Abstract
Background: Increased omega-3 (n–3) fatty acid consumption is reported to benefit patients with metabolic syndrome, possibly due to improved adipose tissue function.
Objective: We tested the effects of high-dose, very-long-chain ω-3 fatty acids on adipose tissue inflammation and insulin regulation of lipolysis.
Design: A double-blind, placebo-controlled study compared 6 mo of 3.9 g eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA)/d (4.2 g total ω-3/d; n = 12) with a placebo (4.2 g oleate/d; n = 9) in insulin-resistant adults. Before and after treatment, the volunteers underwent adipose tissue biopsies to measure the total (CD68+), pro- (CD14+ = M1), and anti- (CD206+ = M2) inflammatory macrophages, crown-like structures, and senescent cells, as well as a 2-step pancreatic clamping with a [U-13C]palmitate infusion to determine the insulin concentration needed to suppress palmitate flux by 50% (IC50(palmitate)f).
Results: In the ω-3 group, the EPA and DHA contributions to plasma free fatty acids increased (P = 0.0003 and P = 0.003, respectively), as did the EPA and DHA content in adipose tissue (P < 0.0001 and P < 0.0001, respectively). Despite increases in adipose and plasma EPA and DHA in the ω-3 group, there were no significant changes in the IC50(palmitate)f (19 ± 2 compared with 24 ± 3 μIU/mL), adipose macrophages (total: 31 ± 2/100 adipocytes compared with 33 ± 2/100 adipocytes; CD14+: 13 ± 2/100 adipocytes compared with 14 ± 2/100 adipocytes; CD206+: 28 ± 2/100 adipocytes compared with 29 ± 3/100 adipocytes), crown-like structures (1 ± 0/10 images compared with 1 ± 0/10 images), or senescent cells (4% ± 1% compared with 4% ± 1%). There were no changes in these outcomes in the placebo group.
Conclusions: Six months of high-dose ω-3 supplementation raised plasma and adipose ω-3 fatty acid concentrations but had no beneficial effects on adipose tissue lipolysis or inflammation in insulin-resistant adults. This trial was registered at clinicaltrials.gov as NCT01686568.
Keywords: EPA, DHA, macrophage, lipolysis, inflammation, insulin resistance
INTRODUCTION
Adipose tissue has many functions that help maintain a healthy metabolic state, including its role to release free fatty acids (FFAs) for utilization by lean tissues. Insulin is a major regulator of this process; it inhibits FFA release into circulation, thereby enhancing insulin’s ability to promote glucose disposal (1). If adipose tissue has a diminished response to insulin, as is the case with insulin resistance and type 2 diabetes, excess FFAs are released, primarily from upper-body subcutaneous adipose tissue (2, 3). Elevated circulating FFA concentrations are associated with metabolic abnormalities, including muscle and liver insulin resistance (4–6) and impaired insulin secretion by pancreatic β cells (7, 8).
Adipose tissue also secretes cytokines. A majority of these are secreted from stromovascular cells, including senescent cells (9) and/or classically activated macrophages (10) that infiltrate adipose tissue. These proinflammatory cells accumulate with adipose tissue expansion (11, 12). Like FFAs, excess proinflammatory cytokines can inhibit insulin signaling, which can exaggerate the negative impact of excess FFAs on systemic metabolism.
By focusing therapeutic strategies on maintaining and promoting healthy adipose tissue function, the signs and symptoms of unhealthy metabolic function can theoretically be improved. Research has focused on changes in dietary fatty acids given the high potential for the achievable efficacy of such an intervention. Fatty acid composition of an individual’s diet is reflected in tissue lipid content (13, 14) and can thus modify adipose tissue function. Fish oils that contain the ω-3 fatty acids EPA and DHA have shown promise to prevent or improve insulin resistance in cell cultures (15) and animal models (16–19). Although data from human studies are conflicting (20–24), evidence suggests that ω-3 supplements reduce adipose macrophage infiltration in adults with metabolic syndrome (25).
