The NHLBI (National Heart, Lung and Blood Institute) T-cell depletion (TCD) randomized phase II–III multi-center trial was conducted to examine the impact of TCD of unrelated BM grafts on specific outcomes. The major findings of the study have been reported earlier;1 however, an analysis of lymphocyte reconstitution for study patients has been recently performed and is presented in this report.
Patients received BM grafts from unrelated donors that were either infused unmanipulated, or were TCD using selective ex vivo depletion of αβ + T cells (αβTCD) or a prescribed T-cell dose of 5 × 105 cells/kg after counterflow centrifugal elutriation. Those randomized to receive un-manipulated grafts were conditioned with 1320–1375 cGy fractional TBI over 4 days and CY 120 mg/kg over 2 days. MTX (15 mg/m2 on day 1 and 10 mg/m2 on days +3, +6 and +11) and CsA were used as GVHD prophylaxis. Patients who received αβTCD grafts received 1410 cGy fractionated TBI over 3 days and 100 mg/kg CY over 2 days. Patients who received elutriated grafts received 1320–1375 cGy fractional TBI over 4 days, CY 120 mg/kg over 2 days, and equine antithymocyte globulin over 2 days. All patients received post-BMT CsA sufficient to achieve trough levels of 200–400 ng/ml. Lymphocyte recovery was assessed at 1, 3, 6, 12, 18 and 24 months post-HSCT. To be included in this analysis, patients had to have survived at least 60 days and completed at least 2 of 3 initial post-BMT immunophenotyping assessments at the 30, 60 or 100-day post-BMT time point. Lymphocyte subsets were enumerated using dual platform flow cytometry with a whole blood lyse/wash procedure and staining with fluorochrome-conjugated MoAbs to CD3, CD4, CD8, CD19, CD20, CD45, CD45RA, CD16, CD56, CD57, HLA-DR, TCR αβ and TCR γδ (BD Biosciences, San Jose, CA, USA). Data were tabulated and analyzed using SAS. Published normal ranges for lymphocyte subsets were used to determine time to complete lymphocyte recovery.2,3 The Wilcoxon’s non-parametric test was used to compare recovery of absolute cell counts between groups at each measurement period. A mixed model estimated the rate of change in cell counts for each subject and its slope estimate for each cell subset was then used as a variable to assess disease-free survival. The Cox proportional hazards model was used to examine associations between recovery of individual cell subsets and disease-free survival.
A total of 195 of 407 patients met the inclusion criteria for this analysis. These represented 100 of 206 (48.5%) recipients of unmanipulated grafts, 67 of 135 (49.6%) αβTCD grafts and 28 of 66 (42.4%) elutriated grafts. Recipients of unmanipulated BMT grafts received a T-cell dose of 2.69±2.09 × 107 T cells/kg compared with 1.79±3.00 × 106 T cells/kg for recipients of αβTCD grafts, and 5.29±4.0 × 105 cells/kg for recipients of elutriated grafts. The T-cell dose differed significantly between the αβTCD group and the elutriated group (P = 0.02).
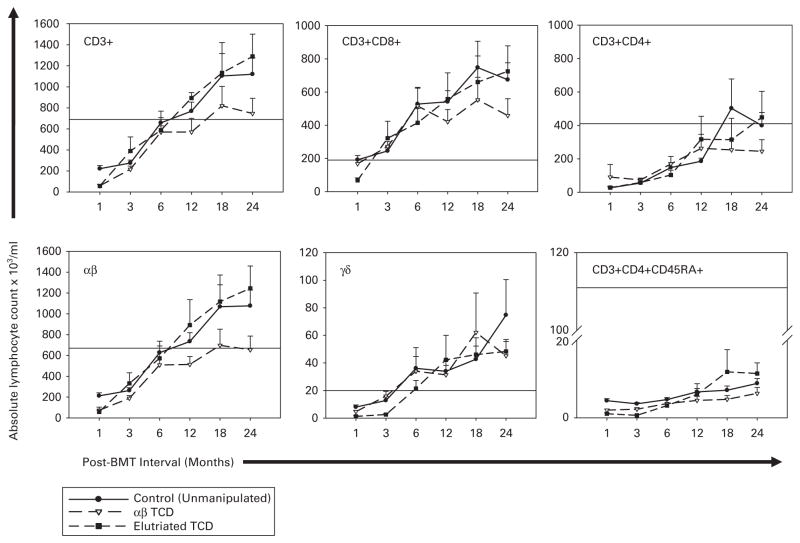
As shown in Figure 1, the total absolute CD3, TCR αβ, TCR γδ, CD3 +CD4 +, CD3 +CD8 + recovery did not differ significantly beyond the first evaluation period for patients who received unmanipulated grafts and those who received TCD grafts. T-cell recovery trended slower after the 12-month evaluation period for all subsets other than γδ T cells in recipients of αβTCD grafts and was significant at 24 months for αβ T-cell counts value (P = 0.03). CD3 +CD4 +CD45RA naive T-cell recovery remained well below the average of 400/μl seen in healthy volunteers3 for all time points. All groups showed prolonged B-cell count recovery (Figure 2a).
