Abstract
Background: Vitamin D may have anticancer activities. The high prevalence of vitamin D deficiency in African Americans (AAs) may be a contributing factor to the cancer health disparities between AAs and European Americans (EAs).
Objectives: We compared concentrations of 25(OH)D and vitamin D–binding protein (VDBP) in AA and EA women and investigated determinants of the vitamin D–biomarker concentrations in both populations.
Design: We used data and biospecimens from 909 AA and 847 EA healthy control subjects from the Carolina Breast Cancer Study (CBCS) and the Women’s Circle of Health Study (WCHS) in the African American Breast Cancer Epidemiology and Risk Consortium. We measured plasma 25(OH)D and VDBP concentrations in all participants and genotyped 67 vitamin D–related genes in AA women only.
Results: AA women had lower 25(OH)D concentrations than did EA women (mean ± SD: 14.2 ± 8.1 compared with 21.1 ± 11.5 ng/mL, respectively; P < 0.0001) but similar concentrations of VDBP (mean ± SD: 344 ± 133 compared with 336 ± 124 μg/mL, respectively; P = 0.25). With VDBP and other factors controlled for, the observed racial difference in 25(OH)D concentrations did not diminish. Relations of demographic and lifestyle factors with 25(OH)D were similar between AA and EA women. Although none of the genetic variants that have been identified in previous genome-wide association studies of 25(OH)D concentrations in EAs were significant (P > 0.05) in AAs, AA women who carried the allele of a functional single nucleotide polymorphism rs4988235, which has been previously associated with lactase expression and lactose tolerance, had higher dietary vitamin D intake and higher measured 25(OH)D concentrations.
Conclusions: AA women have lower concentrations of total 25(OH)D than EA women do, but both groups have similar VDBP concentrations, suggesting that there are lower concentrations of free 25(OH)D in AAs. Although demographic and lifestyle determinants of 25(OH)D concentrations are similar between the 2 groups, genetic determinants may be ethnicity specific. Larger studies in AAs will be needed to fully elucidate the underlying determinants of low vitamin D concentrations in AA populations.
Keywords: African American, European American, racial disparity, vitamin D, vitamin D–binding protein
INTRODUCTION
In humans, vitamin D comes mainly from cutaneous synthesis under sun exposure, limited food sources including fortified dairy products, and supplementary intake (1). African American (AA)9 individuals, for the most part, have notably lower 25-hydroxyvitamin D [25(OH)D] concentrations than those of their European American (EA) counterparts, possibly because of the high content of skin melanin coupled with the relatively low UV-radiation exposure of AAs in North American. In addition, the high prevalence of lactose intolerance in AAs may also contribute to lower intake of fortified dairy products (2).
In recent decades, there has been growing research and public health interest in vitamin D beyond its canonical activities in calcium homeostasis. Vitamin D deficiency has been implicated in a number of chronic diseases including cancer (3, 4). The high prevalence of vitamin D deficiency in AA populations may put them at high risk of these diseases and play a role in the observed health disparities. Paradoxically, despite their lower 25(OH)D concentrations, AAs, compared with EAs, have a higher bone mineral density and lower risk of fractures (5, 6). This discordance might be due to similar concentrations of bioavailable 25(OH)D between AAs and EAs. In blood circulation, a majority of 25(OH)D is bound by vitamin D–binding protein (VDBP), which has a 100-fold higher affinity than that of albumin (7). As a result, only 10–15% of circulating 25(OH)D is either bound by albumin or unbound (8). This small fraction of free 25(OH)D is thought to be bioavailable according to a concept that is similar to that of free steroid hormones such as estradiol and testosterone (9).
It is unclear whether the VDBP-bound fraction of 25(OH)D is indeed unavailable to all target cells and, thus, physiologically unimportant. A study showed that AAs had lower concentrations of both total 25(OH)D and VDBP, which result in similar concentrations of bioavailable 25(OH)D in AAs and EAs (9). These findings could have profound implications in regards to the definition of vitamin D deficiency, which is currently based on total 25(OH)D without distinguishing the VDBP that is bound from bioavailable fractions (10). However, concerns have been raised regarding the method that was used by Powe et al. (9) to measure VDBP (11–14).
In the current study, we measured and compared concentrations of 25(OH)D and VDBP between AA and EA women and investigated demographic and lifestyle determinants of vitamin D concentrations. In addition, because few published studies have examined genetic determinants of 25(OH)D concentrations in the AA population, we also investigated 25(OH)D concentrations with genetic variations in a large number of vitamin D–related genes in AA women in the American Breast Cancer Epidemiology and Risk (AMBER) Consortium.
METHODS
Study populations
For this analysis, we used data and biospecimens from control subjects who were enrolled in the following 2 studies that participate in the AMBER Consortium (15): the Women’s Circle of Health Study (WCHS) and the Carolina Breast Cancer Study (CBCS). The WCHS is a population-based case-control study that began in metropolitan New York City and expanded to New Jersey (15, 16). Enrollment began in 2002 with women aged 25–75 y who had been diagnosed with incident breast cancer in New York City hospitals with large referral patterns of AAs and through the New Jersey State Cancer Registry. Control subjects were matched for state, race, and age and were identified through random digital dialing and at community events. Blood samples were initially collected at the time of enrollment, but in 2007, to reduce costs and increase participation, the sample collection was switched to saliva. Thus, only WCHS control subjects who were enrolled in the first 5 y in New York City with collected blood samples and were all recruited through random digital dialing were included in the analysis. The CBCS is a population-based case-control study in North Carolina that began in 1993 (17). Breast cancer patients aged 20–74 were identified through the North Carolina State Cancer Registry, and control subjects were frequency matched to cases on the basis of age (±5 y) and race and were identified through the Division of Motor Vehicle lists and Health Care Finance Administration lists. Blood samples were collected at the time of enrollment from the majority of cases and controls in phases 1 and 2 of the CBCS. In both the WCHS and CBCS, controls were well-matched to cases for key designing factors including geography, race, and age.
A flowchart of the selection of participants in the WCHS and CBCS is presented in Supplemental Figure 1. All study participants provided consent for the use of their data and specimens for research purposes, and the studies were approved by the institutional review boards at participating institutions.