Our objective was to test whether very-long-chain ω-3 fatty acids improve insulin-mediated suppression of adipose tissue lipolysis and the proinflammatory cellular composition of adipose in insulin-resistant adults with excess body fat. We hypothesized that 6 mo of ω-3 supplements would improve insulin-mediated suppression of adipose tissue lipolysis and reduce proinflammatory cells (i.e., macrophages, crown-like structures, and senescent cells) in adipose tissue compared with the placebo group.
METHODS
This protocol was an addendum to a Mayo Clinic Institutional Review Board–approved study. The primary results of this trial, which was conducted at Mayo Clinic in Rochester (MN) between January 2013 and October 2014, have been published by Lalia et al. (26).
Study design
This prospective, randomized, placebo-controlled, double-blind study evaluated the effects of 6 mo of ω-3 supplements on metabolic health in insulin-resistant (HOMA-IR: ≥2.6), overweight or obese [BMI (in kg/m2): ≥25.0] adults aged 18–65 y. Inclusion and exclusion criteria for recruitment have been published (26). Briefly, potential volunteers were excluded if they were taking medication or supplements or had disorders or diseases (e.g. diabetes) known to affect the outcome measures. Volunteers were weight stable and did not engage in ≥30 min of structured exercise >2 d/wk. Participants were instructed not to systematically alter dietary and physical activity habits throughout their participation in the study. After screening and baseline measurements, the participants were randomly assigned to groups based on a table prepared by a statistician. All study staff and laboratory staff remained blinded to the intervention until the completion of the trial and sample analyses. Those randomly assigned to the ω-3 group were provided with oral supplements containing 3.9 g EPA+DHA/d, with small amounts of α-linolenic, moroctic (stearidonic), eicosatetraenoic, heneicosapentaenoic, and docosapentaenoic acids. The total amount was 4.2 g ω-3 fatty acids/d. Participants randomly assigned to the placebo group were provided with 4.2 g oleic acid/d. Compliance to the intervention was determined by a pill count every 4 wk. Before and after the intervention participants underwent measurements of body composition and insulin regulation of adipose tissue lipolysis. They also had abdominal subcutaneous adipose tissue biopsies for measurement of fatty acid content, adipocyte size, and macrophage and senescent cell content. The trial ended when all participants had either completed their study visits or confirmed that they were unable to continue participation.
Body composition
Body composition was analyzed with DXA (Lunar DPX-L; Lunar Radiation). Abdominal fat areas were measured from the average of 3 images taken at 1-cm intervals centered on the umbilicus with a Signa 3.0 Tesla MRI scanner (GE Health care) and analyzed by a single, trained technician using Analyze Software System (Mayo Clinic Biomedical Imaging Resource). These analyses were used to estimate mass of lower- and upper-body subcutaneous and visceral fat (27). We also used an image analysis tool to measure the anterior-posterior and lateral diameters of the abdomen in ≥2 MRI images to allow us to calculate waist circumference using the formula for an ellipse (waist circumference was not measured as part of the entry criteria for this study).
Insulin regulation of adipose tissue lipolysis
One of our prespecified primary outcome measures was the sensitivity of adipose tissue lipolysis to insulin suppression, which was calculated as the insulin concentration needed to suppress palmitate appearance rates (i.e., flux) by 50% (IC50(palmitate)f) (28). Palmitate flux was measured in the overnight postabsorptive (i.e., basal) state and during a 2-step euglycemic, pancreatic clamp that was also used to measure insulin regulation of glucose metabolism (26). In brief, participants followed a weight-maintaining diet providing 20% protein, 50% carbohydrates, and 30% fat for 3 d. On the third day of the diet, they were admitted in the evening to the Clinical Research and Trials Unit and fasted overnight. For the pancreatic clamp we infused somatostatin (60 ng · kg−1 · min−1), glucagon (0.65 ng · kg−1 · min−1), and human growth hormone (3 ng · kg−1 · min−1). The insulin infusion rates were 0.6 and 2.3 mU · kg fat free mass−1 · min−1 for steps 1 and 2, respectively. Euglycemia was maintained at ∼5.0 mmol/L by infusing 40% dextrose. We infused [U-13C]palmitate (330 nmol/min) beginning 1 h before starting the clamp to measure basal palmitate flux and used a smaller amount (60 nmol/min) during the last hour of each of the two 3-h steps of the clamp to account for the anticipated suppression of lipolysis by insulin.
