Abstract
Objective
This study examines how two key process-of-care tasks of the Collaborative Care Model (CCM) predict patient depression outcomes.
Methods
Registry data were from a large implementation of the CCM in Washington State and included 5,439 patients 18 years or older with a baseline Patient Health Questionnaire 9 (PHQ-9) score of 10 or greater and at least one follow-up contact with the CCM care manager within 24 weeks of the initial contact. Key CCM tasks examined were at least one care manager follow-up contact within 4 weeks of initial contact and at least one psychiatric consultation between weeks 8 and 12 for patients not responding to treatment by week 8. Clinically significant improvement in depression symptoms was defined as achieving a PHQ-9 below 10 or 50% or more reduction in PHQ-9 compared to baseline. Bi-variate and multi-variate (logistic and proportional hazard models) analysis was conducted to examine how fidelity with either task predicted patient outcomes. All analysis was conducted with the original sample and with a propensity-score (PS) matched sample.
Results
4-week follow-up was associated with a greater likelihood of achieving improvement in depression (odds ratio or OR of 1.63, 95% CI = 1.23–2.17) and a shorter time to improvement (hazard ratio or HR of 2.06, CI = 1.67–2.54). Psychiatric consultation was associated with an OR of 1.44, CI = 1.13–1.84 and an HR of 0.99, CI = 0.81–1.21. PS-matched analysis yielded very similar results.
Conclusions
Findings support efforts to improve the fidelity with the two process-of-care tasks and to include these tasks among quality measures for CCM implementation.
Common mental health conditions seen in primary care such as depression and anxiety impose substantial suffering, complicate co-existing medical conditions(1–3), and lead to enormous loss of productivity(4, 5) and increases in total health care costs(6, 7). Primary care has long been recognized as the de facto mental health treatment setting(8, 9). However, the average quality of mental health care in primary care remains lower than optimal(10, 11).
The Collaborative Care Model (CCM) embeds the core concepts of the Chronic Care Model(12, 13) to address the need to integrate high-quality mental health care in primary care. Over 70 randomized controlled trials provided collective and robust evidence on the effectiveness of CCM to improve depression, processes of care, and quality of life outcomes(14, 15) and its potential of reducing long-term health care costs(16). The CCM approaches care with a collaborative team including the primary care physician, a care manager, and a consulting psychiatrist. The key principles of the model include systematic follow-up of the patients by the care manager, measurement-based care that uses symptom rating scales to track clinical improvements or identify patients not improving(17), and “stepped care”(18) with which treatment is systematically adjusted or intensified (by the primary care team but with input from the consulting psychiatrist) for patients not improving(19). The CCM is increasingly recognized as a best practice to integrate evidence-based mental health care into primary care(20–23).
Wide dissemination of the CCM, however, may be hindered by the complexity of the intervention and a lack of knowledge regarding the relative importance of the different components of the model for patient outcomes. The CCM is defined by a set of core components and tasks(24, 25) and has almost always been tested as a package. Provider organizations with limited experience of practice changes may not be able to achieve full fidelity when first implementing CCM(26, 27). Knowing the relative contribution of key elements of the model would help provider organizations direct their resources to maximize the returns to their implementation. Such knowledge would also inform the selection of quality assurance and performance evaluation measures for CCM implementation.
A limited number of studies sought to identify “active ingredients” of CCM that disproportionately contribute to its effectiveness(28–31). The ingredients examined were mostly structural aspects of the intervention (e.g., use of a clinical information system). Studies of process-of-care elements (e.g., intensity and frequency of care manager contacts) are important to guide the operation and quality assurance of the CCM but have been rare(32). Furthermore, analysis of process-of-care measures aggregated to the provider/site level (as seen in Bauer et al.) could be subject to one type of ecological fallacy: average measures that lacked variation across studies or implementations could disguise large variation within these units that might have important implications for patient outcomes(33).
In this paper, we begin to address this knowledge gap by identifying and examining two key process-of-care tasks of CCM and by exploiting variation across patients using data from a large implementation in Washington State. We tested the hypothesis that practice of either of the two tasks predicts clinically meaningful improvement in depression within 6 months of CCM initiation.
Methods
Selection of Key Tasks
We first compiled a list of process-of-care components and key tasks of CCM based on expert consensus(25). We focused on tasks that can be reliably measured using the Clinical Management Tracking System, the clinical information system(34) first developed out of the Improving Mood-Promoting Access to Treatment (IMPACT) study(35), and subsequently used in many CCM implementations (Table 1). We then applied the following criteria to select key tasks to be studied in this paper. First, these tasks should embed one or more of the key principles of the CCM including systematic follow-up, measurement-based care, and stepped care. Second, the tasks occur in the early stage of CCM (first 12 weeks) where care management is most intensive(36) and likely most influential in shaping patient outcome trajectories(32). Restricting to tasks performed in the early stage may also mitigate the selection bias introduced by the stepped-care nature of CCM in that patients whose depression is resistant to treatment subsequently receive more intensive care management but, because of the nature of their conditions, are less likely to achieve good outcomes. Third, the tasks are of significance to all CCM patients regardless of whether they receive medication or psychotherapy (or both). Fourth, substantial variation exists in the practice of these tasks in our data (<90% in fidelity) to enable reliable estimates.
