Abstract
Background: Early menopause, defined as the cessation of ovarian function before the age of 45 y, affects ∼10% of women and is associated with higher risk of cardiovascular disease, osteoporosis, and other conditions. Few modifiable risk factors for early menopause have been identified, but emerging data suggest that high vitamin D intake may reduce risk.
Objective: We evaluated how intakes of vitamin D and calcium are associated with the incidence of early menopause in the prospective Nurses’ Health Study II (NHS2).
Design: Intakes of vitamin D and calcium from foods and supplements were measured every 4 y with the use of a food-frequency questionnaire. Cases of incident early menopause were identified from all participants who were premenopausal at baseline in 1991; over 1.13 million person-years, 2041 women reported having natural menopause before the age of 45 y. We used Cox proportional hazards regression to evaluate relations between intakes of vitamin D and calcium and incident early menopause while accounting for potential confounding factors.
Results: After adjustment for age, smoking, and other factors, women with the highest intake of dietary vitamin D (quintile median: 528 IU/d) had a significant 17% lower risk of early menopause than women with the lowest intake [quintile median: 148 IU/d; HR: 0.83 (95% CI: 0.72, 0.95); P-trend = 0.03]. Dietary calcium intake in the highest quintile (median: 1246 mg/d) compared with the lowest (median: 556 mg/d) was associated with a borderline significantly lower risk of early menopause (HR: 0.87; 95% CI: 0.76, 1.00; P-trend = 0.03). Associations were stronger for vitamin D and calcium from dairy sources than from nondairy dietary sources, whereas high supplement use was not associated with lower risk.
Conclusions: Findings suggest that high intakes of dietary vitamin D and calcium may be modestly associated with a lower risk of early menopause. Further studies evaluating 25-hydroxyvitamin D concentrations, other dairy constituents, and early menopause are warranted.
Keywords: anovulation, calcium, dairy, menopause, menstrual cycle, ovarian function, vitamin D, 25-hydroxyvitamin D
INTRODUCTION
Early menopause, defined as the cessation of ovarian function before the age of 45 y, affects ∼10% of women in Western populations (1). Current research suggests that women who experience early menopause are at increased risk of premature mortality and cognitive decline, osteoporosis, and cardiovascular disease, among other adverse health outcomes (2–5). Women commonly experience an ebb in fertility during the 10 y leading up to natural menopause; for women who have early menopause, this may have substantial financial and psychological consequences for family planning, particularly as women increasingly delay childbearing into the later reproductive years (1, 6). Genetic factors do not fully account for the age at which menopause occurs, and emerging research suggests that modifiable lifestyle factors such as diet may play an important role in ovarian aging (7–14).
Calcium and vitamin D have been implicated in several gynecologic and reproductive conditions including polycystic ovary syndrome, endometriosis, and premenstrual syndrome and appear to play a role in fertility (15–20). Laboratory evidence suggests that the ovary is a target organ for 1,25-dihydroxyvitamin D3, the active metabolite of vitamin D3, and that vitamin D receptors are expressed in reproductive tissues including the ovary (21, 22). Recently, one group observed that plasma 25-hydroxyvitamin D [25(OH)D]9 concentrations were positively associated with ovarian reserve, providing further evidence of a protective role of vitamin D on ovarian aging (23).
Despite biological plausibility, to our knowledge, no epidemiologic studies have specifically investigated the relation of vitamin D and calcium with incident early menopause. One prospective study of total vitamin D and calcium intake and overall age at natural menopause among premenopausal women enrolled in the Nurses’ Health Study found that high intake of low-fat dairy foods, but not total calcium or total vitamin D intake, were associated with later menopause (8). Importantly, because the mean age at the beginning of follow-up for these women was 51.5 y, the relation of vitamin D and calcium intakes and risk of early menopause (<45 y old) remains unclear. In addition, this study did not distinguish calcium and vitamin D from dietary compared with supplemental sources, which has proven to be an important distinction in previous studies of reproductive-related conditions and other outcomes (18, 24, 25).
The aim of the present study was to examine the relation of intakes of vitamin D and calcium from supplemental, dietary, dairy, and nondairy dietary sources and subsequent risk of early menopause in the prospective Nurses’ Health Study II (NHS2). We hypothesized that among participants of the NHS2, total intakes of vitamin D and calcium would be inversely associated with early menopause. We similarly hypothesized that vitamin D and calcium intakes from supplements, foods, and dairy would each be inversely associated with early menopause.
METHODS
The NHS2 is a prospective study of 116,430 female US registered nurses who were 25–42 y old in 1989 when they responded to a mailed baseline questionnaire. Information regarding lifestyle behaviors and medical conditions are collected through biennial questionnaires for which the follow-up rate for each cycle has been ≥89%. The study protocol was approved by the Institutional Review Board at Brigham and Women’s Hospital in Boston, Massachusetts.
Assessment of early menopause
On the 1989 baseline questionnaire, nurses were asked if their periods had ceased permanently with the following response options: 1) no: premenopausal; 2) yes: no menstrual periods; 3) yes: had menopause but now have periods induced by hormones; and 4) not sure (e.g., started hormones before the cessation of periods). Nurses who indicated that their periods had ceased were asked the following questions: 1) At what age did your periods cease? (open response); and 2) For what reason did your periods cease? (response options were surgery; radiation or chemotherapy; and natural). In addition, women were asked about their current and past use of replacement sex hormones. These questions were repeated on all subsequent questionnaires. Age at natural menopause was defined as age after 12 consecutive months of amenorrhea that was not due to radiation, chemotherapy, or surgery. A small number of women reported being postmenopausal on one questionnaire and subsequently reported being premenopausal. For these women, we defined the age at menopause as the age after which periods were absent ≥12 mo and confirmed that this status persisted for ≥3 consecutive questionnaires.