Questionnaire data collection and harmonization
Epidemiologic data were collected through interviewer-administered questionnaires at the time of enrollment along with anthropometric measurements. Because different questionnaires were used across participating studies in the AMBER Consortium, extensive harmonization efforts were invested to reconcile variables that were to be used as main exposures or important covariates in a 2-step process. First, a list of desired variables was identified with data types and formats that were specified for each study to carry out cleaning and recoding. The data were sent to the AMBER statistical core for centralized quality checks and multiple harmonizing recoding in an iterative process to derive common variables that retained information that was as detailed as possible (18). Data on dietary vitamin D intake and moderate and vigorous physical activity were available from the WCHS only, and the use of multivitamins was available from the CBCS only; thus, the data were included in analyses that were restricted to one study or the other. In the WCHS, dietary vitamin D intake was calculated as the micronutrients from a food-frequency questionnaire, which was adopted from food-frequency questionnaires that were used in 2 large prospective studies. Physical activity was assessed as metabolic equivalent of energy expenditure, which was calculated from data on the type of activity and the number of years in total, number of months per year, and mean number of hours per week that the subject performed the activity. The mean number of hours per week was computed for vigorous activity, which was defined as activities with a metabolic equivalent of energy expenditure ≥6.0.
Measurements of 25(OH)D and VDBP
Plasma samples from a total of 909 AA and 847 EA control subjects from the WCHS and the CBCS were available for biomarker measurements. 25(OH)D was measured with the use of a Liasion immunochemiluminometric assay (DiaSorin), and VDBP was measured with the use of an ELISA assay with a Gc-globulin (group-specific component) polyclonal antibody (Assaypro). The assays were performed by Heartland Assays (Ames), which participates in the Vitamin D External Quality Assessment Schema for their 25(OH)D assay. CVs were 13.2% and 15.8% for 25(OH)D and VDBP assays, respectively.
Genetic marker selection, genotyping, quality control, and imputation
Genomic DNA that was used for genotyping was extracted from saliva or blood in the WCHS and from mouthwash-swish samples or blood in the CBCS and were quantitated with the use of NanoDrop and PicoGreen assays (Thermo Fisher Scientific Inc.). Tag single nucleotide polymorphism (SNPs) in a total of 67 vitamin D–related genes from 3 pathways, including the pigmentation synthesis and metabolism pathway, the UV-exposure response pathway, and vitamin D metabolism and signaling pathway as defined in the Molecular Signature Database (19), were selected from the 1000 Genome Project (20) for inclusion as a part of the custom content that was added to the Illumina Human Exome BeadChip array (v1.1; Illumina Inc.) and genotyped at the Center for Inherited Disease Research as previously described (21–23). Imputation to the 1000 Genome reference data was performed at the University of Washington Center for Biomedical Statistics. Markers with a minor allele frequency <0.6% or an imputation quality score (R2) <0.5 were removed. Because the genotyping effort of the AMBER Consortium was focused on AA women, which covered multiple candidate pathways, genotype data were not available from EA women who were included in the current study. Thus, associations between genetic variants and 25(OH)D concentrations were limited to the 807 AA control subjects with both blood biomarker data and genotype data (genotyped and imputed SNPs: n = 25,248). Global genetic ancestry was estimated on the basis of genotypes of ancestry-informative markers that were selected from the genetic data (22).
Statistical analysis
Univariate analyses with the use of Student’s t, chi-square, and ANOVA tests were conducted to compare variables between AA and EA women and to relate these variables to measured 25(OH)D and VDBP concentrations. A generalized linear regression was used to test racial differences (AA compared with EA) in 25(OH)D concentrations while controlling for demographic and lifestyle factors that were significantly associated with 25(OH)D concentrations in univariate analyses and with VDBP concentrations in an additional model. Least-squares means ± SEs of 25(OH)D concentrations were derived from the same linear models after controlling for covariates. To test the association of genetic ancestry with 25(OH)D concentrations in AAs, the percentage of European ancestry was categorized into tertiles (≤9.8%, 9.9-18%, and >18%), and 25(OH)D concentrations were compared across ancestry categories with the use of an ANOVA test. Within AAs, genetic associations of each variant that was coded in an additive model with 25(OH)D and VDBP concentrations were tested with the use of linear regression and controlled for age, study, DNA source, and the percentage of European ancestry (continuous). Observations with missing variables were excluded from the analyses where those variables were used. Genetic association analyses were conducted with the use of the PLINK 1.90 tool set (24), and all other analyses were conducted with the use of R statistical software (R.3.2.3) (https://www.r-project.org/).
RESULTS
Descriptive characteristics of the study population
Descriptive characteristics of AAs and EAs in the WCHS and the CBCS are shown in Table 1, most of which were similar between the 2 studies. In the combined population, the mean age at enrollment was similar between AA and EA women at 51.8 and 52.3 y, respectively. The mean estimated percentage of European ancestry was 17% in AA women from both the CBCS and the WCHS. Compared with EA women, AA women had significantly higher BMI and waist-to-hip ratio (P < 0.0001), fewer women with a family history of breast cancer (P = 0.06), and more women who were never smokers and never drinkers (P < 0.0001). AA women were less likely to walk for exercise (P = 0.03) and, in the WCHS, were less likely to engage in moderate physical activity (P < 0.0005) or vigorous physical activity (P = 0.003). AA women in the WCHS also had lower dietary intake of vitamin D than that of EA women (P = 0.0009). In the CBCS, fewer AA women than EA women were past or current users of multivitamins (P < 0.0001).
TABLE 1.