Table 1.
Core components and key tasks of the collaborative care model measurable with the Mental Health Integration Program (MHIP) registry data.
| Core component | Specific task(s) | Operational definition of task |
|---|---|---|
| Patient identification and diagnosis | Diagnose behavioral health problems and related conditions | >=1 working diagnoses for common behavioral health conditions checked during episode |
| Use valid measurement tools to asses and document baseline severity | >=1 measures completed at baseline, e.g., PHQ-9 used to assess depression severity | |
| Engagement in integrated care program | Initiate patient tracking in population-based registry | Patient has >=1 follow-up contact with the care manger with 4 weeks of initial contact |
| Systematic follow-up, treatment adjustment, and relapse prevention | Use population-based registry to systematically follow all patients | No period(s) of 60 days or more between enrollment and discharge in which no contact occurred |
| Monitor treatment response at each contact with valid outcome measures | Patient has one or more measures based on valid instruments (e.g., PHQ-9 for depression) recorded at each follow-up | |
| Identify patients who are not improving to target them for medication adjustment | Patient, if not improving, has had medication dosage adjustment, switching, or augmentation within 90 days since initial contact | |
| Create and support relapse prevention plan when patients are substantially improved | Patient who has achieved improvement during episode receives a relapse prevention plan as documented in registry | |
| Systematic psychiatric case review and consultation | Conduct regular psychiatric caseload review on patients who are not improving | Patient not improving within 8 weeks since initial contact receives >=1 psychiatric evaluation as documented in registry |
Notes: Adapted and developed from a synthesis of core components and tasks of integrated behavioral health care by the AIMS Center at the University of Washington(25). Table reflects a subset of tasks that can be reliably measured using the MHIP registry.
The two key process-of-care tasks thus selected are: At least one follow-up contact with the patient by the CCM care manager within 4 weeks of the initial contact, either at the clinic (in-person) or by phone (reflecting the principle of systematic follow-up), and, at least one psychiatric consultation of the case between weeks 8 and 12 among patients who do not achieve improvement by week 8 (reflecting measurement-based care and stepped care). Restriction to non-improving patients for the second task was based on the CCM protocol that recommends psychiatric consultation for cases not responding to initial treatment.
Study Population and Data
Our data were from Washington State’s Mental Health Integration Program (MHIP), a publicly funded implementation of the CCM in a network of over 100 community health centers diverse in geographic location, size and patient populations served(37). First started in 2008, the MHIP has served over 35,000 patients with mental health, substance abuse, and comorbid medical conditions who received primary care in participating clinics(38). Eligible patients included enrollees in the state’s Disability Lifeline Program – patients temporarily disabled because of a physical or mental health condition and expected to be unemployed for 90 days or more, veterans and their family members, the uninsured, low-income mothers and their children, and low-income older adults (categories not mutually exclusive). All participating clinics used a web-based registry to systematically document care management activities and clinical outcomes based on standard measurement tools (e.g., Patient Health Questionnaire or PHQ-9 to track depressive symptoms(39), and to assist with population management.
In this study, we focused on patients 18 years or older who initiated care in MHIP between January 1, 2008 and August 15, 2010 in clinics in King and Pierce counties, two most populous counties in Washington State where MHIP implementation first started. We further subset to patients with a baseline PHQ-9 >=10 indicating clinically significant depression and who had at least one follow-up contact with the MHIP care manager within 6 months/24weeks of the initial contact to ensure at least one chance to measure patient depression outcomes. The resulting sample contains 5,439 patient-episodes.
Outcome Measures
Our main outcome measures are a binary outcome of achieving clinically significant improvement in depression defined as having at least one follow-up PHQ-9 score of less than 10 or achieving a 50% or more reduction in PHQ-9(39) within 24 weeks of the initial contact with the MHIP care manager (“improvement” hereafter), and time to achieving improvement.
Statistical Analysis
Bi-variate analysis
We compared the probability of achieving improvement in depression by the receipt of either of the two key tasks defined above in “Selection of Key Tasks”. We generated Kaplan-Meier survival curves to capture cumulative % of patients achieving improvement over the first 24 weeks since initiation by the receipt of either task.
