredjian-Ding I, Pellat-Deceunynck C, et al. Toll-like receptors: lessons to learn from normal and malignant human B cells. Blood. 2008;112(6):2205–2213. 10.1182/blood-2008-02-140673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Connolly DJ, O’Neill LA. New developments in Toll-like receptor targeted therapeutics. Curr Opin Pharmacol. 2012;12(4):510–518. 10.1016/j.coph.2012.06.002 [DOI] [PubMed] [Google Scholar]
- [58].Klinker MW, Lundy SK. Multiple mechanisms of immune suppression by B lymphocytes. Mol Med. 2012;18(1):123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Yang M, Deng J, Liu Y, et al. IL-10–producing regulatory B10 cells ameliorate collagen-induced arthritis via suppressing Th17 cell generation. Am J Pathol. 2012;180(6):2375–2385. 10.1016/j.ajpath.2012.03.010 [DOI] [PubMed] [Google Scholar]
- [60].Lindner S, Dahlke K, Sontheimer K, et al. Interleukin 21–induced granzyme B-expressing B cells infiltrate tumors and regulate T cells. Cancer Res. 2013;73(8):2468–2479. 10.1158/0008-5472.CAN-12-3450 [DOI] [PubMed] [Google Scholar]
- [61].DiLillo DJ, Weinberg JB, Yoshizaki A, et al. Chronic lymphocytic leukemia and regulatory B cells share IL-10 competence and immunosuppressive function. Leukemia. 2013;27(1):170–182. 10.1038/leu.2012.165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Candando KM, Lykken JM, Tedder TF. B10 cell regulation of health and disease. Immunol Rev. 2014;259(1):259–272. 10.1111/imr.12176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Lykken JM, Candando KM, Tedder TF. Regulatory B10 cell development and function. Int Immunol. 2015;27(10):471–477. 10.1093/intimm/dxv046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Inoue S, Leitner WW, Golding B, et al. Inhibitory effects of B cells on antitumor immunity. Cancer Res. 2006;66(15):7741–7747. 10.1158/0008-5472.CAN-05-3766 [DOI] [PubMed] [Google Scholar]
- [65].Das A, Ellis G, Pallant C, et al. IL-10–producing regulatory B cells in the pathogenesis of chronic hepatitis B virus infection. J Immunol. 2012;189(8):3925–3935. 10.4049/jimmunol.1103139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Siewe B, Stapleton JT, Martinson J, et al. Regulatory B cell frequency correlates with markers of HIV disease progression and attenuates anti-HIV CD8⁺ T cell function in vitro. J Leukoc Biol. 2013;93(5):811–818. 10.1189/jlb.0912436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Liu J, Zhan W, Kim CJ, et al. IL-10–producing B cells are induced early in HIV-1 infection and suppress HIV-1–specific T cell responses. PLoS One. 2014;9(2):e89236. 10.1371/journal.pone.0089236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Majlessi L, Lo-Man R, Leclerc C. Regulatory B and T cells in infections. Microbes Infect. 2008;10(9):1030–1035. 10.1016/j.micinf.2008.07.017 [DOI] [PubMed] [Google Scholar]
- [69].Neves P, Lampropoulou V, Calderon-Gomez E, et al. Signaling via the MyD88 adaptor protein in B cells suppresses protective immunity during Salmonella typhimurium infection. Immunity. 2010;33(5):777–790. 10.1016/j.immuni.2010.10.016 [DOI] [PubMed] [Google Scholar]
- [70].Goenka R, Parent MA, Elzer PH, et al. B cell–deficient mice display markedly enhanced resistance to the intracellular bacterium Brucella abortus. J Infect Dis. 2011;203(8):1136–1146. 10.1093/infdis/jiq171 [DOI] [PubMed] [Google Scholar]
- [71].Velupillai P, Secor WE, Horauf AM, et al. B-1 cell (CD5+B220+) outgrowth in murine schistosomiasis is genetically restricted and is largely due to activation by polylactosamine sugars. J Immunol. 1997;158(1):338–344. [PubMed] [Google Scholar]