Descriptive characteristics of study populations1
| Combined |
CBCS |
WCHS |
|||||||
| AA (n = 909) | EA (n = 847) | P | AA (n = 669) | EA (n = 665) | P | AA (n = 240) | EA (n = 182) | P | |
| Age at enrollment, y | 51.8 ± 10.52 | 52.3 ± 10.5 | 0.3 | 52.0 ± 11.3 | 52.7 ± 11.2 | 0.2 | 51.2 ± 8.0 | 50.6 ± 7.7 | 0.4 |
| European ancestry,3 % | 17 (0–98) | NA | — | 17 (0–98) | NA | — | 17 (0–76) | NA | — |
| BMI, kg/m2 | 31.4 ± 7.3 | 26.6 ± 6.1 | <0.0001 | 31.6 ± 7.5 | 26.7 ± 5.8 | <0.0001 | 31.0 ± 6.9 | 26.3 ± 7.1 | <0.0001 |
| <25, n (%) | 163 (18.2) | 408 (49.1) | 116 (17.6) | 307 (47.0) | 47 (19.7) | 101 (56.7) | |||
| 25–29, n (%) | 265 (29.5) | 234 (28.2) | 192 (29.1) | 195 (29.9) | 73 (30.7) | 39 (21.9) | |||
| ≥30, n (%) | 460 (52.3) | 189 (22.7) | 352 (53.3) | 151 (23.1) | 118 (49.6) | 38 (21.3) | |||
| Waist-to-hip ratio | 0.85 ± 0.08 | 0.80 ± 0.08 | <0.0001 | 0.84 ± 0.08 | 0.80 ± 0.08 | <0.0001 | 0.88 ± 0.09 | 0.84 ± 0.08 | <0.0001 |
| Family history of breast cancer, n (%) | 0.06 | 0.16 | 0.17 | ||||||
| No | 811 (89.2) | 731 (86.3) | 597 (89.2) | 577 (86.8) | 214 (89.2) | 154 (84.6) | |||
| Yes | 98 (10.8) | 116 (13.7) | 72 (10.8) | 88 (13.2) | 26 (10.8) | 28 (15.4) | |||
| Cigarette smoking, n (%) | <0.0001 | 0.002 | 0.03 | ||||||
| Never | 554 (61.0) | 455 (53.7) | 399 (59.6) | 352 (52.9) | 155 (64.6) | 103 (56.6) | |||
| Former | 190 (20.9) | 254 (30.0) | 142 (21.2) | 197 (29.6) | 48 (20.0) | 57 (31.3) | |||
| Current | 165 (18.2) | 138 (16.3) | 128 (19.1) | 116 (17.4) | 37 (15.4) | 22 (12.1) | |||
| Alcohol consumption, n (%) | <0.0001 | <0.0001 | <0.0001 | ||||||
| Never | 428 (47.1) | 256 (30.2) | 262 (39.2) | 186 (28.0) | 166 (69.2) | 70 (38.5) | |||
| Ever | 480 (52.9) | 591 (69.8) | 406 (60.8) | 479 (72.0) | 74 (30.8) | 112 (61.5) | |||
| Walking for exercise, n (%) | 0.03 | 0.18 | 0.04 | ||||||
| No | 603 (66.5) | 528 (62.3) | 421 (63.1) | 409 (61.5) | 182 (76.0) | 119 (65.4) | |||
| Yes | 302 (33.4) | 319 (37.7) | 245 (36.8) | 256 (38.5) | 57 (24.0) | 63 (34.6) | |||
| Moderate physical activity, n (%) | 0.0005 | ||||||||
| No | NA | NA | NA | NA | 146 (61.1) | 77 (42.3) | |||
| Yes | NA | NA | NA | NA | 93 (38.9) | 105 (57.7) | |||
| Vigorous physical activity, n (%) | 0.003 | ||||||||
| No | NA | NA | NA | NA | 198 (82.7) | 126 (69.2) | |||
| Yes | NA | NA | NA | NA | 41 (17.3) | 56 (30.8) | |||
| Dietary vitamin D intake, tertile (IU/d), n (%) | 0.0009 | ||||||||
| 1 (<127) | NA | NA | NA | NA | 94 (40.2) | 43 (24.0) | |||
| 2 (127–246) | NA | NA | NA | NA | 76 (32.5) | 62 (34.6) | |||
| 3 (>246) | NA | NA | NA | NA | 64 (27.4) | 74 (41.3) | |||
| Regular use of multivitamins, n (%) | <0.0001 | ||||||||
| No | NA | NA | 398 (59.5) | 1 (0.2) | NA | NA | |||
| Past | NA | NA | 35 (5.2) | 300 (45.1) | NA | NA | |||
| Current | NA | NA | 236 (35.3) | 364 (54.7) | NA | NA | |||
Data on moderate and vigorous physical activity and dietary vitamin D intake were not available for CBCS participants; data on the regular use of multivitamins were not available for WCHS participants. In the WCHS, dietary vitamin D intake was calculated as the micronutrients from a food-frequency questionnaire, which was adopted from food-frequency questionnaires that were used in 2 large prospective studies. Physical activity was assessed as metabolic equivalent of energy expenditure, which was calculated from data on the type of activity and the number of years in total, number of months per year, and mean number of hours per week that the subject performed the activity. The mean number of hours per week was computed for vigorous activity, which was defined as activities with a metabolic equivalent of energy expenditure ≥6.0. P values were derived from either a t test for continuous variables or a chi-square test for categorical variables. AA, African American; CBCS, Carolina Breast Cancer Study; EA, European American; NA, not applicable; WCHS, Women’s Circle of Health Study.
Mean ± SD (all such values).
Medians; ranges in parentheses.
Associations of 25(OH)D concentrations with demographic and lifestyle factors
Participants in the CBCS had higher mean 25(OH)D concentrations than women did who were in the WCHS (mean ± SD: 18.9 ± 10.5 compared with 17.5 ± 8.8 ng/mL, respectively; P = 0.01; data not shown) although the difference by study was limited to EA women [24.0 compared with 21.9 ng/mL (P = 0.007) in the CBCS and WCHS, respectively] (Table 2). Concentrations were similarly low in AA women in both the CBCS and WCHS (14.0 compared with 14.2 ng/mL, respectively; P = 0.72). Older age was associated with higher 25(OH)D concentrations in AA women (P = 0.005), whereas in EA women, the relation was U shaped: Women aged ≤40 y and women aged >60 y had higher concentrations than did women in middle-aged groups (P = 0.008). The associations of 25(OH)D concentrations with demographic and lifestyle factors, including obesity, the season of sample collection, physical activity, and multivitamin use, were similar between AA and EA women and in the expected directions, whereas associations with smoking and alcohol consumption were significant only in EAs, and dietary vitamin D intake was significant only in AAs. In parallel, the analysis of plasma VDBP concentrations with these factors showed that only the study (higher in the WCHS than in the CBCS) and season of blood collection (highest in spring and winter) were significant (data not shown). There was no correlation between plasma 25(OH)D and VDBP concentrations [Pearson correlation coefficient (r) = −0.01, P = 0.78] (Supplemental Figure 2).
TABLE 2.