Multi-variate analysis
We estimated logistic models for achieving clinically significant improvement within 24 weeks and Cox-proportional hazard models for time to improvement. Both models controlled for patient age and gender, baseline PHQ-9 scores and comorbid behavioral health conditions other than depression including anxiety, bipolar, cognitive disorder, psychosis, post traumatic syndrome disorder (PTSD), substance abuse, and suicidal ideation, and categories of eligibility for MHIP. All multivariate analysis included dummy indicators of community health centers/MHIP participating sites (site fixed effects) to control for any between-site differences in quality and outcomes of care and derived robust standard errors taking into account clustering of patients within sites(40, 41).
Propensity score adjustment
Our initial analysis indicated that patient receipt of either task was associated with greater baseline severity of depression, behavioral health co-morbidity, and different MHIP eligibility categories. While such association is consistent with the principle of “stepped care”, the resulting confounding will likely bias our estimates. We thus conducted propensity score-adjusted analysis(42) by constructing propensity score matched control groups for each of the two tasks of interest. Predictors in the propensity score model for each task included all baseline patient demographic and clinical characteristics and MHIP site fixed effects. We used 1:1 nearest-neighbor matching within a defined caliper (0.25 of standard deviation of the log-odds of estimated propensity scores)(43, 44) with replacement, which allowed patients not receiving the task (the “controls”) to serve as matches in more than one pair. This approach, compared to other methods of matching we tested (including 1 to k matching with replacement with and without specified caliper), yielded both the most balanced sample and adequate sample size.
Results
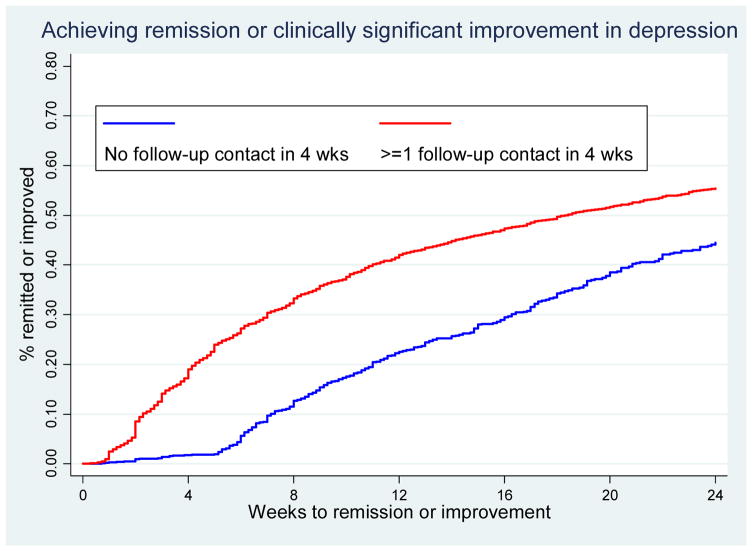
Table 2 reports sample statistics by the receipt of either of the two CCM tasks. Patients receiving at least one follow-up contact in the first 4 weeks (compared to patients who received none) had slightly higher PHQ-9 scores, a greater prevalence of all co-morbid behavioral health conditions, and were more likely to be Disability Lifeline clients and uninsured than from other eligibility categories. There were similar differences in baseline severity and co-morbidities by the receipt of psychiatric consultation among the subsample of patients not achieving improvement by week 8. Overall, 42.8% of patients with at least one follow-up contact in the first 4 weeks achieved clinically significant improvement in 24 weeks, compared to 34.4% among those who had no follow-up within 4 weeks. Among patients not improving by week 8, 24.9% of those with at least one psychiatric consultation between weeks 8 and 12 achieved improvement compared to 20.1% among those who did not receive consultation.
Table 2.
