Abstract
Native ion mobility mass spectrometry (MS) and surface induced dissociation (SID) are applied to study the integral membrane protein complexes AmtB and AqpZ. Fragments produced from SID are consistent with the solved structures of these complexes. SID is, therefore, a promising tool for characterization of membrane protein complexes.
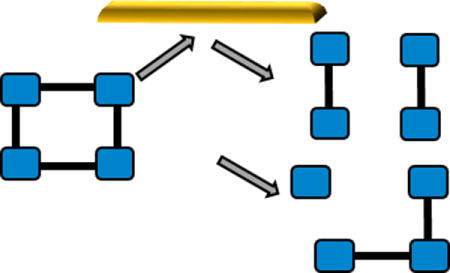
Membrane proteins are essential to mediate the traffic of solutes in and out of the cell, and in translating extracellular stimuli into function. The structural characterization of membrane protein complexes is challenging due to their insolubility in aqueous solution, low expression limits, and propensity for aggregation.1 Mass spectrometry has emerged as a powerful structural biology tool, enabling analysis of intact soluble and membrane protein complexes, as well as membrane protein-lipid complexes.2–7 When coupled with ion mobility (IM), MS can provide an extra dimension of information on the protein shape, in the form of a rotationally averaged collision cross section.8–10 In order to retain native stoichiometry and conformations, membrane proteins are introduced into the gas phase either within a nanodisc, with amphipols, or within a detergent micelle; these assemblies are then disrupted within the mass spectrometer to liberate the protein or protein complex.11–14
To obtain substructural information on the complex using native MS it is necessary to perform dissociation in the gas-phase. The most commonly used activation method is collision induced-dissociation (CID). CID typically partitions effective conversion to products between liberating the complex from the micelle and dissociating the complex, if enough energy is applied. For soluble and membrane protein complexes, when CID does occur it typically produces an unfolded monomer and the corresponding (n-1) multimer.15–17 CID hence provides information on stoichiometry with limited information on substructure and assembly. In contrast, surface induced dissociation (SID) has been shown to selectively disrupt the weaker interfaces of soluble protein complexes, yielding both information on assembly and compact subcomplexes reflective of the native structure.18, 19 Additionally, subunits can retain their ligands if the binding site is not disrupted upon dissociation.20 Here we apply this technique to membrane protein complexes to discern if the fragmentation observed is reflective of the known structure for these protein complexes, even after they have been liberated from a detergent micelle within the mass spectrometer. We chose to study the trimeric ammonia channel (AmtB) and the tetrameric water channel (AqpZ) from Escherichia coli, both of which have solved crystal structures21, 22 making them excellent model systems for this proof-of-concept study. Furthermore, both protein complexes, and the corresponding protein-lipid complexes, have been studied previously with CID, and exhibit only limited dissociation and dissociation consistent with the typical CID pathway i.e. ejection of monomer and (n-1)mer.7, 23 For both membrane proteins, we used the detergent tetraethylene glycol monooctyl ether (C8E4), which has been shown previously to reduce the charge carried by the complex when compared with other MS compatible detergents. Charge reduction is advantageous as it is attributed to more stable and native-like complexes. 7, 24, 25
We first considered the trimeric membrane protein complex AmtB. In order to perform SID studies on a single defined m/z species, the instrument conditions had to first be optimized to enable clean m/z selection in the quadrupole. In typical MS studies the membrane protein complex is liberated from the micelle post introduction into the gas phase. Within a Waters Synapt, an ion-mobility enabled quadrupole time-of-flight instrument, this is most commonly achieved by application of CID in the trap (the first stacked ring ion guide CID cell), which is located after the quadrupole. Here we use a method26 where we disrupt the micelle in the ionization source region, using a raised source temperature (120 °C), and cone voltage (90V), with a correspondingly low trap CID voltage (5 V), which leaves little excess detergent and results in sharp, clean, peaks (Figure S1). The collision cross sections (CCS) of the intact trimeric AmtB generated in this way are on average slightly larger but are within the experimental error of those determined when using a low source temperature (20 °C) and high CID voltage (60 V) and to those previously published7 (Table S1). Hence, this method can enable clean m/z selection, without perturbation of the complex, as has been shown previously.23
With the ability to isolate a selected charge state of membrane protein complexes we then carried out SID studies of the isolated 17+ charge state of AmtB at low collisional energy, 1700 eV (Figure 1). At this energy, monomers and dimers were the main SID products with these products being compact and in good agreement with the theoretical CCS for the subcomplexes generated from the crystal structure (Figure 1). However, the highest charge states of monomer produced are slightly unfolded, as is the highest charge state of the dimer, suggesting that under the conditions required here we do observe some limited unfolding upon dissociation. The most intense products are however consistent with the solved structure of AmtB and highlight that SID, unlike CID, can provide substructural information for this membrane protein complex liberated from the detergent micelle, in the form of primarily folded subproducts. As SID energy is increased, further dissociation can occur, producing monomer from dimer (Figure S2).