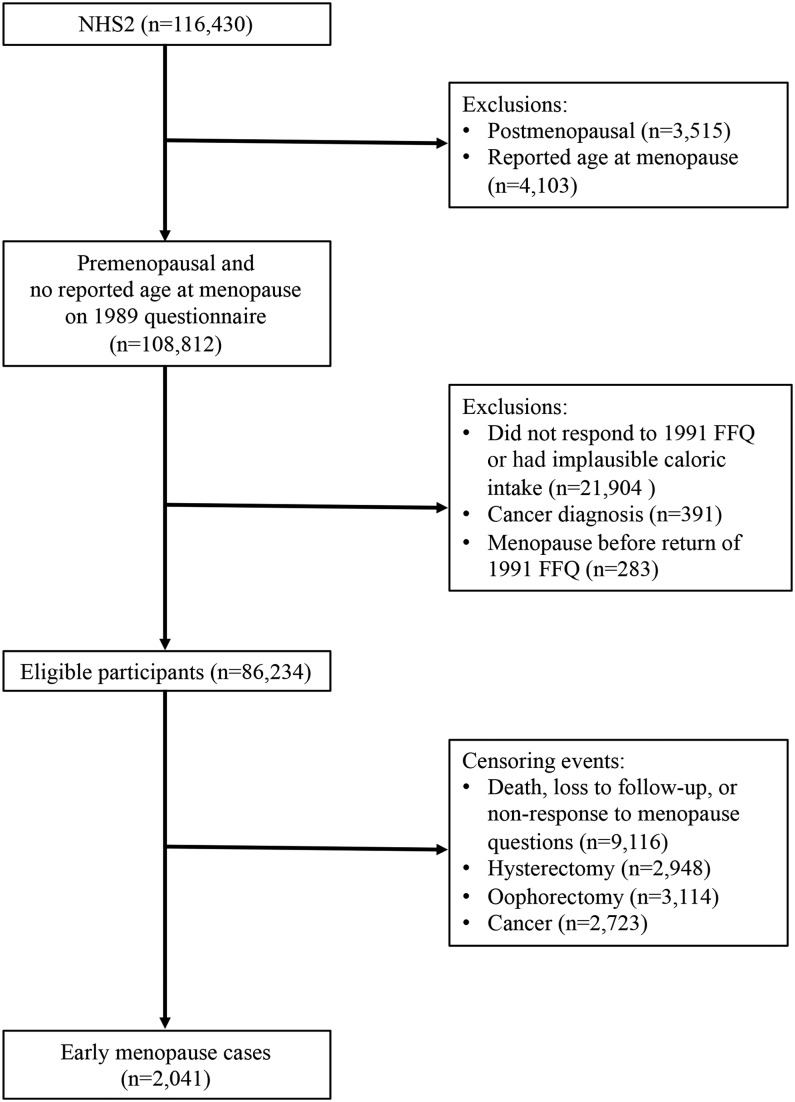
Because we were interested in prospectively evaluating the relation of intakes of vitamin D and calcium with incident early menopause, participants were eligible for inclusion in our study if they indicated that they were premenopausal and reported no age at menopause on the baseline 1989 questionnaire (n = 108,812). We further excluded women who did not respond to or who reported implausible caloric intake (<500 or ≥3500 kcal/d) on the 1991 food-frequency questionnaire (FFQ) (n = 21,904), were diagnosed with cancer before 1991 (n = 391), or whose date of menopause was before their return date of the 1991 FFQ (n = 283). After baseline exclusions, 86,234 women remained in the study sample (Figure 1).
FIGURE 1.
NHS2 participant flowchart: 1989–2011. FFQ, food-frequency questionnaire; NHS2, Nurses’ Health Study II.
Women were followed prospectively until 2011 for self-report of the cessation of menses, as previously defined, or first report of a hysterectomy, bilateral or unilateral oophorectomy, cancer (not including nonmelanoma skin cancer), loss to follow-up, or death. We identified cases of early menopause as women who reported having natural menopause before the age of 45 y.
Dietary assessment
Nurses completed validated semi-quantitative FFQs in 1991, 1995, 1999, 2003, 2007, and 2011, which assessed their average intakes of 131 foods, beverages, and supplements over the preceding year (26–28). Each questionnaire asked participants to estimate, on average, how often they consumed specific foods and beverages. Participants reported their consumption by indicating one of 9 frequency categories for each food and beverage (i.e., <1 serving/mo; 1–3 servings/mo; 1, 2–4, and 5–6 servings/wk; and 1, 2–3, 4–5, and ≥6 servings/d). Calcium intake from food sources was estimated by summing the calcium content per 1 serving of each food and beverage (e.g., skim, low-fat, and whole milk; yogurt; hard cheese; cottage cheese; and spinach) and multiplying it by the frequency of consumption. Intakes of vitamin D, vegetable protein, and alcohol were derived similarly. We calculated the percentage of total calories from vegetable protein by multiplying grams of vegetable protein by 4 kcal/g and dividing by total kilocalories.
Nurses were queried about the average use and dosages of multivitamins, calcium, and vitamin D supplements every 2 y on FFQs or biennial questionnaires, from which we estimated intakes of each nutrient from supplement sources. Total vitamin D and calcium intakes were estimated by summing dietary intakes and supplemental intakes of each nutrient. Intakes of all nutrients were adjusted for total energy by using the residual method (29).
The validity of the FFQ was assessed via comparison with 1-wk diet records in a random subset (n = 100) of women in the Nurses’ Health Study, a comparable population of female health professionals. The deattenuated Pearson correlation coefficient comparing the 2 methods for calcium intake was 0.75 (26).
Assessment of covariates
Information regarding age, height, ethnicity, maternal and paternal education level, and age at menarche was collected at baseline in 1989. Updated information on weight, parity, oral contraceptive use, breastfeeding, hormone therapy use, and smoking was collected biennially throughout follow-up. Baseline height and updated weight were used to calculate updated BMI (in kg/m2) as weight divided by the square of height for each questionnaire cycle. Physical activity was assessed in 1991, 1997, 2001, 2005, and 2009 with the use of the nurses’ responses to questions regarding the average time spent per week participating in specific activities (e.g., walking, running, and biking) from which we calculated metabolic equivalent task hours per week (30). For covariates with missing data, we assigned missing values to a missing indicator category. Results from analyses that were restricted to women with complete covariate data (n = 1956) were identical; thus, we present results from analyses using missing indicator categories to maximize statistical power.
Statistical analysis
We divided participants into quintiles of intakes of total (foods plus supplements), dietary (foods only), and dairy (dairy foods only) vitamin D and calcium according to the distribution of the NHS2 population. For supplemental vitamin D and calcium, we categorized participants according to their specified dosage (0, 1–599, and ≥600 IU vitamin D/d; 0, 1–399, 400–899, and ≥900 mg Ca/d) with nonusers serving as the referent group. We further dichotomized participants according to the adequacy of their total vitamin D and calcium intakes based on current Recommended Daily Allowances (i.e., 600 IU vitamin D/d; 1000 mg Ca/d) to aid in the interpretability of analyses. We assessed baseline characteristics of our study sample according to total calcium and vitamin D intakes using age-adjusted generalized linear models.
For our primary analyses, we used Cox proportional hazards regression to estimate age-adjusted and multivariable HRs for early menopause by vitamin D and calcium intakes. Tests for linear trend were conducted by modeling the median of each category as a continuous variable. For each participant, the accrual of follow-up (in months) began on the date of return of the 1991 questionnaire and continued until menopause, first report of hysterectomy, bilateral or unilateral oophorectomy, cancer (not including nonmelanoma skin cancer), loss to follow-up, or death, whichever occurred first. Analyses were stratified on age (in months) and questionnaire cycle.
For each nutrient, we modeled the timing of intake in 3 ways as follows: 1) baseline (1991) intake only; 2) simple updating of intake every 2–4 y; and 3) cumulative average intake. Cumulative average values for each exposure were calculated as mean intakes estimated from all FFQs up to and including the cycle before menopause. Results from these 3 methods were similar, and because the cumulative average method has been suggested to best represent long-term dietary intake by reducing misclassification due to within-person variation, we have presented only the results from cumulative average models (31).