During the clamp, arterialized blood samples were taken from a catheter placed in retrograde fashion into a hand vein by using the heated-box technique (29). Blood samples were collected at 10-min intervals for the last 30 min of the basal state and each stage of the clamp for measurement of plasma insulin, glucose, and FFA concentrations. Plasma insulin and glucose (26), as well as FFA concentrations and palmitate enrichments (30), were measured as previously described.
Subcutaneous adipose tissue sampling and analysis
At least 1 wk after the pancreatic clamp study, the participants were again admitted to the Clinical Research and Trials Unit and provided a standardized meal before an overnight fast. The next morning an abdominal subcutaneous adipose tissue biopsy was collected under local anesthesia by using a sterile technique. The samples were analyzed for adipocyte size (31). Another one of our prespecified primary outcome measurements was the tissue burden of senescent cells, which we measured by staining for the percentage of positive cells with senescence-associated β-galactosidase activity (32). Our third, prespecified, primary outcome measurement was immunohistochemistry assessments of macrophage burden [total (CD68), M1 (CD14), and M2 (CD206) macrophages/100 adipocytes], as well as the number of crown-like structures/10 images (33).
Materials
[U-13C]palmitate was purchased from Isotec (SigmaAldrich).
Power and statistical analysis
We measured the combined biological and methodologic variability for adipose tissue content for total (CD68), M1 (CD14), and M2 (CD206) macrophages by using immunohistochemistry. Spencer et al. (25) found that adipose tissue macrophages (CD68) were reduced by ∼30% with ω-3 fatty acid supplements. Based on our known adipose macrophage assay reproducibility, our sample size provided >80% power to detect a 30% reduction in total adipose macrophages with a P < 0.05.
Insulin-mediated glucose disposal is 2–3 times greater in lean than in upper-body-obese adults, and the difference in IC50 for insulin-mediated suppression of lipolysis is of similar magnitude (34). Our goal was to detect smaller, yet clinically relevant, effects of ω-3 fatty acids on adipose tissue insulin resistance, such as those observed with a 10% weight loss due to a comprehensive lifestyle intervention. In a previous study that used this approach to help obese adults with insulin resistance, we found that insulin-suppressed fatty acid flux improved by 38% ± 46% and insulin-stimulated glucose disposal increased by 30% (35). Although not “normal,” we estimated that this degree of improvement would be clinically meaningful, in line with the observations of Magkos et al. (36). We calculated that with 12 participants in the ω-3 group and 9 in the placebo group we had >80% power to detect a 38% difference in IC50(palmitate)f with an α at 0.05.
The baseline categorical variables (e.g., sex and race/ethnicity) were tested for between-group differences by using Fisher’s exact test. Continuous variables (e.g., age and body composition parameters) with normal distribution were compared by using t tests, and nonnormally distributed data were analyzed with Wilcoxon’s rank-sums tests. By using the multivariate repeated-measures platform, 2-factor repeated-measures ANOVA tested for group differences in changes in body composition parameters across 6 mo of intervention. This analytic approach also tested whether the changes in adipose tissue outcome measures differed between groups. If data were not normally distributed, the data were log transformed. Post hoc analyses were conducted to test whether EPA and DHA concentrations in plasma and subcutaneous abdominal adipose tissue in response to intervention explained variation in outcome measurements of adipose tissue lipolysis insulin sensitivity and inflammatory markers postintervention. Analyses were run with JMP 10.0.0 (SAS Institute), and significance was set at P ≤ 0.05. Values are expressed as means ± SDs.