Fig 1.
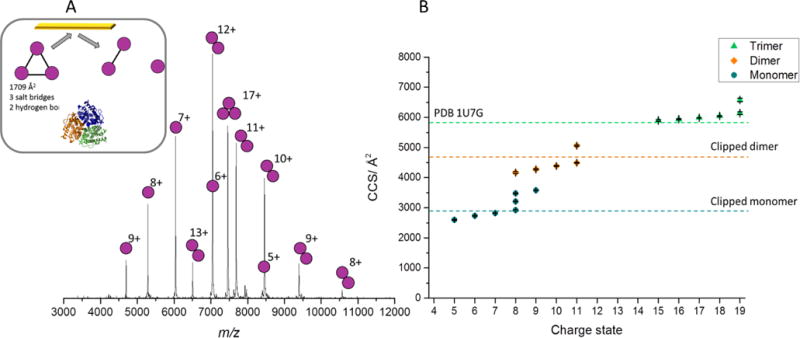
A) SID spectrum for the 17+ AmtB at a collision energy of 1700 eV. Inset is cartoon representation of the structure of AmtB (PDB 1U7G) and interfacial analysis determined from PISA27, with predicted SID products (monomer and dimer), with crystal structure shown below. B) CCS for AmtB determined through the calibration procedure using travelling wave IM. Dashed lines represent the CCS calculated from the crystal structure, for different oligomers. Trimer was determined from the full MS, while dimer and monomer were produced following SID of the 17+ charge state at an energy of 1700 eV. Theoretical CCS were determined using MOBCAL and the previously established scaled PA approach28. For AmtB, a source temperature of 120 °C was used, with a cone voltage of 90 V and a trap CID voltage of 5 V, while a source temperature of 20°C and cone voltage of 20 V was used for the calibration standards, to prevent activation of these soluble complexes. CCS plotted are the average values from three repeats, error bars represent standard deviation between repeats and generally fall within the symbol size.
In order to discern if this trend holds for multiple membrane protein complexes, we then considered the tetrameric AqpZ. As with AmtB, the protein complex was liberated from the micelle before the quadrupole, using a source temperature of 120 °C, producing clean peaks and enabling clean m/z selection (Figure S3). The CCS generated for the tetramer under these conditions are in good agreement with those obtained with a cold source and higher CID energy (Table S2), and to those previously published.7
The 13+ tetrameric AqpZ was selected for SID studies, with low energy SID producing monomer, dimer, and trimer (Figure 2 and S4). Similarly to AmtB, these products are reflective of the solved structure of AqpZ, which has cyclic (C4) symmetry with equal interfaces between all subunits.21 Low energy SID provides substructural information, cleavage to dimer/dimer and monomer/trimer, which is consistent with the atomic structure of this membrane protein complex. Significantly, IM shows that these products are primarily compact with good agreement to the theoretical CCS generated via extracting the coordinates of individual subunits from the atomic structure, PDB 1RC2 (Figure 2B). The trimer is more compact than the theoretical CCS, suggesting it rearranges after dissociation into a more compact and presumably more stable form, as would be expected for a cyclic complex, after one monomer subunit has been lost.
Fig 2.