Baseline patient characteristics by receipt of either task
| Follow-up contacts within 4 weeks | Psychiatric consultation between weeks 8 and 12* | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 (n=968) | >=1 (n=4,471) | 0 (n=3,659) | >=1 (n=358) | |||||||
| Patient demographics | N | % | N | % | N | % | N | % | ||
| Gender | Female (vs. male) | 527 | 55.2 | 2,250 | 51.3 | 1,840 | 51.1 | 178 | 50.4 | |
| Age | 18–29 | 219 | 22.7 | 879 | 19.7 | 696 | 19.1 | 59 | 16.5 | |
| 30–39 | 210 | 21.7 | 967 | 21.7 | 772 | 21.2 | 79 | 22.1 | ||
| 40–49 | 272 | 28.3 | 1,318 | 29.6 | 1,105 | 30.3 | 108 | 30.2 | ||
| 50–59 | 205 | 21.2 | 1,087 | 24.4 | 900 | 24.7 | 95 | 26.5 | ||
| 60+ | 61 | 6.3 | 209 | 4.7 | 177 | 4.9 | 17 | 4.8 | ||
| Baseline clinical indicators | ||||||||||
| PHQ-9 | 10–14 | 258 | 29.9 | 1,101 | 26.2 | 695 | 20.6 | 60 | 17.4 | |
| 15–19 | 298 | 34.5 | 1,343 | 32.0 | 1,133 | 33.6 | 113 | 32.9 | ||
| 20+ | 308 | 35.7 | 154 | 41.8 | 1,547 | 45.8 | 191 | 49.7 | ||
| Comorbid behavioral health conditions | Anxiety | 481 | 49.7 | 2,863 | 64.0 | 2,309 | 63.1 | 256 | 71.5 | |
| Bipolar | 166 | 17.2 | 823 | 18.4 | 669 | 18.3 | 79 | 22.1 | ||
| Cognitive disorder | 9 | 0.9 | 115 | 2.6 | 79 | 2.2 | 12 | 3.4 | ||
| Psychotic disorder | 15 | 1.5 | 133 | 3.0 | 101 | 2.8 | 18 | 5.0 | ||
| PTSD | 142 | 14.7 | 1,067 | 23.9 | 841 | 23.0 | 103 | 28.8 | ||
| Substance abuse | 154 | 15.9 | 938 | 21.0 | 717 | 19.6 | 78 | 21.8 | ||
| Suicidal ideation | 468 | 53.2 | 2,504 | 59.0 | 2,096 | 61.2 | 222 | 64.7 | ||
| Patient eligibility categories† | ||||||||||
| Disability Lifeline | 649 | 67.1 | 3,213 | 71.9 | 2,698 | 73.7 | 294 | 82.1 | ||
| Low-income mothers | 126 | 13.0 | 443 | 9.9 | 302 | 8.3 | 21 | 5.9 | ||
| Low-income older adults | 112 | 11.6 | 479 | 10.7 | 390 | 10.7 | 36 | 10.1 | ||
| Uninsured | 98 | 10.1 | 590 | 13.2 | 435 | 11.9 | 30 | 8.4 | ||
| Veterans | 53 | 5.5 | 129 | 2.9 | 129 | 3.5 | 11 | 3.1 | ||
| Family members of veterans | 18 | 1.9 | 61 | 1.4 | 63 | 1.7 | 3 | 0.8 | ||
| Achieving clinically significant improvement in depression within 24 weeks | 333 | 34.4 | 1,915 | 42.8 | 737 | 20.1 | 89 | 24.9 | ||
Notes:
Among patients not improving within 8 weeks since initial contact with care manager
Categories not mutually exclusive
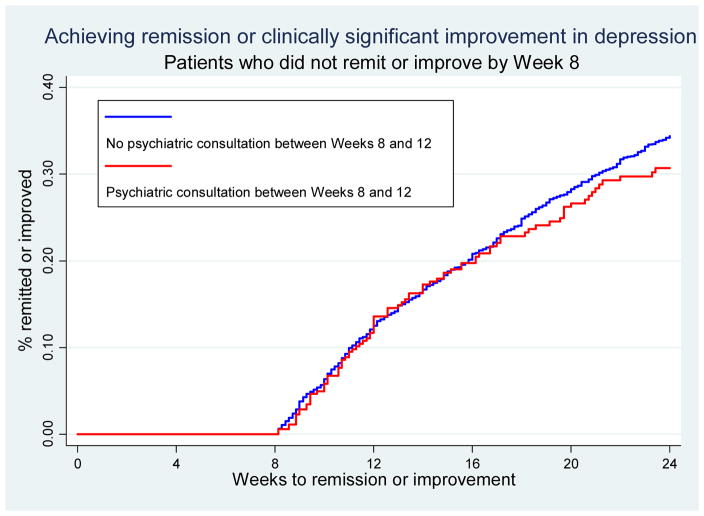
Figures 1–2 are Kaplan-Meier survival curves for time to first clinically significant improvement in depression within 24 weeks. Having had at least one follow-up contact within the first 4 weeks was significantly associated with shorter time to improvement (log rank test, p<0.001); cumulative % of patients achieving improvement was 10–20 percentage point greater among patients with at least one follow-up than among those without (Figure 1). Having had psychiatric consultation, on the other hand, was not associated with a shorter time to improvement as shown by largely overlapping survival curves (Figure 2).
Figure 1.
Kaplan-Meier survival curve for time to first clinically significant improvement in depression by follow-up contacts in the first 4 weeks
Figure 2.