- [72].Gaubert S, Viana da Costa A, Maurage CA, et al. X–linked immunodeficiency affects the outcome of Schistosoma mansoni infection in the murine model. Parasite Immunol. 1999;21(2):89–101. 10.1046/j.1365-3024.1999.00205.x [DOI] [PubMed] [Google Scholar]
- [73].Mangan NE, Fallon RE, Smith P, et al. Helminth infection protects mice from anaphylaxis via IL-10–producing B cells. J Immunol. 2004;173(10):6346–6356. 10.4049/jimmunol.173.10.6346 [DOI] [PubMed] [Google Scholar]
- [74].Amu S, Saunders SP, Kronenberg M, et al. Regulatory B cells prevent and reverse allergic airway inflammation via FoxP3–positive T regulatory cells in a murine model. J Allergy Clin Immunol. 2010;125(5):1114–1124. 10.1016/j.jaci.2010.01.018 [DOI] [PubMed] [Google Scholar]
- [75].Van der Vlugt LE, Labuda LA, Ozir-Fazalalikhan A, et al. Schistosomes induce regulatory features in human and mouse CD1d(hi) B cells: inhibition of allergic inflammation by IL-10 and regulatory T cells. PLoS One. 2012;7(2):e30883. 10.1371/journal.pone.0030883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Van der Vlugt LE, Zinsou JF, Ozir-Fazalalikhan A, et al. Interleukin 10 (IL-10) –producing CD1dhi regulatory B cells from Schistosoma haematobium–infected individuals induce IL-10–positive T cells and suppress effector T-cell cytokines. J Infect Dis. 2014;210(8):1207–1216. 10.1093/infdis/jiu257 [DOI] [PubMed] [Google Scholar]
- [77].Velupillai P, Harn DA. Oligosaccharide-specific induction of interleukin 10 production by B220+ cells from schistosome–infected mice: a mechanism for regulation of CD4+ T–cell subsets. Proc Natl Acad Sci USA. 1994;91(1):18–22. 10.1073/pnas.91.1.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Ronet C, Hauyon-La Torre Y, Revaz-Breton M, et al. Regulatory B cells shape the development of Th2 immune responses in BALB/c mice infected with Leishmania major through IL-10 production. J Immunol. 2010;184(2):886–894. 10.4049/jimmunol.0901114 [DOI] [PubMed] [Google Scholar]
- [79].Bankoti R, Gupta K, Levchenko A, et al. Marginal zone B cells regulate antigen-specific T cell responses during infection. J Immunol. 2012;188(8):3961–3971. 10.4049/jimmunol.1102880 [DOI] [PubMed] [Google Scholar]
- [80].Bao LQ, Huy NT, Kikuchi M, et al. CD19(+) B cells confer protection against experimental cerebral malaria in semi-immune rodent model. PLoS One. 2013;8(5):e64836. 10.1371/journal.pone.0064836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Liu Y, Chen Y, Li Z, et al. Role of IL-10–producing regulatory B cells in control of cerebral malaria in Plasmodium berghei infected mice. Eur J Immunol. 2013;43(11):2907–2918. 10.1002/eji.v43.11 [DOI] [PubMed] [Google Scholar]
- [82].Andreani G, Ouellet M, Menasria R, et al. Leishmania infantum amastigotes trigger a subpopulation of human B cells with an immunoregulatory phenotype. PLoS Negl Trop Dis. 2015;9(2):e0003543. 10.1371/journal.pntd.0003543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Jeong YI, Hong SH, Choa SH, et al. Induction of IL-10–producing regulatory B cells following Toxoplasma gondii infection is important to the cyst formation. Biochem Biophys Rep. 2016;2016(7):91–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Schaut RG, Lamb IM, Toepp AJ, et al. Regulatory IgDhi B cells suppress T cell function via IL-10 and PD-L1 during progressive visceral leishmaniasis. J. Immunol. 2016;196(10):4100–4109. 10.4049/jimmunol.1502678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Tate EW, Bell AS, Rackham MD, et al. N-Myristoyltransferase as a potential drug target in malaria and leishmaniasis. Parasitology. 2014;141(1):37–49. 10.1017/S0031182013000450 [DOI] [PubMed] [Google Scholar]
- [86].World Health Organization (WHO) [Internet]. World Malaria Report 2015; 2015. [updated 2016 April 20]. Avaliable from: http://www.rbm.who.int.