Plasma 25-hydroxyvitamin D concentrations by demographic and lifestyle factors1
| African American (n = 909) |
European American (n = 847) |
|||
| ng/mL | P | ng/mL | P | |
| Study | 0.72 | 0.007 | ||
| CBCS | 14.0 ± 7.7 | 24.0 ± 10.5 | ||
| WCHS | 14.2 ± 7.1 | 21.9 ± 8.8 | ||
| Age at enrollment, y | 0.005 | 0.008 | ||
| ≤40 | 13.4 ± 8.4 | 26.1 ± 11.1 | ||
| 41–50 | 13.2 ± 7.2 | 22.7 ± 9.8 | ||
| 51–60 | 14.2 ± 6.9 | 22.7 ± 10.4 | ||
| >60 | 15.5 ± 8.1 | 24.4 ± 10.0 | ||
| BMI, kg/m2 | 0.001 | <0.0001 | ||
| <25 | 15.4 ± 8.5 | 25.9 ± 10.9 | ||
| 25–29.9 | 14.8 ± 7.7 | 22.7 ± 9.1 | ||
| ≥30 | 13.2 ± 7.1 | 19.9 ± 8.8 | ||
| Waist-to-hip ratio, tertile | 0.03 | <0.0001 | ||
| 1 (<0.785) | 15.0 ± 8.1 | 25.4 ± 10.4 | ||
| 2 (0.785–0.859) | 14.3 ± 8.1 | 23.9 ± 10.5 | ||
| 3 (≥0.860) | 13.3 ± 6.6 | 19.4 ± 8.2 | ||
| Season of blood collection | <0.0001 | <0.0001 | ||
| Spring (March through May) | 13.7 ± 7.7 | 20.8 ± 9.2 | ||
| Summer (June through August) | 15.4 ± 7.9 | 26.2 ± 10.5 | ||
| Fall (September through November) | 14.5 ± 7.4 | 25.0 ± 10.0 | ||
| Winter (December through February) | 11.6 ± 6.3 | 21.9 ± 10.4 | ||
| Family history of breast cancer | 0.63 | 0.56 | ||
| No | 14.1 ± 7.6 | 23.4 ± 10.2 | ||
| Yes | 13.7 ± 7.5 | 24.1 ± 10.0 | ||
| Cigarette smoking | 0.12 | 0.03 | ||
| Never | 14.3 ± 7.8 | 23.7 ± 9.3 | ||
| Former | 14.1 ± 7.0 | 24.4 ± 10.9 | ||
| Current | 12.9 ± 7.1 | 21.6 ± 11.3 | ||
| Alcohol consumption | 0.65 | 0.02 | ||
| Never | 13.9 ± 7.4 | 22.3 ± 9.5 | ||
| Ever | 14.1 ± 7.7 | 24.1 ± 10.5 | ||
| Walking for exercise | 0.03 | 0.003 | ||
| No | 13.6 ± 7.6 | 22.7 ± 10.1 | ||
| Yes | 14.8 ± 7.4 | 24.9 ± 10.3 | ||
| WCHS | ||||
| Moderate physical activity | 0.05 | 0.09 | ||
| No | 13.4 ± 6.9 | 20.6 ± 8.6 | ||
| Yes | 15.3 ± 7.2 | 22.8 ± 9.0 | ||
| Vigorous physical activity | 0.15 | 0.25 | ||
| No | 13.9 ± 7.1 | 21.4 ± 8.8 | ||
| Yes | 15.6 ± 7.0 | 23.0 ± 8.8 | ||
| Dietary vitamin D intake, tertile (IU/d) | 0.02 | 0.23 | ||
| 1 (<127) | 12.9 ± 6.4 | 20.0 ± 9.4 | ||
| 2 (127–246) | 14.2 ± 6.8 | 22.7 ± 9.7 | ||
| 3 (246) | 16.1 ± 8.1 | 22.6 ± 7.7 | ||
| Regular use of multivitamins in the CBCS | <0.0001 | 0.0004 | ||
| No | 13.1 ± 7.1 | 15.1 | ||
| Past | 11.5 ± 5.3 | 22.3 ± 10.0 | ||
| Current | 15.8 ± 8.6 | 25.4 ± 10.7 | ||
All values are means ± SD. Data on moderate and vigorous physical activity and dietary vitamin D intake were not available for CBCS participants; data on the regular use of multivitamins were not available for WCHS participants. In the WCHS, dietary vitamin D intake was calculated as the micronutrients from a food-frequency questionnaire, which was adopted from food-frequency questionnaires that were used in 2 large prospective studies. Physical activity was assessed as metabolic equivalent of energy expenditure, which was calculated from data on the type of activity and the number of years in total, number of months per year, and mean number of hours per week that the subject performed the activity. The mean number of hours per week was computed for vigorous activity, which was defined as activities with a metabolic equivalent of energy expenditure ≥6.0. P values were derived from either a t test or an ANOVA test. CBCS, Carolina Breast Cancer Study; WCHS, Women’s Circle of Health Study.
Differences in plasma 25(OH)D concentrations between AA and EA women
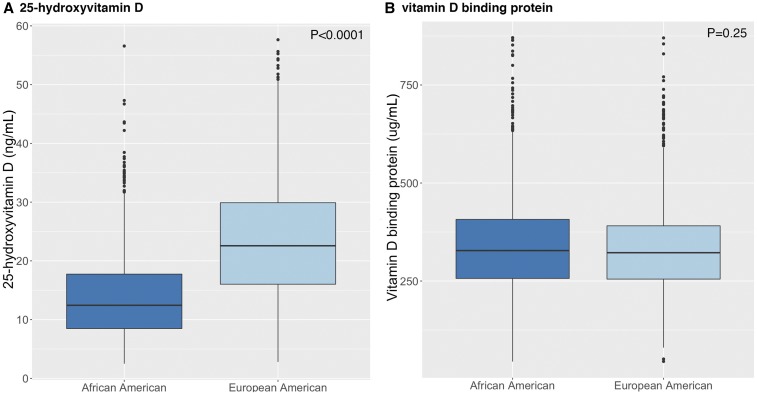
As shown in Figure 1A, AA women compared with EA women had significantly lower 25(OH)D concentrations (mean ± SD: 14.2 ± 8.1 compared with 21.1 ± 11.5 ng/mL, respectively; P < 0.0001) but similar concentrations of VDBP (mean ± SD: 344 ± 133 compared with 336 ± 124 μg/mL, respectively; P = 0.25) (Figure 1B). There were marked differences between AAs and EAs in terms of the prevalence of vitamin D deficiency [i.e., 25(OH)D concentration ≤20 ng/mL (25)] with 83% of AAs being deficient compared with 40% of EA women (Supplemental Figure 3). Only 4% of AAs were vitamin D sufficient [i.e., 25(OH)D concentration ≥30 ng/mL (25)] compared with 24% of EAs. Similar differences were observed when vitamin D deficiency was defined on the basis of a different cutoff [i.e., 25(OH)D concentration ≤12.5 ng/mL (26)] (50.2% in AAs compared with 13.5% in EAs) or when tested in the WCHS and the CBCS separately (data not shown). When adjusted for factors that affected 25(OH)D concentrations, including the study, season of blood collection, age, BMI, smoking, alcohol use, and physical activity, the difference in 25(OH)D concentrations between AAs and EAs persisted after adjustment (least-squares means ± SEs: 14.3 ± 0.3 compared with 22.4 ± 0.3, respectively; P < 0.0001). Additional adjustment for dietary vitamin D intake and multivitamin use in the WCHS and CBCS, respectively, did not substantially change the difference either. Last, further adjustment for VDBP concentrations in the multivariable model did not change associations (least-squares means ± SEs after adjustment: 14.3 ± 0.3 in AAs compared with 22.4 ± 0.3 in EAs; P < 0.0001).
FIGURE 1.