Kaplan-Meier survival curve for time to first clinically significant improvement in depression by psychiatric consultation between weeks 8 and 12 among patients not achieving improvement by Week 8
Based on multivariate logistic analysis of achieving improvement in 24 weeks, having at least one follow-up contact was associated with an odds ratio of 1.63 (95% confidence interval or CI = 1.23–2.17; Table 3). Among patients not improving by week 8, having had psychiatric consultation between weeks 8 and 12 was associated with an odds ratio of 1.44 (95% CI = 1.13–1.84) for achieving improvement in 24 weeks. Based on estimated Cox-proportional hazard models, having had one or more follow-up contacts in the first 4 weeks was associated with a hazard ratio of 2.06 (95% CI = 1.67–2.54; Table 3) of achieving improvement; hazard ratio associated with psychiatric consultation was 0.99 (95% CI = 0.81–1.21). Complete estimates of both models are presented in Appendix A.
Table 3.
Odds ratios of achieving clinically significant improvement in depression and hazard ratios for time to improvement associated with receipt of either task, with and without propensity score adjustment
| Achieving clinically significant improvement in depression | Time to first clinically significant improvement in depression* | |||||||
|---|---|---|---|---|---|---|---|---|
| Original sample | Propensity score matched sample | Original sample | Propensity score matched sample | |||||
| OR | 95% CI | OR | 95% CI | HR | 95% CI | HR | 95% CI | |
| >=1 follow-up contact in 4 weeks | 1.63 | 1.23–2.17 | 1.57 | 1.11–2.24 | 2.06 | 1.67–2.54 | 1.96 | 1.52–2.54 |
| >=1 psychiatric consultation between weeks 8 and 12* | 1.44 | 1.13–1.84 | 1.40 | 1.13–1.74 | 0.99 | 0.81–1.21 | 0.90 | 0.64–1.26 |
OR-Odds Ratio; HR-Hazard Ratio
Analysis conducted on the sub-sample of patients not achieving improvement within 8 weeks since initial contact with the care manager
Our propensity score-matched samples achieved good balance in all observed baseline characteristics. Propensity score adjusted analysis generated very similar results as those based on the original samples (Table 3).
Discussion
In this study, we used data from a large implementation of the CCM to examine whether the practice of two key tasks in the early phase of CCM intervention predicted patient depression outcomes. We found that timely care manager follow-up within the first 4 weeks strongly predicted clinically significant improvement in depression within 6 months and a shorter time to achieving improvement. For patients not achieving improvement by week 8, we found that psychiatric consultation within the next four weeks (weeks 8–12) strongly predicted improvement within 6 months but not time to improvement.
Our findings regarding timely follow-up in the first 4 weeks suggest that patient engagement in the early phase of treatment might have critical implications for patient outcomes. Three pathways are potentially at play. First, as is the case with most CCM protocols, patients were referred to the MHIP program at a time when they were acutely depressed. Timely follow-up at the acute phase provides an opportunity to assess patient response and adjust treatment plans, potentially influencing the trajectory of treatment and patient outcomes. As shown in a previous study of between-site variation in a large CCM demonstration project(32), sites with higher rates of early follow-ups also had better patient depression outcomes that were not explained by patient characteristics. Second, early-stage follow-up enables care managers to conduct patient education and strengthen clinical alliances at a time when patients are at a high risk of becoming non-adherent to antidepressant therapy(45). Third, care manager contact may have therapeutic effects independent of its support of antidepressant or psychotherapy interventions. As seen in several large trials of the CCM, the effect of the intervention on depression outcomes was found as early as 3 months after the start of the program(35, 46), suggesting that increased clinician attention and patient engagement in the early phase of CCM may have had a direct effect before improved antidepressant or psychotherapy could translate into improved patient outcomes.
Our findings also support the value of timely psychiatric consultation of cases that did not experience improvement in the early phase of CCM. Patients who did not achieve improvement in the first 8 weeks were likely more clinically challenging to treat, as indicated by their substantially lower rate of improvement within 6 months (~20%) compared to what was seen among the entire cohort (30–40%). Our results suggest that timely psychiatric consultation to adjust treatment could substantially improve the chances of remission and recovery for patients resistant to initial treatment. On the other hand, psychiatric consultation did not predict more speedy improvement. This could be because of the low rate of improvement (20%) among this cohort and the fact that it took a much longer time for the majority of this cohort to achieve improvement (around 48 weeks; Kaplan-Meier curves shown in Appendix B for reviewers).
Our findings should not be interpreted to indicate that the two CCM tasks studied were the only tasks primary care teams need to practice to achieve improved patient outcomes. Primary care practices need to first undertake the systematic and organizational changes necessary to implement the CCM(27, 47). These include both changes in overall approaches to chronic care (e.g., addition of a care manager to perform care management activities outside of primary care visits) and infrastructure-building to enable operation (e.g., the use of a depression registry). In this paper, we chose to focus on process-of-care measures as they are less studied and are likely subject to greater variation in implementation.