- [87].Schofield L, Grau GE. Immunological processes in malaria pathogenesis. Nat Rev Immunol. 2005;5:722–735. 10.1038/nri1686 [DOI] [PubMed] [Google Scholar]
- [88].Riley EM, Couper KN, Helmby H, et al. Neuropathogenesis of human and murine malaria. Trends Parasitol. 2010;26(6):277–278. 10.1016/j.pt.2010.03.002 [DOI] [PubMed] [Google Scholar]
- [89].Clark IA, Budd AC, Alleva LM, et al. Human malarial disease: a consequence of inflammatory cytokine release. Malar J. 2006;5(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Haque A, Best SE, Ammerdorffer A, et al. Type I interferons suppress CD4+ T-cell–dependent parasite control during blood–stage Plasmodium infection. Eur J Immunol. 2011;41(9):2688–2698. 10.1002/eji.201141539 [DOI] [PubMed] [Google Scholar]
- [91].Asito AS, Moormann AM, Kiprotich C, et al. Alterations on peripheral B cell subsets following an acute uncomplicated clinical malaria infection in children. Malar J. 2008;7(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Nduati E, Gwela A, Karanja H, et al. The plasma concentration of the B cell activating factor is increased in children with acute malaria. J Infect Dis. 2011;204(6):962–970. 10.1093/infdis/jir438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Scholzen A, Teirlinck AC, Bijker EM, et al. BAFF and BAFF receptor levels correlate with B cell subset activation and redistribution in controlled human malaria infection. J Immunol. 2014;192(8):3719–3729. 10.4049/jimmunol.1302960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Scholzen A, Sauerwein RW. How malaria modulates memory: activation and dysregulation of B cells in Plasmodium infection. Trends Parasitol. 2013;29(5):252–262. 10.1016/j.pt.2013.03.002 [DOI] [PubMed] [Google Scholar]
- [95].Borhis G, Richard Y. Subversion of the B-cell compartment during parasitic, bacterial, and viral infections. BMC Immunol. 2015;16(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Rest JR. Cerebral malaria in inbred mice. I. A new model and its pathology. Trans R Soc Trop Med Hyg. 1982;76(3):410–415. 10.1016/0035-9203(82)90203-6 [DOI] [PubMed] [Google Scholar]
- [97].Good MF, Xu H, Wykes M, et al. Development and regulation of cell–mediated immune responses to the blood stages of malaria: implications for vaccine research. Annu Rev Immunol. 2005;23:69–99. 10.1146/annurev.immunol.23.021704.115638 [DOI] [PubMed] [Google Scholar]
- [98].Amante FH, Haque A, Stanley AC, et al. Immune–mediated mechanisms of parasite tissue sequestration during experimental cerebral malaria. J Immunol. 2010;185(6):3632–3642. 10.4049/jimmunol.1000944 [DOI] [PubMed] [Google Scholar]
- [99].Fillatreau S, Gray D, Anderton SM. Not always the bad guys: B cells as regulators of autoimmune pathology. Nat Rev Immunol. 2008;8(5):391–397. [DOI] [PubMed] [Google Scholar]
- [100].Margry B, Kersemakers SC, Hoek A, et al. Activated peritoneal cavity B-1a cells possess regulatory B cell properties. PLoS One. 2014;9(2):e88869. 10.1371/journal.pone.0088869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Bismuth G, Gary-Gouy H. Signaling functions of the CD5 molecule. Curr Trends Immunol. 1998;1:79–87. [Google Scholar]
- [102].Carsetti R, Rosado MM, Wardmann H. Peripheral development of B cells in mouse and man. Immunol Rev. 2004;197:179–191. 10.1111/imr.2004.197.issue-1 [DOI] [PubMed] [Google Scholar]
- [103].Lee J, Kuchen S, Fischer R, et al. Identification and characterization of a human CD5+ pre-naive B cell population. J Immunol. 2009;182 (7):4116-4126. 10.4049/jimmunol.0803391 [DOI] [PubMed] [Google Scholar]