Box plots of circulating 25-hydroxyvitamin D concentrations (A) and vitamin D–binding protein concentrations (B) in African American and European American control subjects in the African American Breast Cancer Epidemiology and Risk Consortium. In each box, the horizontal line in the middle of the box indicates the median, and the 2 other horizontal lines indicate 25th and 75th quartiles. Lower and upper whiskers indicate ranges, and dots indicate outlier values.
Associations of 25(OH)D concentrations with genetic ancestry and variants in vitamin D–related pathways in AA women
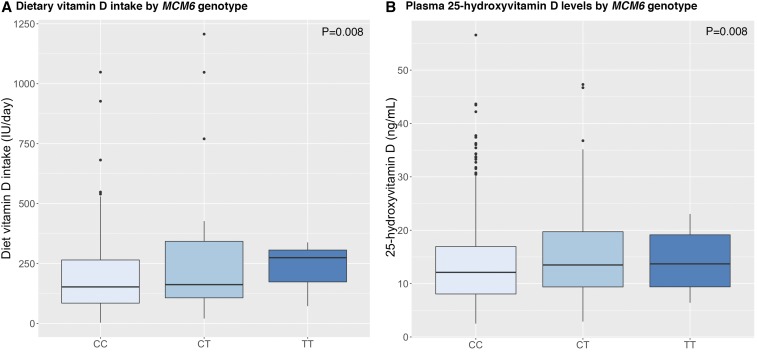
Global genetic ancestry, which was estimated on the basis of ancestry-informative markers, was significantly associated with 25(OH)D concentrations. Women in the highest tertile of European ancestry (>18%) had the highest 25(OH)D concentrations (mean ± SD: 14.9 ± 8.0, 13.4 ± 6.9, and 13.5 ± 7.6 ng/mL for women in tertiles 3–1 of European ancestry, respectively; P = 0.03) (Supplemental Figure 4). None of the identified variants in previous genome-wide association studies (GWASs) of 25(OH)D concentrations in populations of European ancestry were significant in AA women after adjustment for multiple testing in this study (Table 3). Nevertheless, the T allele of SNP rs4988235 (−13910 C→T) in minichromosome maintenance complex component 6 gene (MCM6), which is associated with elevated lactase gene expression and lactase persistence and is less common in individuals of African ancestry, was nominally associated with both higher dietary vitamin D intake and measured 25(OH)D concentrations in AA women [β ± SE dietary vitamin D intake of 101 ± 38 IU/d per copy of the variant allele (P = 0.008); β ± SE 25(OH)D concentrations: 1.6 ± 0.6 ng/mL (P = 0.008)] (Figure 2) although these associations were NS after adjustment for multiple comparisons. When dietary vitamin D intake was adjusted in the model, the association between the SNP and 25(OH)D concentrations was no longer significant (P = 0.27). The frequency of the T allele of the SNP was 0.12 in our AA population, which was similar to that in African ancestry individuals in the International HapMap Consortium (0.14) but much lower than in HapMap European ancestry individuals (0.73).
TABLE 3.
Associations of selected genetic variants with plasma 25-hydroxyvitamin D concentrations1
| SNP | Gene | A1 | A2 | A1-allele frequency | Info score | β ± SE | P |
| rs1155563 | GC | C | T | 0.12 | 0.92 | −0.82 ± 0.63 | 0.19 |
| rs2282679 | GC | G | T | 0.09 | 1.02 | −0.62 ± 0.66 | 0.35 |
| rs7041 | GC | C | A | 0.16 | 1.03 | 0.31 ± 0.52 | 0.55 |
| rs2060793 | CYP2R1 | G | A | 0.64 | 1.04 | 0.22 ± 0.40 | 0.58 |
| rs10741657 | CYP2R1 | G | A | 0.72 | 1.05 | −0.27 ± 0.42 | 0.52 |
| rs4988235 | MCM6 | T | C | 0.12 | 0.98 | 1.60 ± 0.60 | 0.008 |
A1 is the minor allele, and A2 is the reference allele. β Regression coefficients ± SEs and P values were derived from linear regression models of 25-hydroxyvitamin D with each genetic variant. Info score is a measure of SNP imputation quality ranging from 0 to 1, with a higher score indicating better imputation quality. SNPs with info score of 1 or higher are directly genotyped. CYP2R1, cytochrome P450 family 2 subfamily R member 1; GC, group-specific component (vitamin D binding protein); MCM6, minichromosome maintenance complex component 6; SNP, single nucleotide polymorphism.
FIGURE 2.
Box plots of dietary vitamin D intake in control subjects in the WCHS (A) and 25-hydroxyvitamin D concentrations in control subjects in the WCHS and Carolina Breast Cancer Study from the African American Breast Cancer Epidemiology and Risk Consortium (B) according to MCM6 rs4988235 (−13910 C→T) genotype. The variant T allele is associated with an elevated expression of lactase and lactose intolerance. In each box, the horizontal line in the middle of the box indicates the median, and the 2 other horizontal lines indicate 25th and 75th quartiles. Lower and upper whiskers indicate ranges, and dots indicate outlier values. MCM6, minichromosome maintenance complex component 6; WCHS, Women’s Circle of Health Study.
Of all 25,248 genotyped and imputed variants in 67 genes in vitamin D–related pathways, the most significant marker that was identified in association with 25(OH)D concentrations in AA women was rs113091724 in heme A:farnesyltransferase cytochrome C oxidase assembly factor gene (COX10), which is involved in the pigmentation synthesis and metabolism pathway. This SNP is an imputed low-frequency SNP [variant G allele frequency: 0.007; imputation R2 = 0.90; β ± SE: 12.2 ± 2.4 ng/mL per copy of the variant allele (P = 4 × 10−7)] (Supplemental Figure 5), which remained significant after adjustment for multiple comparisons with the use of the Bonferroni method (corrected P = 0.01).
No significant genetic associations were shown between vitamin D–related pathway SNPs and VDBP concentrations in AA women, including the 2 functional SNPs rs7041 (P = 0.22) and rs4588 (P = 0.11) in group-specific component (vitamin D binding protein) (GC), which encodes for VDBP. VDBP haplotypes on the basis of the combinations of these 2 SNPs (Gc1s, Gc1f, and Gc2) were not associated with VDBP concentrations either (P = 0.40) (Supplemental Figure 6).