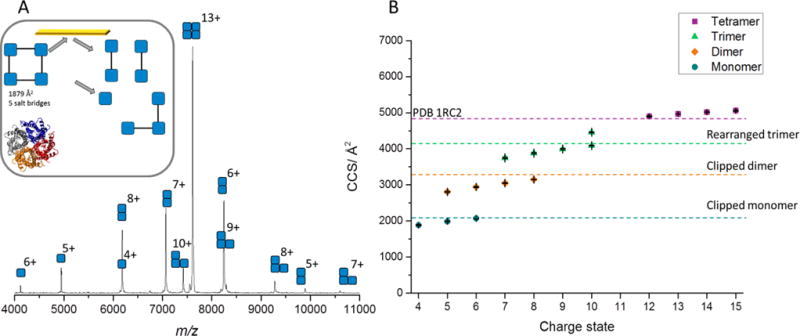
A) SID spectrum for the 13+ AqpZ at a collision energy of 1300 eV. Inset is cartoon representation of the structure of AqpZ (PDB 1RC2) and interfacial analysis determined from PISA27, with predicted SID products and crystal structure shown below. B) CCS for AqpZ determined through the calibration procedure using travelling wave IM. Tetramer was determined from full MS, while trimer, dimer, and monomer were produced following SID of the 13+ charge state at an energy of 1300 eV. Theoretical CCS were determined using MOBCAL and the previously established scaled PA approach.28 For AqpZ, a source temperature of 120 °C was used, with a cone voltage of 90 V and a trap CID voltage of 5 V, while a source temperature of 20°C and cone voltage of 20 V was used for the calibration standards, to prevent activation of these soluble complexes. CCS plotted are the average values from three repeats, error bars represent standard deviation between repeats and generally fall within the symbol size.
We also applied SID to study the interactions of AmtB and AqpZ with three lipids: cardiolipin (CDL), phosphatidylethanolamine (PE) and phosphatidylglycerol (PG). We first considered AmtB and the lipid PG, a phospholipid with known binding sites to the channel.7 In this MS study, AmtB was observed to bind up to six PG molecules (Figure S5A). The most intense holo species was AmtB(PG)1, and therefore the 17+ charge state of this species was mass selected for SID studies. SID, performed at an energy of 1700 eV, of the isolated 17+ ion of AmtB(PG)1 primarily produces compact monomers and dimers, with some precursor remaining also (Figure S5B), similar to the observations for the apo protein at this SID energy. Significantly, lipid is observed bound to a portion of all products and ~30% of PG is lost from the precursor ion upon collision with the surface. The fact that lipid is retained on the subcomplexes is interesting, and suggests these interactions are preferentially retained, in comparison to the protein-protein interactions.
We then applied the same approach for AmtB(CDL)1 and AmtB(PE)1 (Figure S5 C–F). 53% of the precursor is observed to lose PE following SID with no significant lipid loss observed for CDL. The fact that lipid is lost from the precursor following surface collision may be due to partial unfolding following collision with the surface, as observed with IM, or may be due to a weaker interaction for PG and PE in comparison to CDL. However, the strong retention of bound CDL is an interesting observation and is in agreement with recent gas-phase unfolding experiments23. For AmtB(CDL)1 and AmtB(PE)1 a portion of all subcomplexes retain the lipid. Interestingly, for all lipids we see no significant difference in the onset energy for fragmentation in comparison to the apo protein (Figure 3), suggesting that although lipid binding can stabilize the complex with respect to unfolding7, no significant effect is observed here for dissociation of the complex (lipid is unlikely to be located at the interface between subunits).
Fig 3.
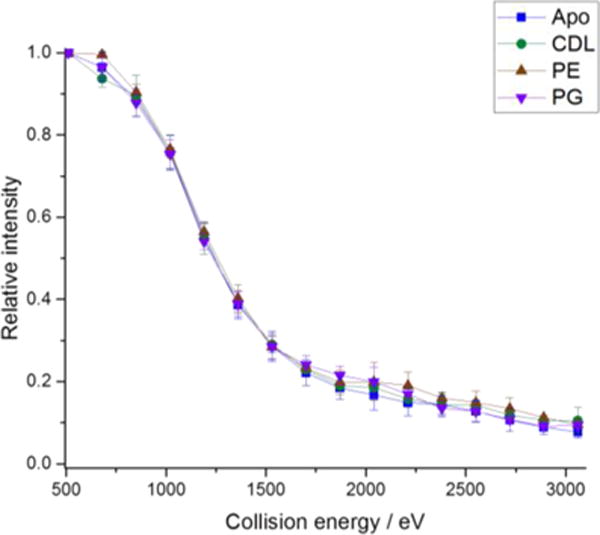
Fragmentation efficiency plot for the 17+ trimeric apo AmtB, AmtB(PG)1, AmtB(PE)1, and AmtB(CDL)1. Apo and holo trimer intensity following surface collision were summed; values shown are the average of three repeats and error bars represent the standard deviation.