- [104].Griffin DO, Holodick NE, Rothstein TL. Human B1 cells in umbilical cord and adult peripheral blood express the novel phenotype CD20+CD27+CD43+CD70-. J Exp Med. 2011;208(1):67–80. 10.1084/jem.20101499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Dalloul A. CD5: a safeguard against autoimmunity and a shield for cancer cells. Autoimmun Rev. 2009;8(4):349–353. 10.1016/j.autrev.2008.11.007 [DOI] [PubMed] [Google Scholar]
- [106].Gary-Gouy H, Sainz-Perez A, Marteau JB, et al. Natural phosphorylation of CD5 in chronic lymphocytic leukemia B cells and analysis of CD5-regulated genes in a B cell line suggest a role for CD5 in malignant phenotype. J Immunol. 2007;179(7):4335–4344. 10.4049/jimmunol.179.7.4335 [DOI] [PubMed] [Google Scholar]
- [107].Maseda D, Candando KM, Smith SH, et al. 2013. Peritoneal cavity regulatory B cells (B10 cells) modulate IFN-γ+ CD4+ T cell numbers during colitis development in mice. J Immunol. 2013;191(5):2780-2795. 10.4049/jimmunol.1300649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Jeong YI, Hong SH, Cho SH, et al. Induction of IL-10–producing CD1dhighCD5+ regulatory B cells following Babesia microti-infection. PLoS One. 2012;7(10):e46553. 10.1371/journal.pone.0046553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Sakkas H, Gartzonika C, Levidiotou S. Laboratory diagnosis of human visceral leishmaniasis. J Vector Borne Dis. 2016;53(1):8–16. [PubMed] [Google Scholar]
- [110].World Health Organization (WHO) [Internet] [updated 2017 March 09]. Available from: http://www.who.int/leishmaniasis/en/.
- [111].Chappuis F, Sundar S, Hailu A, et al. Visceral leishmaniasis: what are the needs for diagnosis, treatment and control? Nat Rev Microbiol. 2007;5(11):873–882. [DOI] [PubMed] [Google Scholar]
- [112].Stanley AC, Engwerda CR. Balancing immunity and pathology in visceral leishmaniasis. Immunol Cell Biol. 2007;85(2):138–147. 10.1038/sj.icb7100011 [DOI] [PubMed] [Google Scholar]
- [113].Reithinger R, Dujardin JC, Louzir H, et al. Cutaneous leishmaniasis. Lancet Infect Dis. 2007;7(9):581–596. 10.1016/S1473-3099(07)70209-8 [DOI] [PubMed] [Google Scholar]
- [114].Silveira FT, Lainson R, De Castro Gomes CM, et al. Immunopathogenic competences of Leishmania (V.) braziliensis and L. (L.) amazonensis in American cutaneous leishmaniasis. Parasite Immunol. 2009;31(8):423–431. 10.1111/pim.2009.31.issue-8 [DOI] [PubMed] [Google Scholar]
- [115].Amezcua Vesely MC, Bermejo DA, Montes CL, et al. B-cell response during protozoan parasite infections. J Parasitol Res. 2012;2012:362131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Kumar R, Nylén S. Immunobiology of visceral leishmaniasis. Front Immunol. 2012;3(251):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Nylén S, Sacks D. Interleukin-10 and the pathogenesis of human visceral leishmaniasis. Trends Immunol. 2007;28(9):378–384. 10.1016/j.it.2007.07.004 [DOI] [PubMed] [Google Scholar]
- [118].Wanasen N, Xin L, Soong L. Pathogenic role of B cells and antibodies in murine Leishmania amazonensis infection. Int J Parasitol. 2008;38(3–4):417–429. 10.1016/j.ijpara.2007.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Deak E, Jayakumar A, Cho KW, et al. Murine visceral leishmaniasis: IgM and polyclonal B-cell activation lead to disease exacerbation. Eur J Immunol. 2010;40(5):1355–1368. 10.1002/eji.200939455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Rodrigues V, Cordeiro-da-Silva A, Laforge M, et al. Regulation of immunity during visceral Leishmania infection. Parasit Vectors. 2016;9(118):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Bankoti R, Stäger S. Differential regulation of the immune response in the spleen and liver of mice infected with Leishmania donovani. J Trop Med. 2012;2012:639304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Schurr E, Kidane K, Yemaneberhan T, et al. Cutaneous leishmaniasis in Ethiopia: I. Lymphocyte transformation and antibody titre. Trop Med Parasitol. 1986;37(4):403–408. [PubMed] [Google Scholar]