DISCUSSION
Our study confirms that there are marked differences between EAs and AAs in circulating 25(OH)D concentrations but no differences in circulating VDBP concentrations. When VDBP was controlled for, there was no impact on the observed racial differences in 25(OH)D. Although we did not have the albumin data that were needed to calculate free 25(OH)D concentrations, these results suggest that free or bioavailable 25(OH)D concentrations may still be lower in AA women than in EA women. Our results are contrary to those that were observed previously by Powe et al. (9), which showed similar concentrations of free 25(OH)D between AA and EA individuals. However, the monoclonal antibody used (R&D Systems) in the study of Powe et al. (9) recognizes an epitope enclosing the polymorphic region that is affected by the 2 missense SNPs rs7041 and rs4588. Because the Gc1f haplotype, which consists of the 2 SNPs that are associated with a lower affinity, has a far-higher frequency in AAs than in EAs (93% compared with 6%, respectively) (9), VDBP concentrations in a majority of the AA group might have been underestimated. In our study, a different polyclonal VDBP antibody (Assaypro) was used, which showed no difference by GC genotypes in our analysis. Our findings are consistent with several other studies (11–14), including one study that measured VDBP with the use of liquid chromatography–tandem mass spectrometry and another study that measured free 25(OH)D directly with the use of an ELISA. Therefore, the evidence that has been accumulated to date does not support the notion that AAs have similar free 25(OH)D concentrations as those of EAs. The findings provide support for further investigations of whether vitamin D is a contributing factor to health disparities in cancer and other chronic diseases.
Our analyses reveal that associations between plasma concentrations and demographic and lifestyle determinants of 25(OH)D concentrations are largely similar between AA and EA women. Note that dietary vitamin D intake is a stronger determinant in AAs than in EAs. This difference is possibly due to the limited source of vitamin D from sun exposure that is available for AAs, and thus, dietary sources may be relatively more important. VDBP displays an opposite seasonal trend to that of 25(OH)D with the concentration being higher in the spring and winter and lower in the summer and fall. In an earlier study, it was reported that VDBP concentrations were lower in the summer in prostate cancer cases but the concentrations were constant throughout the year in male control subjects (27). If seasonal changes in VDBP concentrations exist, our finding suggests that seasonal changes in free 25(OH)D would be even greater than in total 25(OH)D concentrations, which might have some physiologic implications.
Although several studies have examined nongenetic determinants of vitamin D concentrations in AA and EA populations (28–31), to our knowledge, few studies have examined genetic determinants. In 2 previous GWASs of circulating 25(OH)D concentrations in populations of European descent, several associations with genetic variants in GC, cytochrome P450 family 2 subfamily R member 1 (CYP2R1), and 7-dehydrocholesterol reductase gene (DHCR7)/nicotinamide adenine dinucleotide synthetase 1 gene (NADSYN1) were identified at a genome-wide significance level (32, 33). To our knowledge, no GWAS of 25(OH)D concentrations has been done in AA populations to date. Data from our group and other groups have shown an inverse relation between the proportion of African ancestry and measured 25(OH)D (34, 35), thereby supporting a genetic component in determining 25(OH)D concentrations in AAs. In addition, in our analysis, racial differences in 25(OH)D concentrations persisted after controlling for all available nongenetic determinants, which also suggested that genetic factors may have contributed to the racial difference.
However, the identification of genetic determinants of 25(OH)D concentrations in AA populations can be challenging. In general, genetic associations can be race specific. For example, a majority of GWAS hits for breast cancer risk that were initially identified in populations of European or Asian populations were not replicated in AA populations (36–39). This difference could be particularly true for genetic variants that are associated with vitamin D concentrations. According to the “Out of Africa” theory of human evolution and diaspora (2), lighter skin color was favorably selected for when human ancestors immigrated to Northern latitudes at least partially to compensate for compromised vitamin D synthesis that was due to weaker sun exposure (40–42). Thus, genetic variants that are associated with 25(OH)D concentrations in EAs, particularly those variants in genes that are related to skin pigmentation, might occur or become more common only in EAs. This effect would suggest that genetic determinants of 25(OH)D concentrations may be race specific. However, because of the relatively limited sample size of AAs in our study, future larger studies are warranted to either confirm or refute this possibility.
We showed that rs4988235 in MCM6, which is a functional variant that affects the expression of lactase and a putative causal variant for lactase persistence (43), was nominally associated with both dietary vitamin D intake and plasma 25(OH)D concentrations in AAs. The association with 25(OH)D concentrations appeared to be dependent on its association with dietary vitamin D intake. Although the association was NS after correction for multiple comparisons, the consistency with the expected functionality of the variant provides an endorsement for the validity of the finding. Note that this variant has a much-higher frequency in EAs than in AAs (0.73 compared with 0.14, respectively), which is likely due strong positive-selection pressures at a time that was coincident with dairy farming (44, 45) and is consistent with the high prevalence of lactose intolerance in AAs. As a result, a majority of AAs who do not carry the variant might limit their dairy food intake and, subsequently, vitamin D intake. In European populations, this SNP was associated with intake of dairy products and dietary calcium (46, 47) but not strongly associated with serum 25(OH)D concentrations (47). Note that this SNP was not associated with 25(OH)D concentrations in previous GWASs in European populations (32, 33). The reason for this outcome might be that dietary intake is only a minor source of vitamin D in Europeans, whereas it is a relatively more-important source for AAs as shown in our data.
Our study has some limitations. First, data and samples in this analysis came from 2 case-control studies. Measured 25(OH)D concentrations were higher in EA women in the CBCS than in the WCHS, which were probably due to the lower latitude region in which the CBCS participants resided. Although the CBCS and WCHS questionnaires were not exactly the same, there were many similarities. Because of the similarities and the data-harmonization efforts in the AMBER Consortium, most of the examined associations were consistent between the 2 studies, which suggested that there were limited between-study heterogeneities. Second, we included a large number of genetic variants in candidate genes from vitamin D–related pathways in analyses of plasma 25(OH)D concentrations but did not identify any common-variant associations after correcting for multiple comparisons. Our sample size (n = 807) was moderate and likely underpowered to detect small effects. However, to our knowledge, our study is the first trial to investigate genetic determinants of vitamin D in AAs, and our findings of the MCM6 SNP rs4988235 with dietary vitamin D intake and plasma 25(OH)D concentrations make a contribution to the literature. Because of the design of the AMBER Consortium, genotype data were not available from EA women. Third, the CVs for 25(OH)D and VDBP assays were at the high end of a normally expected range. Last, study participants were drawn from 3 states (New York, New Jersey, and North Carolina) and, thus, may not represent all AA women nationwide.
In conclusion, in the study population, AA women have lower 25(OH)D concentrations and a much higher prevalence of vitamin D deficiency than EA women do; however, there is no difference in VDBP concentrations. Although demographic and lifestyle factors have similar effects on 25(OH)D concentrations between AAs and EAs in our study, genetic determinants may be race specific. In addition, we identify that the functional variant rs4988235 affects lactase gene expression in association with dietary vitamin D intake and plasma 25(OH)D concentrations in AA women. Future larger studies will be needed to fully delineate genetic determinants of vitamin D concentrations in AA populations.
Acknowledgments
We thank Robert Millikan who was instrumental in the creation of the Consortium.