In addition to analyses adjusted only for age, we fit a full multivariable model adjusting for variables including age, pack-years of smoking, BMI, parity, lifetime duration of breastfeeding, age at menarche, physical activity, percentage of total calories from vegetable protein, and alcohol intake [multivariable model 1 (MV1)]. Because none of the covariates we tested were associated with a >10% change in exposure HRs, covariate selection was based on factors identified a priori (i.e., age, smoking, BMI, parity, age at menarche, and physical activity) and factors associated with early menopause in our population (i.e., the duration of breastfeeding and intakes of alcohol and vegetable protein). To account for the high correlation of vitamin D and calcium intakes, we mutually adjusted vitamin D for calcium, and vice versa, and supplemental intake for dietary intake, and vice versa, in a second multivariable model.
To investigate the potential variation in associations by timing of exposure, we evaluated whether vitamin D and calcium intakes at age 35 compared with age 40 y were differently associated with early menopause by using logistic regression. Because not all participants completed questionnaires at exactly age 35 or 40 y, dietary exposures and covariates on the questionnaire completed closest in time before ages 35 and 40 y were used. These analyses were restricted to women for whom diet data were available at both ages 35 and 40 y and evaluated risk of early menopause from age 40 to <45 y (n = 534).
Because vitamin D is sequestered in adipose tissue (32), and BMI might plausibly behave as an effect modifier, we further tested the multiplicative interaction of BMI (3 categories) and dietary vitamin D (continuous quintile medians) on risk of early menopause by using cross-product terms. We also conducted a number of sensitivity analyses to evaluate the stability of the estimates. First, to ensure the adequacy of our control for confounding by smoking, we restricted our sample to never smokers (n for analysis = 1533 cases). Second, to evaluate the degree to which misclassification of early menopause may have occurred in our study, we conducted further analyses excluding noncases who experienced menopause before the age of 48 y. We were also concerned that conditions indicated for hysterectomy that are also related to vitamin D and calcium intakes may have selectively excluded noncases with low intakes of these nutrients. To evaluate this potential selection bias, we conducted a sensitivity analysis censoring at date of laparoscopy-confirmed endometriosis and ultrasound-confirmed uterine fibroid diagnosis (n for analysis = 1996 cases).
All statistical analyses were conducted with SAS v9.4 software (SAS Institute Inc.). We used 2-sided statistical tests performed at the 0.05 significance level for all analyses.
RESULTS
During 1.13 million person-years of follow-up, 2041 women experienced incident early menopause. Baseline age-adjusted descriptive statistics according to quintile of total vitamin D and calcium are presented in Table 1. On average, women who had the highest intakes of calcium and vitamin D were younger, were more physically active, had lower BMI, drank less alcohol, and were less likely to be current smokers than women who had the lowest intakes.
TABLE 1.
Age-adjusted characteristics of premenopausal women according to category of total vitamin D and calcium intakes (intakes from foods and supplements combined) at baseline: NHS2, 19911
| Total vitamin D, quintiles |
Total calcium, quintiles |
|||||||||
| Characteristic | 1 (n = 17,000) | 2 (n = 17,069) | 3 (n = 17,438) | 4 (n = 17,421) | 5 (n = 17,306) | 1 (n = 17,024) | 2 (n = 17,333) | 3 (n = 17,440) | 4 (n = 17,476) | 5 (n = 16,961) |
| Calcium intake,2 mg/d | 684 ± 2.7 | 842 ± 2.7 | 1024 ± 2.7 | 1156 ± 2.7 | 1364 ± 2.7 | 570 | 753 | 923 | 1163 | 1560 |
| Vitamin D intake,3 IU/d | 128 | 217 | 317 | 473 | 742 | 214 ± 1.7 | 289 ± 1.7 | 370 ± 1.7 | 459 ± 1.7 | 617 ± 1.7 |
| Age,4 y | 36.1 ± 4.6 | 36.1 ± 4.6 | 35.9 ± 4.6 | 35.5 ± 4.6 | 35.3 ± 4.5 | 36.2 ± 4.6 | 35.9 ± 4.6 | 35.7 ± 4.6 | 35.5 ± 4.6 | 35.6 ± 4.7 |
| BMI, kg/m2 | 24.8 ± 0.04 | 24.6 ± 0.04 | 24.5 ± 0.04 | 24.4 ± 0.04 | 24.3 ± 0.04 | 24.6 ± 0.04 | 24.6 ± 0.04 | 24.6 ± 0.04 | 24.4 ± 0.04 | 24.4 ± 0.04 |
| Age at menarche, y | 12.4 ± 0.01 | 12.4 ± 0.01 | 12.4 ± 0.01 | 12.4 ± 0.01 | 12.4 ± 0.01 | 12.4 ± 0.01 | 12.4 ± 0.01 | 12.4 ± 0.01 | 12.4 ± 0.01 | 12.4 ± 0.01 |
| Full-term pregnancies, n | 1.6 ± 0.01 | 1.6 ± 0.01 | 1.6 ± 0.01 | 1.6 ± 0.01 | 1.5 ± 0.01 | 1.5 ± 0.01 | 1.6 ± 0.01 | 1.5 ± 0.01 | 1.6 ± 0.01 | 1.5 ± 0.01 |
| Physical activity, MET-h/wk | 20.6 ± 0.5 | 23.1 ± 0.5 | 24.9 ± 0.5 | 25.0 ± 0.5 | 27.5 ± 0.5 | 21.3 ± 0.5 | 23.2 ± 0.5 | 24.8 ± 0.5 | 24.9 ± 0.5 | 26.9 ± 0.5 |
| Vegetable protein intake, % total kcal | 5.0 ± 0.01 | 5.1 ± 0.01 | 5.0 ± 0.01 | 5.0 ± 0.01 | 5.0 ± 0.01 | 4.8 ± 0.01 | 5.1 ± 0.01 | 5.2 ± 0.01 | 5.0 ± 0.01 | 4.9 ± 0.01 |
| Alcohol intake, g/d | 3.5 ± 0.05 | 3.3 ± 0.05 | 3.2 ± 0.05 | 3.1 ± 0.05 | 2.5 ± 0.05 | 3.5 ± 0.05 | 3.6 ± 0.05 | 3.3 ± 0.05 | 2.9 ± 0.05 | 2.5 ± 0.05 |
| Ever used OCs, % | 84.8 | 84.7 | 84.3 | 82.9 | 83.4 | 84.2 | 84.6 | 84.0 | 83.4 | 83.8 |
| Current smoker, % | 16.4 | 12.9 | 10.9 | 10.0 | 9.1 | 17.1 | 12.9 | 10.9 | 9.7 | 8.7 |
Values are means ± SEs unless otherwise indicated. All characteristics were calculated by using generalized linear models adjusted, unless otherwise specified, for the age of participants in 1991. MET-h, metabolic equivalent task hours; NHS2, Nurses’ Health Study II; OC, oral contraceptive.
Values are means ± SEs of total calcium intake per quintile of total vitamin D and quintile medians for total calcium.
Values are means ± SEs of total vitamin D intake per quintile of total calcium and quintile medians for total vitamin D.
All values are means ± SDs. Values are not age adjusted.