Figure 1.
T-lymphocyte recovery over 2 years for patients who received unmodified marrow grafts (solid lines and filled circles) vs patients who received grafts depleted of αβ +T cells using T10B9-A1.31 +complement (dashed lines and open triangles) or grafts processed by elutriation (dashed lines and filled squares). Shown are total T cells (CD3), major subsets (CD3 +CD4 +, CD3 +CD8 +, TCR-αβ, TCR-γδ) and naive CD4 + T cells (CD3 +CD4 +CD45RA +). Horizontal lines designate lower normal values. The upper normal range was not exceeded and is omitted to allow more detail for the plots. Mean TCR-αβ and CD3 +CD4 +CD45RA + T-cell counts were consistently higher in the elutriated group but not so significant. Indeed, with the exception of αβ T-cell numbers at 24 months post-BMT, no group showed statistically superior T-cell or T cell subset recovery at any time point.
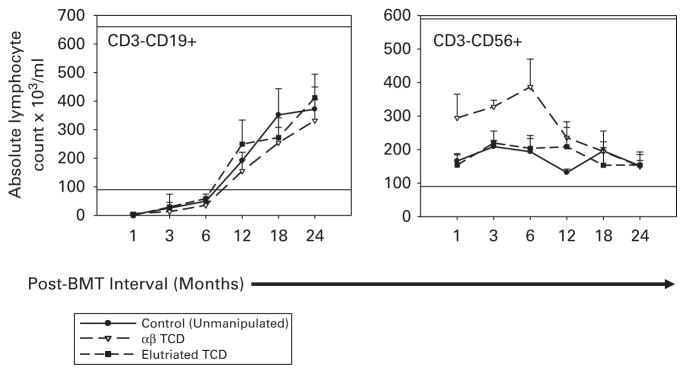
Figure 2.
B-lymphocyte (CD3–CD19 +) and NK cell (CD3–CD56 +) recovery over 2 years for patients who received unmodified marrow grafts (black lines and filled circles) vs patients who received grafts depleted of T cells using T10B9-A1.31 +complement (dashed lines and open triangles) or grafts processed by elutriation (dashed lines and filled squares). Horizontal lines represent the upper and lower normal range. B-cell absolute count reached the lower normal range at ~6 months for both groups and recovery did not differ between groups at any time point post-BMT. The NK cell absolute count was within the normal range at the first measurement period for both groups. NK recovery was significantly more robust in patients who received αβTCD grafts for the initial 12 months post-BMT but did not exceed the upper normal range.
Interestingly, natural killer (NK) cell absolute count was significantly higher in patients who received αβTCD grafts through the first four evaluation periods than those who received unmanipulated grafts (Figure 2b). NK recovery did not differ between patients who received elutriated grafts and patients who received unmanipulated grafts. The more robust recovery of NK cells has been observed in the setting of TCD4 and is likely a result of homeostatic proliferation caused by enrichment of the graft for NK cells and the initial absence of T cells, which compete for similar growth factors.4,5
OS for this patient subset did not differ between recipients of unmanipulated grafts and recipients of TCD grafts as was shown for the total patient cohort. Cox proportional hazards analysis revealed that the slope (rate) of recovery of T cells (P = 0.03) and CD19 + B cells (P = 0.04) was associated with improved disease-free survival independent of treatment group, findings that are consistent with published reports.6–10 A limitation of this analysis, however, was that the small number of patients in this subset prevented a meaningful stratification of cell subset recovery by disease-specific or demographic parameters.