By design, different process-of-care tasks of CCM are often practiced together and enable each other. For example, using PHQ-9 to continuously assess patient outcomes (Table 1), another key task of CCM that is not studied in this paper, is an important part of each care manager contact with the patient and provides the basis for psychiatric consultation. This study provides evidence to support two key tasks in the first 4–12 weeks of CCM without negating the importance of the other key tasks of CCM identified by expert consensus (Table 1).
Several limitations should be noted. First, despite propensity-score adjusted analysis, unobserved and unmeasured differences between patients who received either task and those who did not might be substantial and might have led to biases in our estimates. As discussed earlier, selection by patient baseline severity and complexity could have biased our estimates towards the null. Selection could also have occurred through patient motivation: patients with greater motivation were more likely to follow through with care management appointments but, at the same time, all else equal, more likely to achieve improvement in depression. Thus, selection by motivation could have biased the effect of the 4-week follow-up away from the null (i.e., towards suggesting a positive effect of follow-up), offsetting the selection bias by severity to an unknown extent.
Second, practice of either task may reflect the overall quality or fidelity to CCM at a given implementation site. This concern, however, is alleviated because our adjusted analysis controlled for all time-invariant, between-site differences by inclusion of site fixed effects. Results of our analysis thus reflect how variation in task performance (either over time or across patients) within a given site predicted patient outcomes. Meanwhile, practice of either task may be correlated with the practice of other related tasks to the same patient. For example, timely follow-up in the early phase may increase the likelihood that patients being continuously engaged and followed up in the rest of the treatment episode. Thus, estimated odds ratios and hazard ratios associated with either of the two tasks may reflect a combination of their direct effects and indirect effects mediated through other tasks and need to be interpreted with caution.
In conclusion, our study provided strong evidence that timely care manager follow-up in the first 4 weeks of CCM predicted substantial improvement in patient depression outcomes. Evidence regarding timely psychiatric consultation of cases not responding to initial course of treatment was less consistent but suggestive of the benefits of this task. Our findings support efforts to improve the fidelity with these two process-of-care tasks and to include these two tasks among quality measures for CCM implementation. Future studies should seek to assess the relative importance of other key tasks of CCM and test implementation strategies (e.g., pay-for-performance) to encourage and enable high fidelity to tasks found to contribute to good patient outcomes.
Supplementary Material
Acknowledgments
Grant Support
Dr. Bao is funded by the National Institute of Mental Health (K01MH090087; 1R01MH104200). Drs. Unützer and Chan received salary support from Community Health Plan of Washington for training, clinical consultation, and quality improvement efforts related to the Mental Health Integration Program (MHIP). The authors report no other competing interests.
Footnotes
Disclosures
MHIP registry data were originally collected for quality improvement purposes and were funded by Community Health Plan of Washington and Public Health of Seattle and King County.
References
- 1.Moussavi S, Chatterji S, Verdes E, et al. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet. 2007;370:851–8. doi: 10.1016/S0140-6736(07)61415-9. [DOI] [PubMed] [Google Scholar]
- 2.Druss BG, Zhao L, Cummings JR, et al. Mental comorbidity and quality of diabetes care under Medicaid: a 50-state analysis. Medical care. 2012;50:428–33. doi: 10.1097/MLR.0b013e318245a528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Katon WJ, Lin E, Russo J, et al. Increased medical costs of a population-based sample of depressed elderly patients. Arch Gen Psychiatry. 2003;60:897–903. doi: 10.1001/archpsyc.60.9.897. [DOI] [PubMed] [Google Scholar]
- 4.Wang PS, Simon GE, Kessler RC. Making the business case for enhanced depression care: the National Institute of Mental Health-harvard Work Outcomes Research and Cost-effectiveness Study. Journal of occupational and environmental medicine/American College of Occupational and Environmental Medicine. 2008;50:468–75. doi: 10.1097/JOM.0b013e31816a8931. [DOI] [PubMed] [Google Scholar]
- 5.Katon W. The impact of depression on workplace functioning and disability costs. Am J Manag Care. 2009;15:S322–7. [PubMed] [Google Scholar]
- 6.Unutzer J, Patrick DL, Simon G, et al. Depressive symptoms and the cost of health services in HMO patients aged 65 years and older. A 4-year prospective study. JAMA. 1997;277:1618–23. doi: 10.1001/jama.1997.03540440052032. [DOI] [PubMed] [Google Scholar]
- 7.Unutzer J, Schoenbaum M, Katon WJ, et al. Healthcare costs associated with depression in medically Ill fee-for-service medicare participants. Journal of the American Geriatrics Society. 2009;57:506–10. doi: 10.1111/j.1532-5415.2008.02134.x. [DOI] [PubMed] [Google Scholar]
- 8.Regier DA, Narrow WE, Rae DS, et al. The de facto US mental and addictive disorders service system. Epidemiologic catchment area prospective 1-year prevalence rates of disorders and services. Archives of general psychiatry. 1993;50:85–94. doi: 10.1001/archpsyc.1993.01820140007001. [DOI] [PubMed] [Google Scholar]
- 9.Wang PS, Demler O, Olfson M, et al. Changing profiles of service sectors used for mental health care in the United States. The American journal of psychiatry. 2006;163:1187–98. doi: 10.1176/appi.ajp.163.7.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rush AJ, Trivedi M, Carmody TJ, et al. One-year clinical outcomes of depressed public sector outpatients: a benchmark for subsequent studies. Biological psychiatry. 2004;56:46–53. doi: 10.1016/j.biopsych.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 11.National Committee for Quality Assurance: 2014 State of Health Care Quality. [Date accessed: April 16, 2015];Antidepressant Medication Management. Available from: http://www.ncqa.org/ReportCards/HealthPlans/StateofHealthCareQuality/2014TableofContents/Antidepressant.aspx.