Results from analyses of vitamin D intake are presented in Table 2. In age-adjusted analyses, total vitamin D intake was not associated with early menopause (HR: 0.95; 95% CI: 0.83, 1.09; P-trend = 0.80). Supplemental vitamin D intake ≥600 IU/d was also not associated with risk (HR: 1.30; 95% CI: 0.94, 1.78; P-trend = 0.23), whereas intakes of vitamin D from dietary and dairy sources were associated with lower risk of early menopause [HR: 0.79 (95% CI: 0.69, 0.91; P-trend <0.01) and 0.82 (95% CI: 0.71, 0.94; P-trend = 0.02), respectively]. For reference, this level of vitamin D intake from dairy sources corresponds to ∼2.5 servings (8 oz/serving) of vitamin D–fortified milk/d. Intake of nondairy dietary vitamin D was not associated with risk of early menopause (quintile 5 compared with quintile 1: HR: 0.94; 95% CI: 0.81, 1.07; P-trend = 0.32).
TABLE 2.
HRs (95% CIs) for early menopause by cumulatively averaged vitamin D intake: NHS2 (1991–2011)1
| HR (95% CI) |
||||
| Vitamin D | Median, IU/d | Cases, n | Age-adjusted | Multivariable 12 |
| Total, quintile | ||||
| 1 | 145 | 438 | 1 | 1 |
| 2 | 241 | 392 | 0.86 (0.75, 0.99) | 0.89 (0.78, 1.02) |
| 3 | 341 | 419 | 0.90 (0.79, 1.03) | 0.95 (0.83, 1.08) |
| 4 | 474 | 399 | 0.89 (0.77, 1.02) | 0.94 (0.82, 1.07) |
| 5 | 695 | 393 | 0.95 (0.83, 1.09) | 0.99 (0.86, 1.13) |
| P-trend | — | — | 0.80 | 0.76 |
| RDA, IU/d | ||||
| <600 | 301 | 1711 | 1 | 1 |
| ≥600 | 721 | 330 | 1.02 (0.90, 1.15) | 1.02 (0.91, 1.15) |
| Dietary, quintile | ||||
| 1 | 148 | 441 | 1 | 1 |
| 2 | 232 | 424 | 0.94 (0.82, 1.07) | 0.97 (0.85, 1.11) |
| 3 | 301 | 375 | 0.81 (0.71, 0.93) | 0.85 (0.74, 0.98) |
| 4 | 383 | 441 | 0.96 (0.84, 1.09) | 1.01 (0.88, 1.15) |
| 5 | 528 | 360 | 0.79 (0.69, 0.91) | 0.83 (0.72, 0.95) |
| P-trend | — | — | <0.01 | 0.03 |
| From dairy sources | ||||
| 1 | 25 | 436 | 1 | 1 |
| 2 | 62 | 403 | 0.88 (0.77, 1.01) | 0.90 (0.79, 1.03) |
| 3 | 101 | 399 | 0.86 (0.75, 0.99) | 0.90 (0.78, 1.03) |
| 4 | 153 | 419 | 0.88 (0.77, 1.01) | 0.92 (0.80, 1.05) |
| 5 | 254 | 384 | 0.82 (0.71, 0.94) | 0.85 (0.74, 0.98) |
| P-trend | — | — | 0.02 | 0.06 |
| Nondairy dietary, quintile | ||||
| 1 | 46 | 436 | 1 | 1 |
| 2 | 75 | 421 | 0.96 (0.84, 1.10) | 1.02 (0.89, 1.16) |
| 3 | 101 | 401 | 0.93 (0.81, 1.07) | 0.99 (0.86, 1.14) |
| 4 | 135 | 397 | 0.93 (0.81, 1.06) | 0.98 (0.86, 1.13) |
| 5 | 196 | 386 | 0.94 (0.81, 1.07) | 0.97 (0.84, 1.12) |
| P-trend | — | — | 0.32 | 0.55 |
| Supplemental, IU/d | ||||
| 0 | 0 | 899 | 1 | 1 |
| 1–599 | 209 | 1102 | 1.01 (0.92, 1.11) | 1.04 (0.95, 1.15) |
| ≥600 | 800 | 40 | 1.30 (0.94, 1.78) | 1.29 (0.94, 1.77) |
| P-trend | — | — | 0.23 | 0.10 |
NHS2, Nurses’ Health Study II; RDA, Recommended Daily Allowance.
Multivariable Cox proportional hazards model adjusted for age, pack-years of smoking (0–10, 11–20, or ≥21), BMI (in kg/m2; <18.5, 18.5 to <25, 25 to <30, or ≥30), age at menarche (continuous), parity (nulliparous, 1–2, or ≥3), breastfeeding duration (months; continuous), physical activity (continuous metabolic equivalent task-hours per week), percentage of total calories from vegetable protein (quintiles 1–3 or 4 + 5), and alcohol intake (<10 or ≥10 g/d).
After further adjustment for risk factors for early menopause, estimates were similar to those from age-adjusted models. For example, in MV1, in which we controlled for age, BMI, smoking, and other risk factors, total vitamin D intake in the highest compared with the lowest quintile was not associated with risk of early menopause (quintile 5 compared with quintile 1: HR: 0.99; 95% CI: 0.86, 1.13; P-trend = 0.76). Women with dietary vitamin D intake in the highest compared with the lowest quintile were 17% (95% CI: 0.72, 0.95; P-trend = 0.03) less likely to experience early menopause. To evaluate whether this association may have been driven by intakes of these nutrients from dairy foods, we ran MV1 separately, evaluating dairy compared with nondairy dietary vitamin D. The HR comparing the highest and the lowest quintiles of dairy vitamin D intake was 0.85 (95% CI: 0.74, 0.98; P-trend = 0.06), whereas nondairy dietary vitamin D was not associated with risk (quintile 5 compared with quintile 1: HR: 0.97; 95% CI: 0.84, 1.12; P-trend = 0.55). Vitamin D supplement use was also not associated with increased risk of early menopause (P-trend = 0.10).
Results from analyses of calcium intake and early menopause are presented in Table 3. Age-adjusted and MV1 estimates for total, dietary, and dairy calcium intakes were similar to those for vitamin D. For example, in MV1, dietary calcium intake in the highest quintile was associated with a borderline significant 13% lower risk of early menopause (95% CI: 0.76, 1.00; P-trend = 0.03) than in the lowest quintile. High calcium intake from dairy sources was also associated with a borderline significantly lower risk (quintile 5 compared with quintile 1: HR: 0.87, 95% CI: 0.75, 1.00; P-trend = 0.03), whereas nondairy dietary calcium intake was not associated with risk of early menopause (quintile 5 compared with quintile 1: HR: 1.01, 95% CI: 0.85, 1.20; P-trend = 0.99). Calcium supplement intake was positively associated with risk of early menopause (P-trend < 0.01).
TABLE 3.