RESULTS
Subject characteristics
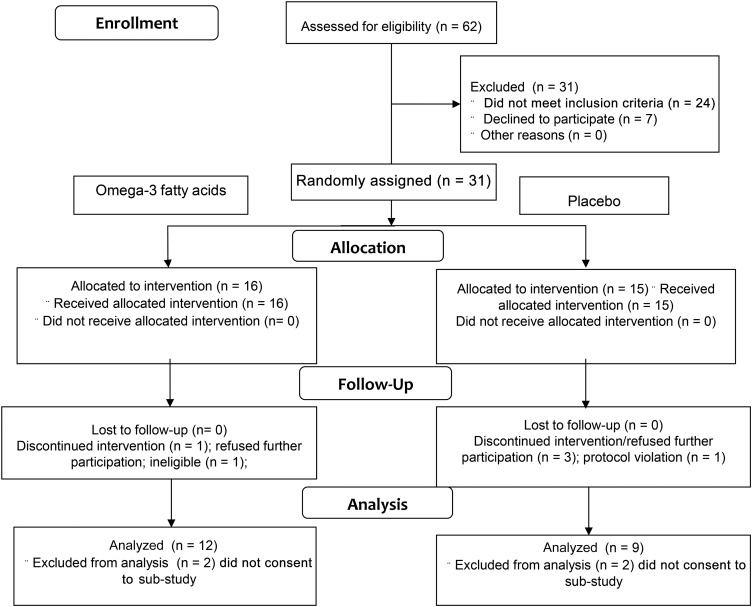
Twenty-seven of the 31 participants enrolled in the study and provided written, informed consent to undergo the lipolysis measures and adipose biopsies in addition to the methods of the main study (26). Six participants completed measurements at baseline but did not return for the second studies; therefore, their data were not included in this analysis. The 12 participants randomly assigned to the ω-3 group and 9 participants randomly assigned to the placebo group who completed the intervention and studies were included in this analysis. Figure 1 presents these details in a flow diagram. Table 1 presents subject characteristics. Six participants did not have a complete MRI scan at baseline and/or postintervention and are missing upper-body subcutaneous and visceral fat data. The 2 groups had generally similar characteristics at baseline. At baseline all participants had ≥1 and, on average, 2 metabolic syndrome criteria according to NIH guidelines for diagnosis of metabolic syndrome. The most prevalent risk factors were a large waist circumference (76%; 101 ± 7 cm) and hypertriglyceridemia (48%; 151 ± 68 mg/dL). We found that abdominal subcutaneous adipocyte size was greater in the placebo group than in the ω-3 group at baseline (P = 0.02, not adjusted for multiple post hoc comparisons of baseline characteristics). However, adipocyte size was not different between groups postintervention, and the change in size from baseline was also not different between groups (Table 1). Fat-free mass, upper-body subcutaneous fat mass, and visceral fat mass also did not differ across the intervention or between groups. However, BMI (+0.7; P = 0.03), percentage of body fat (0.9%; P = 0.009), and leg fat mass (0.5 kg; P = 0.02) increased for participants in both groups at the end of the intervention, but the changes were not different between groups (Table 1).
FIGURE 1.
Flow diagram of the study participants.
TABLE 1.
Subject characteristics1
| ω-3 (n = 12) |
Placebo (n = 9) |
P |
|||||
| Baseline | Postintervention | Baseline | Postintervention | Group | Time | Interaction | |
| Sex, M/F | 4/8 | — | 2/7 | — | — | — | — |
| Race/ethnicity, % Caucasian | 92 | — | 89 | — | — | — | — |
| Age, y | 36 ± 11 | — | 34 ± 9 | — | — | — | — |
| BMI, kg/m2 | 34.4 ± 3.6 | 35.2 ± 4.4 | 34.7 ± 5.0 | 35.4 ± 5.0 | 0.88 | 0.03 | 0.82 |
| Fat-free mass, kg | 57.4 ± 9.0 | 57.7 ± 8.9 | 55.0 ± 9.2 | 55.6 ± 9.1 | 0.55 | 0.33 | 0.70 |
| Body fat, % | 42.2 ± 7.5 | 43.2 ± 8.0 | 42.7 ± 7.0 | 43.5 ± 7.2 | 0.91 | 0.009 | 0.83 |
| Visceral fat, kg | 2.5 ± 0.92 | 3.1 ± 1.23 | 3.0 ± 1.44 | 2.8 ± 1.14 | 0.92 | 0.73 | 0.06 |
| Upper-body subcutaneous fat | |||||||
| Fat mass, kg | 25.7 ± 6.22 | 26.6 ± 7.63 | 25.3 ± 9.14 | 27.6 ± 7.54 | 0.75 | 0.16 | 0.81 |
| Adipocyte size, μg lipid/cell | 0.87 ± 0.25 | 1.00 ± 0.34 | 1.12 ± 0.18 | 1.14 ± 0.27 | 0.06 | 0.30 | 0.45 |
| Lower-body fat | |||||||
| Fat mass, kg | 12.8 ± 4.3 | 13.3 ± 4.9 | 14.4 ± 4.4 | 13.7 ± 4.7 | 0.83 | 0.02 | 0.90 |
Values are means ± SDs, unless otherwise noted, for subject characteristics for both intervention groups supplemented with ω-3 fatty acids or a placebo at baseline and postintervention, along with analysis results from multivariate models with repeated measures.
n = 9.
n = 8.
n = 7.