We next considered the interactions between AqpZ and CDL, PG, and PE. In these MS studies, AqpZ was observed to bind up to two CDL molecules or up to three PG or PE molecules (Figure S6). In all cases, however, the most intense holo complex observed was the species with one lipid-bound, and therefore this molecular species was selected for SID studies. SID was performed at a collision energy of 1300 eV in all cases as this energy yielded significant fragmentation of precursor to subcomplexes consistent with the structure for the apo protein. SID of the 13+ charge state of AqpZ(CDL)1 at 1300 eV primarily produces compact monomers, dimers, and trimers, with some precursor remaining (Figure S6B), similar to the observations for the apo protein at this SID energy. Significantly, lipid is observed to be bound to a portion of all subcomplexes and no lipid is lost from the precursor upon collision with the surface.
Similar studies were performed on AqpZ(PG)1 and AqpZ(PE)1 (Figure S6 C–F) and, for both of these lipids, a fraction of lipid is lost from the precursor following SID. However, 81% (PG) and 64% (PE) of the total remaining tetramer retains the lipid at 1300 eV collision energy. This is similar to the observations made for AmtB in complex with these lipids, however, the extent of lipid loss is much lower. For both AqpZ(PG)1 and AqpZ(PE)1, monomer/trimer and dimer/dimer are produced and in a fraction of all subcomplexes the lipid is observed to be bound, even to monomer products (Figure S6D and F). As with AmtB there is no significant difference in the onset energy for fragmentation in comparison to the apo protein (Figure S7) again suggesting that although lipid binding can stabilize AqpZ with respect to unfolding, no significant effect on disassembly is observed. In conclusion, SID of membrane protein complexes produces subcomplexes consistent with their solved structure. We therefore believe that SID has potential in the study of membrane protein complexes and their binding partners.
Supplementary Material
Acknowledgments
The authors would like to acknowledge financial support from the National Institute of Health (R01GM113658) awarded to VHW and partial support from National Institute Of Neurological Disorders And Stroke of the National Institutes of Health (R21NS094882) and National Institute Of General Medical Sciences of the National Institutes of Health (DP2GM123486) awarded to AL. SRH is supported by a UK Engineering and Physical Sciences Research Council (EPSRC) Doctoral Prize Fellowship awarded through the University of Manchester. Part of this work was supported by new faculty startup funds from the Texas A&M Health Science Center.
Footnotes
Electronic Supplementary Information (ESI) available: [Experimental methods, additional tables and additional figures]. See DOI: 10.1039/x0xx00000x
References
- 1.Booth PJ, Templer RH, Meijberg W, Allen SJ, Curran AR, Lorch M. Critical reviews in biochemistry and molecular biology. 2001;36:501–603. doi: 10.1080/20014091074246. [DOI] [PubMed] [Google Scholar]
- 2.Barrera NP, Robinson CV. Annual review of biochemistry. 2011;80:247–271. doi: 10.1146/annurev-biochem-062309-093307. [DOI] [PubMed] [Google Scholar]
- 3.Heck AJ. Nature methods. 2008;5:927–933. doi: 10.1038/nmeth.1265. [DOI] [PubMed] [Google Scholar]
- 4.Barrera NP, Zhou M, Robinson CV. Trends in cell biology. 2013;23:1–8. doi: 10.1016/j.tcb.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 5.Bechara C, Nöll A, Morgner N, Degiacomi MT, Tampé R, Robinson CV. Nature chemistry. 2015;7:255–262. doi: 10.1038/nchem.2172. [DOI] [PubMed] [Google Scholar]