HRs (95% CIs) for early menopause by cumulatively averaged calcium intake: NHS2 (1991–2011)1
| HR (95% CI) |
||||
| Median, mg/d | Cases, n | Age-adjusted | Multivariable 12 | |
| Calcium | ||||
| Total, quintile | ||||
| 1 | 609 | 419 | 1 | 1 |
| 2 | 802 | 477 | 1.09 (0.95, 1.24) | 1.16 (1.01, 1.32) |
| 3 | 982 | 421 | 0.96 (0.84, 1.10) | 1.04 (0.90, 1.19) |
| 4 | 1205 | 337 | 0.79 (0.68, 0.91) | 0.86 (0.74, 0.99) |
| 5 | 1566 | 387 | 1.01 (0.88, 1.15) | 1.09 (0.94, 1.25) |
| P-trend | — | — | 0.11 | 0.60 |
| RDA, mg/d | ||||
| <1000 | 771 | 1177 | 1 | 1 |
| ≥1000 | 1270 | 864 | 0.89 (0.82, 0.97) | 0.93 (0.85, 1.01) |
| Dietary, quintile | ||||
| 1 | 556 | 426 | 1 | 1 |
| 2 | 705 | 414 | 0.91 (0.79, 1.04) | 0.96 (0.84, 1.10) |
| 3 | 832 | 437 | 0.95 (0.83, 1.09) | 1.02 (0.89, 1.17) |
| 4 | 987 | 390 | 0.83 (0.73, 0.96) | 0.90 (0.78, 1.03) |
| 5 | 1246 | 374 | 0.81 (0.70, 0.93) | 0.87 (0.76, 1.00) |
| P-trend | — | — | <0.01 | 0.03 |
| From dairy sources, quintile | ||||
| 1 | 246 | 426 | 1 | 1 |
| 2 | 382 | 410 | 0.90 (0.79, 1.03) | 0.94 (0.82, 1.08) |
| 3 | 503 | 426 | 0.93 (0.81, 1.06) | 0.97 (0.85, 1.11) |
| 4 | 657 | 397 | 0.85 (0.74, 0.97) | 0.90 (0.78, 1.03) |
| 5 | 926 | 382 | 0.83 (0.72, 0.95) | 0.87 (0.75, 1.00) |
| P-trend | — | — | <0.01 | 0.03 |
| Nondairy dietary, quintile | ||||
| 1 | 235 | 415 | 1 | 1 |
| 2 | 280 | 430 | 1.02 (0.89, 1.17) | 1.11 (0.96, 1.27) |
| 3 | 312 | 398 | 0.92 (0.80, 1.06) | 1.03 (0.89, 1.20) |
| 4 | 347 | 421 | 0.98 (0.85, 1.12) | 1.12 (0.96, 1.31) |
| 5 | 410 | 377 | 0.89 (0.77, 1.02) | 1.01 (0.85, 1.20) |
| P-trend | — | — | 0.06 | 0.99 |
| Supplemental, mg/d | ||||
| 0 | 0 | 1019 | 1 | 1 |
| 1–399 | 139 | 714 | 0.98 (0.88, 1.08) | 1.01 (0.91, 1.12) |
| 400–899 | 512 | 252 | 1.31 (1.14, 1.50) | 1.36 (1.18, 1.56) |
| ≥900 | 1015 | 56 | 0.99 (0.75, 1.29) | 1.03 (0.78, 1.35) |
| P-trend | — | — | 0.02 | <0.01 |
| Vitamin D or calcium supplement use | ||||
| Nonuser | NA | 772 | 1 | 1 |
| Vitamin D only | NA | 247 | 0.99 (0.86, 1.14) | 1.01 (0.87, 1.17) |
| Calcium only | NA | 127 | 1.06 (0.88, 1.28) | 1.09 (0.90, 1.32) |
| Calcium and vitamin D | NA | 895 | 1.04 (0.94, 1.16) | 1.09 (0.98, 1.21) |
NA, not applicable; NHS2, Nurses’ Health Study II; RDA, Recommended Daily Allowance.
Multivariable Cox proportional hazards model was adjusted for age, pack-years of smoking (0–10, 11–20, or ≥21), BMI (in kg/m2; <18.5, 18.5 to <25, 25 to <30, or ≥30), age at menarche (continuous), parity (nulliparous, 1–2, or ≥3), breastfeeding duration (months; continuous), physical activity (continuous metabolic equivalent task-hours per week), percentage of total calories from vegetable protein (quintiles 1–3 or 4 + 5), and alcohol intake (<10 or ≥10 g/d).
In an attempt to disentangle the individual associations of vitamin D and calcium with risk of early menopause, we mutually adjusted for corresponding sources of vitamin D and calcium in a second multivariable model. After adjustment, estimates were similar, while 95% CIs were wider and no longer significant (complete results not shown). For example, in this model, calcium intake from dairy foods in quintile 5 compared with quintile 1 was associated with a nonsignificant 12% decreased risk of early menopause (HR: 0.88; 95% CI: 0.67, 1.14; P-trend = 0.29). The association for vitamin D intake from dairy foods was also attenuated (quintile 5 compared with quintile 1: HR: 0.96; 95% CI: 0.74, 1.25; P-trend = 0.94).
Results from MV1 logistic regression models assessing intakes of vitamin D and calcium from dietary and supplemental sources at ages 35 and 40 y are presented in Table 4. At age 35 y, intake of ≥600 IU supplemental vitamin D/d compared with no supplement use was associated with higher risk (OR: 1.93; 95% CI: 1.15, 3.22), whereas calcium supplement use was not associated with risk of early menopause. Intakes of dietary vitamin D and calcium were both inversely associated with risk of early menopause. Similarly, at age 40 y, intakes of both dietary vitamin D and calcium were inversely associated with early menopause. Supplemental vitamin D intake was not associated with risk, whereas high intake of supplemental calcium was associated with increased risk (≥900 mg/d compared with nonusers: OR: 1.60; 95% CI: 1.19, 2.17; P-trend = 0.02).
TABLE 4.