- 12.Von Korff M, Gruman J, Schaefer J, et al. Collaborative management of chronic illness. Annals of internal medicine. 1997;127:1097–102. doi: 10.7326/0003-4819-127-12-199712150-00008. [DOI] [PubMed] [Google Scholar]
- 13.Wagner EH, Austin BT, Von Korff M. Organizing care for patients with chronic illness. The Milbank quarterly. 1996;74:511–44. [PubMed] [Google Scholar]
- 14.Gilbody S, Bower P, Fletcher J, et al. Collaborative care for depression: a cumulative meta-analysis and review of longer-term outcomes. Archives of internal medicine. 2006;166:2314–21. doi: 10.1001/archinte.166.21.2314. [DOI] [PubMed] [Google Scholar]
- 15.Williams JW, Jr, Gerrity M, Holsinger T, et al. Systematic review of multifaceted interventions to improve depression care. General hospital psychiatry. 2007;29:91–116. doi: 10.1016/j.genhosppsych.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 16.Unutzer J, Katon WJ, Fan MY, et al. Long-term cost effects of collaborative care for late-life depression. Am J Manag Care. 2008;14:95–100. [PMC free article] [PubMed] [Google Scholar]
- 17.Trivedi MH. Tools and strategies for ongoing assessment of depression: a measurement-based approach to remission. The Journal of clinical psychiatry. 2009;70(Suppl 6):26–31. doi: 10.4088/JCP.8133su1c.04. [DOI] [PubMed] [Google Scholar]
- 18.Von Korff M, Tiemens B. Individualized stepped care of chronic illness. The Western journal of medicine. 2000;172:133–7. doi: 10.1136/ewjm.172.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katon W, Unutzer J, Wells K, et al. Collaborative depression care: history, evolution and ways to enhance dissemination and sustainability. General hospital psychiatry. 2010;32:456–64. doi: 10.1016/j.genhosppsych.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Butler M, Kane RL, McAlpine D, et al. Integration of mental health/substance abuse and primary care. Evidence report/technology assessment. 2008:1–362. [PMC free article] [PubMed] [Google Scholar]
- 21.Finch RA, Phillips K Center for Prevention and Health Services. [Date accessed: April 16, 2015];An Employer’s Guide to Behavioral Health Services: A Roadway and Recommendations for Evaluating, Designing, and Implementing Behavioral Health Services. Available from: www.businessgrouphealth.org/pub/f3139c4c-2354-d714-512d-355c09ddcbc4.
- 22. [Date accessed: April 16, 2015];National Institute of Mental Health: Mental Health: A Report of the Surgeon General. Available from: http://www.surgeongeneral.gov/library/reports/
- 23.Substance Abuse and Mental Health Services Administration. [Date accessed: April 16, 2015];Achieving the Promise: Transforming Mental Health Care in America. Available from: http://store.samhsa.gov/product/SMA03-3831.
- 24.Fortney J, Pyne J, Smith J, et al. Steps for implementing collaborative care programs for depression. Population health management. 2009;12:69–79. doi: 10.1089/pop.2008.0023. [DOI] [PubMed] [Google Scholar]
- 25.AIMS Center Advancing Integrated Mental Health Solutions. [Date accessed: April 16, 2015];Checklist of Collaborative Care Principles and Components. Available from: http://aims.uw.edu/resource-library/checklist-collaborative-care-principles-and-components.