- 6.Konijnenberg A, Bannwarth L, Yilmaz D, Koçer A, Venien‐Bryan C, Sobott F. Protein Science. 2015 doi: 10.1002/pro.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laganowsky A, Reading E, Allison TM, Ulmschneider MB, Degiacomi MT, Baldwin AJ, Robinson CV. Nature. 2014;510:172–175. doi: 10.1038/nature13419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leney AC, McMorran LM, Radford SE, Ashcroft AE. Analytical chemistry. 2012;84:9841–9847. doi: 10.1021/ac302223s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Konijnenberg A, Yilmaz D, Ingólfsson HI, Dimitrova A, Marrink SJ, Li Z, Vénien-Bryan C, Sobott F, Koçer A. Proceedings of the National Academy of Sciences. 2014;111:17170–17175. doi: 10.1073/pnas.1413118111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pacholarz KJ, Barran PE. Analytical chemistry. 2015 doi: 10.1021/acs.analchem.5b01063. [DOI] [PubMed] [Google Scholar]
- 11.Laganowsky A, Reading E, Hopper JT, Robinson CV. Nature protocols. 2013;8:639–651. doi: 10.1038/nprot.2013.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watkinson TG, Calabrese AN, Giusti F, Zoonens M, Radford SE, Ashcroft AE. International Journal of Mass Spectrometry. 2015;391:54–61. doi: 10.1016/j.ijms.2015.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marty MT, Hoi KK, Gault J, Robinson CV. Angewandte Chemie. 2016;128:560–564. doi: 10.1002/ange.201508289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hopper JT, Yu YTC, Li D, Raymond A, Bostock M, Liko I, Mikhailov V, Laganowsky A, Benesch JL, Caffrey M. Nature methods. 2013;10:1206–1208. doi: 10.1038/nmeth.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beardsley RL, Jones CM, Galhena AS, Wysocki VH. Analytical chemistry. 2009;81:1347–1356. doi: 10.1021/ac801883k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sinelnikov I, Kitova EN, Klassen JS. Journal of the American Society for Mass Spectrometry. 2007;18:617–631. doi: 10.1016/j.jasms.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 17.Sciuto SV, Liu J, Konermann L. Journal of The American Society for Mass Spectrometry. 2011;22:1679–1689. doi: 10.1007/s13361-011-0205-x. [DOI] [PubMed] [Google Scholar]
- 18.Zhou M, Dagan S, Wysocki VH. Angewandte Chemie International Edition. 2012;51:4336–4339. doi: 10.1002/anie.201108700. [DOI] [PubMed] [Google Scholar]
- 19.Quintyn RS, Yan J, Wysocki VH. Chemistry & biology. 2015;22:583–592. doi: 10.1016/j.chembiol.2015.03.019. [DOI] [PubMed] [Google Scholar]
- 20.Y. Ju, R. S. Quintyn and V. H. Wysocki, In preparation.
- 21.Savage DF, Egea PF, Robles-Colmenares Y, O’Connell JD, III, Stroud RM. PLoS Biol. 2003;1:e72. doi: 10.1371/journal.pbio.0000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khademi S, O’Connell J, Remis J, Robles-Colmenares Y, Miercke LJ, Stroud RM. Science. 2004;305:1587–1594. doi: 10.1126/science.1101952. [DOI] [PubMed] [Google Scholar]
- 23.Liu Y, Cong X, Liu W, Laganowsky A. Journal of the American Society of Mass Spectrometry. 2016 in press. [Google Scholar]
- 24.Reading E, Liko I, Allison TM, Benesch JL, Laganowsky A, Robinson CV. Angewandte Chemie. 2015;127:4660–4664. doi: 10.1002/anie.201411622. [DOI] [PubMed] [Google Scholar]
- 25.Zhou M, Dagan S, Wysocki VH. Analyst. 2013;138:1353–1362. doi: 10.1039/c2an36525a. [DOI] [PubMed] [Google Scholar]
- 26.Cong X, Liu Y, Liu W, Liang X, Russell DH, Laganowsky A. Journal of the American Chemical Society. 2016 doi: 10.1021/jacs.6b01771. [DOI] [PubMed] [Google Scholar]
- 27.Krissinel E, Henrick K. Journal of molecular biology. 2007;372:774–797. doi: 10.1016/j.jmb.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 28.Benesch JL, Ruotolo BT. Current opinion in structural biology. 2011;21:641–649. doi: 10.1016/j.sbi.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