Multivariable ORs (95% CIs) for early menopause according to vitamin D and calcium intakes assessed at ages 35 and 40 y: NHS2 (1991–2011)1
| Intake assessed at age 35 y |
Intake assessed at age 40 y |
|||||
| Median | Cases, n | OR (95% CI) | Median | Cases, n | OR (95% CI) | |
| Vitamin D, IU/d | ||||||
| Dietary, quintile | ||||||
| 1 | 107 | 114 | 1 | 76 | 119 | 1 |
| 2 | 170 | 122 | 1.04 (0.80, 1.35) | 137 | 96 | 0.76 (0.58, 1.00) |
| 3 | 226 | 89 | 0.71 (0.54, 0.95) | 190 | 107 | 0.89 (0.68, 1.17) |
| 4 | 295 | 103 | 0.81 (0.62, 1.07) | 254 | 108 | 0.86 (0.66, 1.13) |
| 5 | 403 | 106 | 0.80 (0.61, 1.05) | 363 | 104 | 0.82 (0.63, 1.08) |
| P-trend | — | — | 0.04 | — | — | 0.38 |
| Supplemental | ||||||
| 0 | 0 | 306 | 1 | 0 | 258 | 1 |
| 1–599 | 400 | 211 | 0.81 (0.68, 0.98) | 228 | 266 | 1.10 (0.92, 1.31) |
| ≥600 | 800 | 17 | 1.93 (1.15, 3.22) | 800 | 10 | 0.89 (0.47, 1.70) |
| P-trend | — | — | 0.33 | — | — | 0.67 |
| Calcium, mg/d | ||||||
| Dietary, quintile | ||||||
| 1 | 527 | 106 | 1 | 509 | 104 | 1 |
| 2 | 680 | 93 | 0.77 (0.57, 1.02) | 661 | 109 | 1.07 (0.81, 1.43) |
| 3 | 814 | 113 | 0.87 (0.66, 1.14) | 797 | 117 | 1.08 (0.82, 1.42) |
| 4 | 988 | 108 | 0.76 (0.58, 1.01) | 973 | 102 | 0.90 (0.68, 1.19) |
| 5 | 1284 | 114 | 0.77 (0.59, 1.01) | 1280 | 102 | 0.89 (0.67, 1.18) |
| P-trend | — | — | 0.12 | — | — | 0.17 |
| Supplemental | ||||||
| 0 | 0 | 353 | 1 | 0 | 286 | 1 |
| 1–399 | 162 | 117 | 0.89 (0.72, 1.10) | 162 | 131 | 1.08 (0.88, 1.34) |
| 400–899 | 500 | 44 | 1.07 (0.77, 1.48) | 500 | 62 | 0.93 (0.70, 1.23) |
| ≥900 | 1055 | 20 | 1.15 (0.72, 1.82) | 1000 | 55 | 1.60 (1.19, 2.17) |
| P-trend | — | — | 0.59 | — | — | 0.02 |
| Vitamin D or calcium supplement | ||||||
| Nonuser | NA | 282 | 1 | NA | 225 | 1 |
| User | NA | 252 | 0.88 (0.74, 1.05) | NA | 309 | 1.08 (0.90, 1.29) |
Analysis was limited to women with vitamin D and calcium intakes assessed at both ages 35 and 40 y; case ascertainment was limited to women with early menopause occuring after diet assessment at age 40 y. Multivariable Cox proportional hazards model was adjusted for age, pack-years of smoking (0–10, 11–20, or ≥21), BMI (in kg/m2; <18.5, 18.5 to <25, 25 to <30, or ≥30), age at menarche (continuous), parity (nulliparous, 1–2, or ≥3), breastfeeding duration (months; continuous), physical activity (continuous metabolic equivalent task-hours per week), percentage of total calories from vegetable protein (quintiles 1–3 or 4 + 5), and alcohol intake (<10 or ≥10 g/d). NA, not applicable; NHS2, Nurses’ Health Study II; RDA, Recommended Daily Allowance.
Results from analyses among never smokers were similar but slightly stronger in magnitude than in the main analyses (Supplemental Table 1). The likelihood ratio test comparing models with and without total vitamin D–BMI multiplicative interaction terms was NS (P-interaction = 0.22). BMI also did not modify the dietary vitamin D–early menopause relation (P-interaction = 0.41). Estimates from analyses excluding noncases with menopause occurring before age 48 y and analyses censoring at diagnosis of endometriosis and uterine fibroids were similar to estimates from the main analyses (data not shown). Tests of proportional hazards were not statistically significant, indicating that the proportional hazards assumption was met.
DISCUSSION
In this prospective study, we found high intakes of vitamin D and calcium from food sources to be modestly associated with lower risk of early menopause. Contrarily, supplemental vitamin D intake was not associated with risk of early menopause, and supplemental calcium intake was positively associated with early menopause.
The inverse associations for vitamin D and calcium from food sources appear to be driven largely by dairy sources of these nutrients, and mutual adjustment of vitamin D for calcium, and vice versa, attenuated the findings for each. Although we are aware of no previous studies that have assessed dairy vitamin D and calcium specifically, Carwile et al. (8) observed that intake of low-fat dairy but not high-fat dairy was associated with later age of menopause among women <51 y of age. A similar association was reported in an abstract from the Japan Nurses’ Health Study, which observed an inverse association of milk and dairy consumption on risk of early menopause; however, specific details about exposure and covariate measurements in this unpublished study are unavailable (14). In contrast, dairy consumption was not associated with menopausal timing in a study within the European Prospective Investigation into Cancer and Nutrition.
It is notable that Carwile et al. (8) similarly did not find total vitamin D or calcium intake to be related to age at menopause. However, their study did not separately evaluate dietary compared with supplemental sources of these nutrients, which may have attenuated associations for vitamin D from foods. In our study, high doses of supplemental calcium were associated with higher risk of early menopause. The observed higher risk among supplement users was unexpected, and we postulate that these women may have experienced conditions related to sex-steroid hormone concentrations before early menopause for which their doctors indicated a calcium supplement, such as autoimmune diseases or a family history of osteoporosis.
To further address this potential bias, we also conducted a post hoc analysis (n for analysis = 1757 cases) excluding women with conditions potentially related to both vitamin D and supplement use (e.g., lupus, multiple sclerosis, rheumatoid arthritis, low bone density, hip fracture, and osteoporosis). Estimates from these analyses were essentially identical to those from the main analyses. We also considered the possibility that women were taking multivitamins that contained supplemental vitamin D and calcium to treat perimenopause symptoms before early menopause. Nevertheless, after further controlling for multivitamin use, the positive association for supplemental calcium intake persisted.
The observed inverse association of dairy calcium and the borderline significant inverse association of dairy vitamin D specifically suggest that other constituents of dairy products may also influence menopause timing. Bovine milk is a rich source of steroid hormones, particularly of progesterone; for example, whole milk contains 10 μg progesterone/L (33). Milk consumption has also been shown to increase concentrations of plasma estradiol (34) and insulin-like growth factor I (35–37) in previous epidemiologic studies. Therefore, it is biologically plausible that these components, which are highly correlated with intakes of vitamin D and calcium in dairy, also contributed to the inverse associations observed. Thus, studies of dairy foods and dairy constituents, such as phosphorus, potassium, and vitamin B-12, and early menopause are warranted.
Because vitamin D and calcium are typically present together in specific foods, the attenuation of the estimates for each nutrient after mutual adjustment is likely due to the collinearity of vitamin D and calcium. In this population, the Pearson correlation coefficient for dairy vitamin D and calcium was 0.86 (P < 0.001). It is likely that vitamin D and calcium each had an individual contribution to the observed estimates, but separating out associations for each nutrient is statistically impossible because of near-perfect collinearity.
The observed inverse association for dietary vitamin D also may be due to a modest protective effect of vitamin D and calcium on ovarian aging. Several potential mechanisms supporting this hypothesis have been proposed, namely the ability of vitamin D to modify messenger-RNA expression of anti-Müllerian hormone, a glycoprotein secreted by granulosa cells during early follicle development that correlates with overall age at menopause and may play a role in regulating ovarian aging (38–42). Because the majority of vitamin D is obtained via cutaneous synthesis during solar UV-ray exposure, dietary and supplemental intakes of vitamin D represent a small overall contribution to circulating plasma 25(OH)D (43). To better understand how vitamin D status is related to early menopause, future biomarker studies of anti-Müllerian hormone, plasma 25(OH)D concentrations, and menopause timing are warranted.