The authors’ responsibilities were as follows—SY, CBA, JRP, and AFO: research design; SY, C-CH, CBA, GZ, SAH, KLL, EAR-N, MAT, LR, JRP, AFO, and CBA: research conduct; SY, C-CH, CBA, GZ, SAH, KLL, EAR-N, MAT, LR, JRP, AFO, and CBA: provision of essential materials; SY, QZ, and SL: data analysis; SY and CBA: primary responsibility for the final content of the manuscript; and all authors: original and substantial contributions to the manuscript, writing of the manuscript, and reading and approval of the final manuscript. None of the authors reported a conflict of interest related to the study.
Footnotes
Abbreviations used: AA, African American; AMBER, African American Breast Cancer Epidemiology and Risk; CBCS, Carolina Breast Cancer Study; EA, European American; GC, group-specific component (vitamin D binding protein); GWAS, genome-wide association study; MCM6, minichromosome maintenance complex component 6; SNP, single nucleotide polymorphism; VDBP, vitamin D–binding protein; WCHS, Women’s Circle of Health Study; 25(OH)D, 25-hydroxyvitamin D.
REFERENCES
- 1.Lips P. Worldwide status of vitamin D nutrition. J Steroid Biochem Mol Biol 2010;121:297–300. [DOI] [PubMed] [Google Scholar]
- 2.Yao S, Ambrosone CB. Associations between vitamin D deficiency and risk of aggressive breast cancer in African-American women. J Steroid Biochem Mol Biol 2013;136:337–41. [DOI] [PubMed] [Google Scholar]
- 3.Deeb KK, Trump DL, Johnson CS. Vitamin D signalling pathways in cancer: potential for anticancer therapeutics. Nat Rev Cancer 2007;7:684–700. [DOI] [PubMed] [Google Scholar]
- 4.Feldman D, Krishnan AV, Swami S, Giovannucci E, Feldman BJ. The role of vitamin D in reducing cancer risk and progression. Nat Rev Cancer 2014;14:342–57. [DOI] [PubMed] [Google Scholar]
- 5.Cauley JA, Lui LY, Ensrud KE, Zmuda JM, Stone KL, Hochberg MC, Cummings SR. Bone mineral density and the risk of incident nonspinal fractures in black and white women. JAMA 2005;293:2102–8. [DOI] [PubMed] [Google Scholar]
- 6.Aloia JF. African Americans, 25-hydroxyvitamin D, and osteoporosis: a paradox. Am J Clin Nutr 2008;88:545S–50S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Speeckaert M, Huang G, Delanghe JR, Taes YE. Biological and clinical aspects of the vitamin D binding protein (Gc-globulin) and its polymorphism. Clin Chim Acta 2006;372:33–42. [DOI] [PubMed] [Google Scholar]
- 8.Bikle DD, Gee E, Halloran B, Kowalski MA, Ryzen E, Haddad JG. Assessment of the free fraction of 25-hydroxyvitamin D in serum and its regulation by albumin and the vitamin D-binding protein. J Clin Endocrinol Metab 1986;63:954–9. [DOI] [PubMed] [Google Scholar]
- 9.Powe CE, Evans MK, Wenger J, Zonderman AB, Berg AH, Nalls M, Tamez H, Zhang D, Bhan I, Karumanchi SA, et al. Vitamin D-binding protein and vitamin D status of black Americans and white Americans. N Engl J Med 2013;369:1991–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.LeFevre ML, U.S. Preventive Services Task Force. Screening for vitamin D deficiency in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2015;162:133–40. [DOI] [PubMed] [Google Scholar]
- 11.Nielson CM, Jones KS, Bouillon R, Osteoporotic Fractures in Men Research Group, Chun RF, Jacobs J, Wang Y, Hewison M, Adams JS, Swanson CM, et al. Role of assay type in determining free 25-hydroxyvitamin D levels in diverse populations. N Engl J Med 2016;374:1695–6. [DOI] [PMC free article] [PubMed]
- 12.Hoofnagle AN, Eckfeldt JH, Lutsey PL. Vitamin D-binding protein concentrations quantified by mass spectrometry. N Engl J Med 2015;373:1480–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Denburg MR, Hoofnagle AN, Sayed S, Gupta J, de Boer IH, Appel LJ, Durazo-Arvizu R, Whitehead K, Feldman HI, Leonard MB, et al. Comparison of two ELISA methods and mass spectrometry for measurement of vitamin D-binding protein: implications for the assessment of bioavailable vitamin D concentrations across genotypes. J Bone Miner Res 2016;31:1128–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henderson CM, Lutsey PL, Misialek JR, Laha TJ, Selvin E, Eckfeldt JH, Hoofnagle AN. Measurement by a novel LC-MS/MS methodology reveals similar serum concentrations of vitamin D-binding protein in blacks and whites. Clin Chem 2016;62:179–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ambrosone CB, Ciupak GL, Bandera EV, Jandorf L, Bovbjerg DH, Zirpoli G, Pawlish K, Godbold J, Furberg H, Fatone A, et al. Conducting molecular epidemiological research in the age of HIPAA: a multi-institutional case-control study of breast cancer in African-American and European-American women. J Oncol 2009;2009:871250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bandera EV, Chandran U, Zirpoli G, McCann SE, Ciupak G, Ambrosone CB. Rethinking sources of representative controls for the conduct of case-control studies in minority populations. BMC Med Res Methodol 2013;13:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Newman B, Moorman PG, Millikan R, Qaqish BF, Geradts J, Aldrich TE, Liu ET. The Carolina Breast Cancer Study: integrating population-based epidemiology and molecular biology. Breast Cancer Res Treat 1995;35:51–60. [DOI] [PubMed] [Google Scholar]
- 18.Palmer JR, Ambrosone CB, Olshan AF. A collaborative study of the etiology of breast cancer subtypes in African American women: the AMBER consortium. Cancer Causes Control 2014;25:309–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA 2005;102:15545–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abecasis GR, Auton A, Brooks LD, DePristo MA, Durbin RM, Handsaker RE, Kang HM, Marth GT, McVean GA. An integrated map of genetic variation from 1,092 human genomes. Nature 2012;491:56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haddad SA, Lunetta KL, Ruiz-Narvaez EA, Bensen JT, Hong CC, Sucheston-Campbell LE, Yao S, Bandera EV, Rosenberg L, Haiman CA, et al. Hormone-related pathways and risk of breast cancer subtypes in African American women. Breast Cancer Res Treat 2015;154:145–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yao S, Haddad SA, Hu Q, Liu S, Lunetta KL, Ruiz-Narvaez EA, Hong CC, Zhu Q, Sucheston-Campbell L, Cheng TY, et al. Genetic variations in vitamin D-related pathways and breast cancer risk in African American women in the AMBER consortium. Int J Cancer 2016;138:2118–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruiz-Narváez EA, Haddad SA, Lunetta KL, Yao S, Bensen JT, Sucheston-Campbell LE, Hong CC, Haiman CA, Olshan AF, Ambrosone CB, et al. Gene-based analysis of the fibroblast growth factor receptor signaling pathway in relation to breast cancer in African American women: the AMBER consortium. Breast Cancer Res Treat 2016;155:355–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 2007;81:559–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holick MF. Vitamin D deficiency. N Engl J Med 2007;357:266–81. [DOI] [PubMed] [Google Scholar]