- 26.Huang H, Bauer A, Wasse J, et al. Care managers’ experiences in a collaborative care program for high risk mothers with depression. Psychosomatics. 2013;54:272–6. doi: 10.1016/j.psym.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nutting PA, Gallagher KM, Riley K, et al. Implementing a depression improvement intervention in five health care organizations: experience from the RESPECT-Depression trial. Adm Policy Ment Health. 2007;34:127–37. doi: 10.1007/s10488-006-0090-y. [DOI] [PubMed] [Google Scholar]
- 28.Bower P, Gilbody S, Richards D, et al. Collaborative care for depression in primary care: Making sense of a complex intervention: systematic review and meta-regression. The British Journal of Psychiatry. 2006;189:484–93. doi: 10.1192/bjp.bp.106.023655. [DOI] [PubMed] [Google Scholar]
- 29.Miller C, Grogan Kaylor A, Perron B, et al. Collaborative chronic care models for mental health conditions: cumulative meta-analysis and metaregression to guide future research and implementation. Medical care. 2013;51:922–30. doi: 10.1097/MLR.0b013e3182a3e4c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Whitebird RR, Solberg LI, Jaeckels NA, et al. Effective implementation of collaborative care for depression: What is needed? Am J Pharm Benefits. 2014;6:217–24. [PMC free article] [PubMed] [Google Scholar]
- 31.Coventry PA, Hudson JL, Kontopantelis E, et al. Characteristics of effective collaborative care for treatment of depression: a systematic review and meta-regression of 74 randomised controlled trials. PLoS One. 2014;9:e108114. doi: 10.1371/journal.pone.0108114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bauer AM, Azzone V, Goldman HH, et al. Implementation of Collaborative Depression Management at Community-Based Primary Care Clinics: An Evaluation. Psychiatric services. 2011;62:1047–53. doi: 10.1176/appi.ps.62.9.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thompson SG, Higgins JPT. How should meta-regression analyses be undertaken and interpreted? Statistics in Medicine. 2002;21:1559–73. doi: 10.1002/sim.1187. [DOI] [PubMed] [Google Scholar]
- 34.Unutzer J, Choi Y, Cook IA, et al. A web-based data management system to improve care for depression in a multicenter clinical trial. Psychiatric services. 2002;53:671–3. doi: 10.1176/ps.53.6.671. [DOI] [PubMed] [Google Scholar]
- 35.Unutzer J, Katon W, Callahan CM, et al. Collaborative care management of late-life depression in the primary care setting: a randomized controlled trial. JAMA: the journal of the American Medical Association. 2002;288:2836–45. doi: 10.1001/jama.288.22.2836. [DOI] [PubMed] [Google Scholar]
- 36.Bao Y, Casalino LP, Ettner SL, et al. Designing payment for Collaborative Care for Depression in primary care. Health services research. 2011;46:1436–51. doi: 10.1111/j.1475-6773.2011.01272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. [Date accessed: April 16, 2015];Washington State Mental Health Integration Program (MHIP) Available from: http://integratedcare-nw.org/index.html.
- 38. [Date accessed: April 16, 2015];AIMS Center Advancing Integrated Mental Health Solutions: Washington State’s Mental Health Integration Program (MHIP) Available from: http://aims.uw.edu/washington-states-mental-health-integration-program-mhip.
- 39.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. Journal of general internal medicine. 2001;16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Froot KA. Consistent Covariance-Matrix Estimation with Cross-Sectional Dependence and Heteroskedasticity in Financial Data. J Financ Quant Anal. 1989;24:333–55. [Google Scholar]
- 41.Williams RL. A note on robust variance estimation for cluster-correlated data. Biometrics. 2000;56:645–6. doi: 10.1111/j.0006-341x.2000.00645.x. [DOI] [PubMed] [Google Scholar]
- 42.Rubin DB, Thomas N. Matching using estimated propensity scores: Relating theory to practice. Biometrics. 1996;52:249–64. [PubMed] [Google Scholar]
- 43.Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharmaceutical statistics. 2011;10:150–61. doi: 10.1002/pst.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garrido MM, Kelley AS, Paris J, et al. Methods for constructing and assessing propensity scores. Health services research. 2014;49:1701–20. doi: 10.1111/1475-6773.12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bao Y, Ryan AM, Shao H, et al. Generic initiation and antidepressant therapy adherence under Medicare Part D. Am J Manag Care. 2013;19:989–98. [PMC free article] [PubMed] [Google Scholar]
- 46.Bruce ML, Ten Have TR, Reynolds CF, 3rd, et al. Reducing suicidal ideation and depressive symptoms in depressed older primary care patients a randomized controlled trial. JAMA. 2004;291:1081–91. doi: 10.1001/jama.291.9.1081. [DOI] [PubMed] [Google Scholar]
- 47.Solberg L, Crain AL, Jaeckels N, et al. The DIAMOND initiative: implementing collaborative care for depression in 75 primary care clinics. Implementation Science. 2013;8:135. doi: 10.1186/1748-5908-8-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.