Strengths of our study include a prospective design with 20 y of follow-up and a large sample size that allowed us to consider a variety of potential confounding variables. During this follow-up period, we were able to assess intakes of vitamin D and calcium 5 times and to use cumulative averages of exposures, which allowed us to capture within-person variation in diet and to reduce misclassification (31). Our study expanded on previous studies by assessing early menopause specifically rather than overall age at menopause as an outcome. Menopausal timing follows a normal distribution with a mean of 51 y of age (6). In a previous study of other factors and menopausal timing, associations of nutrients varied across age strata (8), supporting the use of the binary early menopause outcome rather than a continuous distribution of age at menopause with respect to evaluating risk factors.
Our study also has several limitations. First, because a biochemical confirmation of age at menopause was not feasible in a study of this size, we relied on self-reported menopausal status to determine timing of menopause. However, in a study of 6591 women in the comparable Nurses’ Health Study population, among women who were premenopausal in 1976 and reported having natural menopause on the 1978 questionnaire, 82% of women reported their age at menopause ≤1 y on the following 2 questionnaires (44). Any misclassification of early menopause would most likely have been biased toward null. Moreover, in a sensitivity analysis that restricted noncases to women with menopause after age 48 y, we found estimates to be identical, suggesting that our results are robust to misclassification of the outcome. Furthermore, although we cannot rule out the possibility of systematic overreporting or underreporting of total energy, alcohol, and nutrients including vitamin D, calcium, and vegetable protein, it is unlikely that reporting patterns for dietary variables would be related to case status. Misclassification across extreme categories is improbable and would not explain the observed inverse associations for intakes of dietary and dairy vitamin D and calcium.
Because the NHS2 is a fairly heterogeneous population with regard to dietary variables and other lifestyle factors related to early menopause, we anticipate that our results are generalizable to other populations of premenopausal women. However, because the NHS2 is racially homogenous, our ability to assess differences in the relation between vitamin D and calcium and early menopause across racial and ethnic groups is limited, and our findings should be replicated in more racially diverse populations. It is also important to note that in the NHS2, dietary vitamin D intake in the highest category is higher than what has been reported in other population-based studies (45) and, thus, may not reflect typical intake in the United States. Findings from our study suggest that vitamin D and calcium from food sources, particularly from dairy, are modestly associated with lower risk of early menopause. Further studies examining risk of early menopause with regard to dairy constituents and plasma vitamin D concentrations are warranted.
Acknowledgments
The authors’ responsibilities were as follows—JEM, SEH, BAR, KBM, and ERB-J: designed the research; ACP-S, BWW, KLS, MEB, and ERB-J: conducted the research; JEM, SEH, BAR, and KBM: provided essential materials; ACP-S, BWW, and ERB-J: performed the statistical analysis; ACP-S, BWW, JEM, and ERB-J: wrote the manuscript; LMT and KBM: interpreted the study results, reviewed the manuscript for important intellectual content, and contributed knowledge of the underlying biological mechanisms; ACP-S, JEM, and ERB-J: had primary responsibility for the final content of the manuscript; and all authors: read and approved the final manuscript. None of the authors reported a conflict of interest related to the study.
Footnotes
Abbreviations used: FFQ, food-frequency questionnaire; MV1, multivariable model 1; NHS2, Nurses’ Health Study II; 25(OH)D, 25-hydroxyvitamin D.
REFERENCES
- 1.Shuster LT, Rhodes DJ, Gostout BS, Grossardt BR, Rocca WA. Premature menopause or early menopause: long-term health consequences. Maturitas 2010;65:161–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wellons M, Ouyang P, Schreiner PJ, Herrington DM, Vaidya D. Early menopause predicts future coronary heart disease and stroke: the multi-ethnic study of atherosclerosis. Menopause 2012;19:1081–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Der Voort DJ, van Der Weijer PH, Barentsen R. Early menopause: increased fracture risk at older age. Osteoporos Int 2003;14:525–30. [DOI] [PubMed] [Google Scholar]
- 4.Fauser BC. Trilogy 8: premature ovarian failure and perimenopause. Female health implications of premature ovarian insufficiency. In: Tarlatzis BC, Bulun SE, editors. Proceedings of the International Federation of Fertility Societies 21st World Congress on Fertility and Sterility and the 69th Annual Meeting of the American Society for Reproductive Medicine. Boston (MA): American Society for Reproductive Medicine; 2013. p. 137–8. [Google Scholar]
- 5.Bleil ME, Gregorich SE, McConnell D, Rosen MP, Cedars MI. Does accelerated reproductive aging underlie premenopausal risk for cardiovascular disease? Menopause 2013;20:1139–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Broekmans FJ, Soules MR, Fauser BC. Ovarian aging: mechanisms and clinical consequences. Endocr Rev 2009;30:465–93. [DOI] [PubMed] [Google Scholar]
- 7.Simpson JL. Trilogy 8: premature ovarian failure and perimenopause. Genetics of premature ovarian failure. In: Tarlatzis BC, Bulun SE, editors. Proceedings of the International Federation of Fertility Societies 21st World Congress on Fertility and Sterility and the 69th Annual Meeting of the American Society for Reproductive Medicine. Boston (MA): American Society for Reproductive Medicine; 2013. p. 123–36. [Google Scholar]
- 8.Carwile JL, Willett WC, Michels KB. Consumption of low-fat dairy products may delay menopause. J Nutr 2013;143:1642–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bromberger JT, Matthews KA, Kuller LH, Wing RR, Meilahn EN, Plantinga P. Prospective study of the determinants of age at menopause. Am J Epidemiol 1997;145:124–33. [DOI] [PubMed] [Google Scholar]
- 10.Dorjgochoo T, Kallianpur A, Gao YT, Cai H, Yang G, Li H, Zheng W, Shu XO. Dietary and lifestyle predictors of age at natural menopause and reproductive span in the Shanghai Women’s Health Study. Menopause 2008;15:924–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagata C, Takatsuka N, Kawakami N, Shimizu H. Association of diet with the onset of menopause in Japanese women. Am J Epidemiol 2000;152:863–7. [DOI] [PubMed] [Google Scholar]
- 12.Nagel G, Altenburg HP, Nieters A, Boffetta P, Linseisen J. Reproductive and dietary determinants of the age at menopause in EPIC-Heidelberg. Maturitas 2005;52:337–47. [DOI] [PubMed] [Google Scholar]