- 26.Manson JE, Brannon PM, Rosen CJ, Taylor CL, Vitamin D. Deficiency - is there really a pandemic? N Engl J Med 2016;375:1817–20. [DOI] [PubMed] [Google Scholar]
- 27.Corder EH, Friedman GD, Vogelman JH, Orentreich N. Seasonal variation in vitamin D, vitamin D-binding protein, and dehydroepiandrosterone: risk of prostate cancer in black and white men. Cancer Epidemiol Biomarkers Prev 1995;4:655–9. [PubMed] [Google Scholar]
- 28.Shea MK, Houston DK, Tooze JA, Davis CC, Johnson MA, Hausman DB, Cauley JA, Bauer DC, Tylavsky F, Harris TB, et al. Correlates and prevalence of insufficient 25-hydroxyvitamin D status in black and white older adults: the health, aging and body composition study. J Am Geriatr Soc 2011;59:1165–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Freedman DM, Cahoon EK, Rajaraman P, Major JM, Doody MM, Alexander BH, Hoffbeck RW, Kimlin MG, Graubard BI, Linet MS. Sunlight and other determinants of circulating 25-hydroxyvitamin D levels in black and white participants in a nationwide U.S. study. Am J Epidemiol 2013;177:180–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rajakumar K, Holick MF, Jeong K, Moore CG, Chen TC, Olabopo F, Haralam MA, Nucci A, Thomas SB, Greenspan SL. Impact of season and diet on vitamin D status of African American and Caucasian children. Clin Pediatr (Phila) 2011;50:493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tseng M, Giri V, Bruner DW, Giovannucci E. Prevalence and correlates of vitamin D status in African American men. BMC Public Health 2009;9:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ahn J, Yu K, Stolzenberg-Solomon R, Simon KC, McCullough ML, Gallicchio L, Jacobs EJ, Ascherio A, Helzlsouer K, Jacobs KB, et al. Genome-wide association study of circulating vitamin D levels. Hum Mol Genet 2010;19:2739–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang TJ, Zhang F, Richards JB, Kestenbaum B, van Meurs JB, Berry D, Kiel DP, Streeten EA, Ohlsson C, Koller DL, et al. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet 2010;376:180–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Signorello LB, Williams SM, Zheng W, Smith JR, Long J, Cai Q, Hargreaves MK, Hollis BW, Blot WJ. Blood vitamin D levels in relation to genetic estimation of African ancestry. Cancer Epidemiol Biomarkers Prev 2010;19:2325–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yao S, Zirpoli G, Bovbjerg DH, Jandorf L, Hong CC, Zhao H, Sucheston LE, Tang L, Roberts M, Ciupak G, et al. Variants in the vitamin D pathway, serum levels of vitamin D, and estrogen receptor negative breast cancer among African-American women: a case-control study. Breast Cancer Res 2012;14:R58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu Q, Shepherd L, Lunetta KL, Yao S, Liu Q, Hu Q, Haddad SA, Sucheston-Campbell L, Bensen JT, Bandera EV, et al. Trans-ethnic follow-up of breast cancer GWAS hits using the preferential linkage disequilibrium approach. Oncotarget 2016;7:83160–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feng Y, Stram DO, Rhie SK, Millikan RC, Ambrosone CB, John EM, Bernstein L, Zheng W, Olshan AF, Hu JJ, et al. A comprehensive examination of breast cancer risk loci in African American women. Hum Mol Genet 2014;23:5518–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huo D, Zheng Y, Ogundiran TO, Adebamowo C, Nathanson KL, Domchek SM, Rebbeck TR, Simon MS, John EM, Hennis A, et al. Evaluation of 19 susceptibility loci of breast cancer in women of African ancestry. Carcinogenesis 2012;33:835–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Long J, Zhang B, Signorello LB, Cai Q, Deming-Halverson S, Shrubsole MJ, Sanderson M, Dennis J, Michailidou K, Easton DF, et al. Evaluating genome-wide association study-identified breast cancer risk variants in African-American women. PLoS One 2013;8:e58350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Key FM, Fu Q, Romagne F, Lachmann M, Andres AM. Human adaptation and population differentiation in the light of ancient genomes. Nat Commun 2016;7:10775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yuen AW, Jablonski NG. Vitamin D: in the evolution of human skin colour. Med Hypotheses 2010;74:39–44. [DOI] [PubMed] [Google Scholar]
- 42.Clemens TL, Adams JS, Henderson SL, Holick MF. Increased skin pigment reduces the capacity of skin to synthesise vitamin D3. Lancet 1982;1:74–6. [DOI] [PubMed] [Google Scholar]
- 43.Smith GD, Lawlor DA, Timpson NJ, Baban J, Kiessling M, Day IN, Ebrahim S. Lactase persistence-related genetic variant: population substructure and health outcomes. Eur J Hum Genet 2009;17:357–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ranciaro A, Campbell MC, Hirbo JB, Ko WY, Froment A, Anagnostou P, Kotze MJ, Ibrahim M, Nyambo T, Omar SA, et al. Genetic origins of lactase persistence and the spread of pastoralism in Africa. Am J Hum Genet 2014;94:496–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schlebusch CM, Sjodin P, Skoglund P, Jakobsson M. Stronger signal of recent selection for lactase persistence in Maasai than in Europeans. Eur J Hum Genet 2013;21:550–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Travis RC, Appleby PN, Siddiq A, Allen NE, Kaaks R, Canzian F, Feller S, Tjonneland A, Fons Johnsen N, Overvad K, et al. Genetic variation in the lactase gene, dairy product intake and risk for prostate cancer in the European Prospective Investigation into Cancer and Nutrition. Int J Cancer 2013;132:1901–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koek WN, van Meurs JB, van der Eerden BC, Rivadeneira F, Zillikens MC, Hofman A, Obermayer-Pietsch B, Lips P, Pols HA, Uitterlinden AG, et al. The T-13910C polymorphism in the lactase phlorizin hydrolase gene is associated with differences in serum calcium levels and calcium intake. J Bone Miner Res 2010;25:1980–7. [DOI] [PubMed] [Google Scholar]