- [123].Mengistu G, Akuffo HO, Yemane-Berhan T, et al. Serum antibody specificities to Leishmania aethiopica antigens in patients with localized and diffuse cutaneous leishmaniasis. Parasite Immunol. 1990;12(5):495–495. 10.1111/pim.1990.12.issue-4-5 [DOI] [PubMed] [Google Scholar]
- [124].Galvao-Castro B, Sá Ferreira JA, Marzochi KF, et al. Polyclonal B cell activation, circulating immune complexes and autoimmunity in human American visceral leishmaniasis. Clin Exp Immunol. 1984;56(1):58–66. [PMC free article] [PubMed] [Google Scholar]
- [125].Costa CH, Stewart JM, Gomes RB, et al. Asymptomatic human carriers of Leishmania chagasi. Am J Trop Med Hyg. 2002;66(4):334–7l. [DOI] [PubMed] [Google Scholar]
- [126].Gidwani K, Picado A, Ostyn B, et al. Persistence of Leishmania donovani antibodies in past visceral leishmaniasis cases in India. Clin Vaccine Immunol. 2011;18(2):346–348. 10.1128/CVI.00473-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Hasker E, Malaviya P, Gidwani K, et al. Strong association between serological status and probability of progression to clinical visceral leishmaniasis in prospective cohort studies in India and Nepal. PLoS Negl Trop Dis. 2014;8(1):e2657. 10.1371/journal.pntd.0002657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Soulas P, Koenig-Marrony S, Julien S, et al. A role for membrane IgD in the tolerance of pathological human rheumatoid factor B cells. Eur J Immunol. 2002;32(9):2623–2634. [DOI] [PubMed] [Google Scholar]
- [129].Maity PC, Blount A, Jumaa H, et al. B cell antigen receptors of the IgM and IgD classes are clustered in different protein islands that are altered during B cell activation. Sci Signal. 2015; 8(394):ra93. 10.1126/scisignal.2005887 [DOI] [PubMed] [Google Scholar]
- [130].Geisberger R, Lamers M, Achatz G. The riddle of the dual expression of IgM and IgD. Immunology. 2006;118(4):429–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Ranatunga D, Hedrich CM, Wang F, et al. A human IL10 BAC transgene reveals tissue–specific control of IL-10 expression and alters disease outcome. Proc Natl Acad Sci USA. 2009;106(40):17123–17128. 10.1073/pnas.0904955106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Gonzaga WF, Xavier V, Vivanco BC, et al. B-1 cells contribute to susceptibility in experimental infection with Leishmania (Leishmania) chagasi. Parasitology. 2015;142(12):1506–1515. 10.1017/S0031182015000943 [DOI] [PubMed] [Google Scholar]
- [133].Murray HW. Interleukin 10 receptor blockade–pentavalent antimony treatment in experimental visceral leishmaniasis. Acta Trop. 2005;93(3):295–295. 10.1016/j.actatropica.2004.11.008 [DOI] [PubMed] [Google Scholar]
- [134].134. Faleiro RJ, Kumar R, Hafner LM, et al. Immune regulation during chronic visceral leishmaniasis. PLoS Negl Trop Dis. 2014;8(7):e2914. 10.1371/journal.pntd.0002914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Gollob KJ, Viana AG, Dutra WO. Immunoregulation in human American leishmaniasis: balancing pathology and protection. Parasite Immunol. 2014;36(8):367–376. 10.1111/pim.2014.36.issue-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Singh OP, Sundar S. Immunotherapy and targeted therapies in treatment of visceral leishmaniasis: current status and future prospects. Front Immunol. 2014;5(296):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Khadem F, Uzonna JE. Immunity to visceral leishmaniasis: implications for immunotherapy. Future Microbiol. 2014;9(7):901–915. 10.2217/fmb.14.43 [DOI] [PubMed] [Google Scholar]