Earlier analysis of the total patient cohort revealed faster neutrophil recovery, decreased incidence of acute GVHD and reduced grade III–IV toxicities in the TCD group, however, TCD was associated with an increased risk of relapse in CML patients.1 In this report, we have shown no differences in lymphocyte subset recovery beyond 60 days for patients in the therapy groups who received unmanipulated grafts and those who received a fixed lymphocyte dose after elutriation. Patients who received αβTCD grafts showed significantly faster NK recovery but fewer αβT cells at later time points. Taken together as an aggregate of the reports on this trial, we have shown that partial ex vivo TCD remains a reasonable platform for development of cell-based immunotherapies of cancer that permits timely lymphocyte recovery while minimizing the risk for acute GVHD.
Acknowledgments
The trial was supported by a contract from the National Heart, Lung and Blood Institute N01-HB-47195 (JEW), N01-HB-47097 (JST), N01-HB-47094 (SLC) and N01-HB-47098 (NAK).
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Wagner JE, Thompson JS, Carter SL, Kernan NA. Effect of graft-versus-host disease prophylaxis on 3-year disease-free survival in recipients of unrelated donor bone marrow (T-cell Depletion Trial): a multi-centre, randomised phase II–III trial. Lancet. 2005;366:733–741. doi: 10.1016/S0140-6736(05)66996-6. [DOI] [PubMed] [Google Scholar]
- 2.Reichert T, DeBruyere M, Deneys V, Totterman T, Lydyard P, Yuksel F, et al. Lymphocyte subset reference ranges in adult Caucasians. Clin Immunol Immunopathol. 1991;60:190–208. doi: 10.1016/0090-1229(91)90063-g. [DOI] [PubMed] [Google Scholar]
- 3.Novitzky N, Davison GM, Hale G, Waldmann H. Immune reconstitution at 6 months following T-cell depleted hematopoietic stem cell transplantation is predictive for treatment outcome. Transplantation. 2002;74:1551–1559. doi: 10.1097/00007890-200212150-00012. [DOI] [PubMed] [Google Scholar]
- 4.Savani BN, Mielke S, Adams S, Uribe M, Rezvani K, Yong AS, et al. Rapid natural killer cell recovery determines outcome after T-cell-depleted HLA-identical stem cell transplantation in patients with myeloid leukemias but not with acute lymphoblastic leukemia. Leukemia. 2007;21:2145–2152. doi: 10.1038/sj.leu.2404892. [DOI] [PubMed] [Google Scholar]
- 5.Barrett AJ. Understanding and harnessing the graft-versus-leukaemia effect. Br J Haematol. 2008;142:877–888. doi: 10.1111/j.1365-2141.2008.07260.x. [DOI] [PubMed] [Google Scholar]
- 6.Koehl U, Bochennek K, Zimmermann SY, Lehrnbecher T, Sorensen J, Esser R, et al. Immune recovery in children undergoing allogeneic stem cell transplantation: absolute CD8+ CD3+ count reconstitution is associated with survival. Bone Marrow Transplant. 2007;39:269–278. doi: 10.1038/sj.bmt.1705584. [DOI] [PubMed] [Google Scholar]
- 7.Small TN, Papadopoulos EB, Boulad F, Black P, Castro-Malaspina H, Childs BH, et al. Comparison of immune reconstitution after unrelated and related T-cell-depleted bone marrow transplantation: effect of patient age and donor leukocyte infusions. Blood. 1999;93:467–480. [PubMed] [Google Scholar]
- 8.Auletta JJ, Lazarus HM. Immune restoration following hematopoietic stem cell transplantation: an evolving target. Bone Marrow Transplant. 2005;35:835–857. doi: 10.1038/sj.bmt.1704966. [DOI] [PubMed] [Google Scholar]
- 9.Keever CA, Small TN, Flomenberg N, Heller G, Pekle K, Black P, et al. Immune reconstitution following bone marrow transplantation: comparison of recipients of T-cell depleted marrow with recipients of conventional marrow grafts. Blood. 1989;73:1340–1350. [PubMed] [Google Scholar]
- 10.Storek J, Saxon A. Reconstitution of B cell immunity following bone marrow transplantation. Bone Marrow Transplant. 1992;9:395–408. [PubMed] [Google Scholar]