- 13.Torgerson DJ, Thomas RE, Campbell MK, Reid DM. Alcohol consumption and age of maternal menopause are associated with menopause onset. Maturitas 1997;26:21–5. [DOI] [PubMed] [Google Scholar]
- 14.Yasui T. Endometriosis as a risk factor for early menopause. Climacteric 2008;2(Suppl 2):SM-32–02. [Google Scholar]
- 15.Yildizhan R, Kurdoglu M, Adali E, Kolusari A, Yildizhan B, Sahin HG, Kamaci M. Serum 25-hydroxyvitamin D concentrations in obese and non-obese women with polycystic ovary syndrome. Arch Gynecol Obstet 2009;280:559–63. [DOI] [PubMed] [Google Scholar]
- 16.Wehr E, Pilz S, Schweighofer N, Giuliani A, Kopera D, Pieber TR, Obermayer-Pietsch B. Association of hypovitaminosis D with metabolic disturbances in polycystic ovary syndrome. Eur J Endocrinol 2009;161:575–82. [DOI] [PubMed] [Google Scholar]
- 17.Li HW, Brereton RE, Anderson RA, Wallace AM, Ho CK. Vitamin D deficiency is common and associated with metabolic risk factors in patients with polycystic ovary syndrome. Metabolism 2011;60:1475–81. [DOI] [PubMed] [Google Scholar]
- 18.Harris HR, Chavarro JE, Malspeis S, Willett WC, Missmer SA. Dairy-food, calcium, magnesium, and vitamin D intake and endometriosis: a prospective cohort study. Am J Epidemiol 2013;177:420–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bertone-Johnson ER, Hankinson SE, Bendich A, Johnson SR, Willett WC, Manson JE. Calcium and vitamin D intake and risk of incident premenstrual syndrome. Arch Intern Med 2005;165:1246–52. [DOI] [PubMed] [Google Scholar]
- 20.Rojansky N, Brzezinski A, Schenker JG. Seasonality in human reproduction: an update. Hum Reprod 1992;7:735–45. [DOI] [PubMed] [Google Scholar]
- 21.Dokoh S, Donaldson CA, Marion SL, Pike JW, Haussler MR. The ovary: a target organ for 1,25-dihydroxyvitamin D3. Endocrinology 1983;112:200–6. [DOI] [PubMed] [Google Scholar]
- 22.Halloran BP, DeLuca HF. Effect of vitamin D deficiency on fertility and reproductive capacity in the female rat. J Nutr 1980;110:1573–80. [DOI] [PubMed] [Google Scholar]
- 23.Jukic AM, Steiner AZ, Baird DD. Association between serum 25-hydroxyvitamin D and ovarian reserve in premenopausal women. Menopause 2015;22:312–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anderson JJ, Kruszka B, Delaney J, He K, Burke GL, Alonso A, Bild DE, Budoff M, Michos ED. Calcium from diet and supplements and the risk of coronary artery calcification and its progression among older adults: 10-year follow-up of the Multi-Ethnic Study of Atherosclerosis (MESA). J Am Heart Assoc 2016;5:e003815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Curhan GC, Willett WC, Knight EL. Dietary factors and the risk of incident kidney stones in younger women: Nurses’ Health Study II. Arch Intern Med 2004;164:885–91. [DOI] [PubMed] [Google Scholar]
- 26.Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol 1985;122:51–65. [DOI] [PubMed] [Google Scholar]
- 27.Salvini S, Hunter DJ, Sampson L, Stampfer MJ, Colditz GA, Rosner B, Willett WC. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. Int J Epidemiol 1989;18:858–67. [DOI] [PubMed] [Google Scholar]
- 28.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol 1992;135:1114–26. [DOI] [PubMed] [Google Scholar]
- 29.Willett WC. Nutritional epidemiology. 2nd ed. New York: Oxford University Press; 1998. [Google Scholar]
- 30.Wolf AM, Hunter DJ, Colditz GA, Manson JE, Stampfer MJ, Corsano KA, Rosner B, Kriska A, Willett WC. Reproducibility and validity of a self-administered physical activity questionnaire. Int J Epidemiol 1994;23:991–9. [DOI] [PubMed] [Google Scholar]
- 31.Hu FB, Stampfer MJ, Rimm E, Ascherio A, Rosner BA, Spiegelman D, Willett WC. Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol 1999;149:531–40. [DOI] [PubMed] [Google Scholar]
- 32.Wortsman J, Matsuoka LY, Chen TC, Zhiren L, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr 2000;72:690–3. [DOI] [PubMed] [Google Scholar]
- 33.Hartmann S, Lacorn M, Steinhart H. Natural occurrence of steroid hormones in food. Food Chem 1998;62:7–20. [Google Scholar]
- 34.Brinkman MT, Baglietto L, Krishnan K, English DR, Severi G, Morris HA, Hopper JL, Giles GG. Consumption of animal products, their nutrient components and postmenopausal circulating steroid hormone concentrations. Eur J Clin Nutr 2010;64:176–83. [DOI] [PubMed] [Google Scholar]
- 35.Holmes MD, Pollack MN, Willett WC, Hankinson SE. Dietary correlates of plasma insulin-like growth factor I and insulin-like growth factor binding protein 3 concentrations. Cancer Epidemiol Biomarkers Prev 2002;11:852–61. [PubMed] [Google Scholar]
- 36.Ma J, Giovannucci E, Pollack M, Chan JM, Gaziano JM, Willett WC, Stampfer MJ. Milk intake, circulating levels of insulin-like growth factor-I, and risk of colorectal cancer in men. J Natl Cancer Inst 2001;93:1330–6. [DOI] [PubMed] [Google Scholar]
- 37.Giovannucci E, Pollack M, Liu Y, Platz EA, Majeed N, Rimm EB, Willett WC. Nutritional predictors of insulin-like growth factor I and their relationships to cancer in men. Cancer Epidemiol Biomarkers Prev 2003;12:84–9. [PubMed] [Google Scholar]
- 38.Wojtusik J, Johnson PA. Vitamin D regulates anti-Mullerian hormone expression in granulosa cells of the hen. Biol Reprod 2012;86:91. [DOI] [PubMed] [Google Scholar]
- 39.Durlinger AL, Kramer P, Karels B, de Jong FH, Ulenbroek JTH, Grootegoed JA, Themmen APN. Control of primordial follicle recruitment by anti-Müllerian hormone in the mouse ovary. Endocrinology 1999;140:5789–96. [DOI] [PubMed] [Google Scholar]
- 40.Visser JA, Schipper I, Laven JS, Themmen AP. Anti-Mullerian hormone: an ovarian reserve marker in primary ovarian insufficiency. Nat Rev Endocrinol 2012;8:331–41. [DOI] [PubMed] [Google Scholar]
- 41.Dennis NA, Houghton LA, Jones GT, van Rij AM, Morgan K, McLennan IS. The level of serum anti-Müllerian hormone correlates with vitamin D status in men and women but not in boys. J Clin Endocrinol Metab 2012;97:2450–5. [DOI] [PubMed] [Google Scholar]
- 42.Merhi ZO, Seifer DB, Weedon J, Adeyemi O, Holman S, Anastos K, Golub ET, Young M, Karim R, Greenblatt R, et al. Circulating vitamin D correlates with serum anti-Müllerian hormone levels in late-reproductive-aged women: women’s interagency HIV study. Fertil Steril 2012;98:228–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Holick MF. Vitamin D: a d-lightful solution for health. J Investig Med 2011;59:872–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Colditz GA, Stampfer MJ, Willett WC, Stason WB, Rosner B, Hennekens CH, Speizer FE. Reproducibility and validity of self-reported menopausal status in a prospective cohort study. Am J Epidemiol 1987;126:319–25. [DOI] [PubMed] [Google Scholar]
- 45.Bailey RL, Dodd KW, Goldman JA, Gahche JJ, Dwyer JT, Moshfegh AJ, Sempos CT, Picciano MF. Estimates of total usual calcium and vitamin D intakes in the United States. J Nutr 2010;140:817–22. [DOI] [PMC free article] [PubMed] [Google Scholar]