Plasma and adipose tissue fatty acid content
Fasting plasma total FFA concentrations did not change in either group (P = 0.42); however, the FFA profile changed in a manner consistent with the intervention. The EPA and DHA contributions to plasma FFAs increased dramatically in the ω-3 group and did not change in the placebo group (Table 2). The interaction effects in EPA (P = 0.0002) and DHA (P = 0.0004) reflected the more robust increases in these plasma FFAs in the ω-3 group compared with the minimal change in the placebo group. The percentage of adipose tissue FFAs as EPA (P < 0.0001) and DHA (P < 0.0001) likewise increased substantially in the ω-3 group but not in the placebo group (Table 2).
TABLE 2.
Plasma and adipose tissue responses to ω-3 fatty acid or placebo supplements1
| ω-3 (n = 12) |
Placebo (n = 9) |
P |
|||||
| Baseline | Postintervention | Baseline | Postintervention | Group | Time | Interaction | |
| Plasma, % of total FFAs | |||||||
| EPA | 0.95 ± 0.22 | 6.0 ± 0.92 | 1.2 ± 0.27 | 1.1 ± 0.19 | 0.002 | 0.0006 | 0.0002 |
| DHA | 0.89 ± 0.23 | 3.5 ± 0.84 | 1.2 ± 0.39 | 0.90 ± 0.18 | 0.12 | 0.002 | 0.0004 |
| Adipose tissue, % of total FFAs | |||||||
| EPA | 0.06 ± 0.00 | 0.19 ± 0.02 | 0.07 ± 0.01 | 0.07 ± 0.01 | 0.0002 | <0.0001 | <0.0001 |
| DHA | 0.14 ± 0.01 | 0.28 ± 0.02 | 0.15 ± 0.01 | 0.16 ± 0.02 | 0.03 | <0.0001 | <0.0001 |
| IC50(palmitate)f2 | 19 ± 2 | 24 ± 3 | 29 ± 43 | 27 ± 63 | 0.17 | 0.94 | 0.11 |
| Senescent cells, % | 4 ± 1 | 4 ± 1 | 4 ± 3 | 4 ± 2 | 0.84 | 0.65 | 0.68 |
| Macrophages, n/100 adipocytes | |||||||
| Total (CD68) | 31 ± 2 | 33 ± 2 | 33 ± 2 | 31 ± 2 | 0.97 | 0.74 | 0.25 |
| M1 (CD14) | 13 ± 2 | 14 ± 2 | 13 ± 1 | 12 ± 2 | 0.49 | 0.93 | 0.49 |
| M2 (CD206) | 28 ± 2 | 29 ± 3 | 29 ± 2 | 29 ± 2 | 0.84 | 0.78 | 0.87 |
| Crown-like structures (n/10 images) | 1 ± 0 | 1 ± 0 | 1 ± 1 | 1 ± 0 | 0.33 | 0.77 | 0.31 |
Values are means ± SEs for effects of intervention groups’ ω-3 fatty acid or placebo supplement on insulin regulation of lipolysis and inflammation markers in subcutaneous abdominal adipose tissue, along with analysis results from multivariate models with repeated measures. FFA, free fatty acid.
IC50(palmitate)f: the insulin concentration (μIU/mL) resulting in a 50% suppression of palmitate FFA flux is a measure of insulin-mediated suppression of adipose tissue lipolysis.
n = 8.
Adipose tissue lipolysis and inflammation
Despite the increases in tissue and plasma EPA and DHA content and despite robust statistical power to detect clinically meaningful changes in IC50(palmitate)f, the between-group differences in response to the 6-mo intervention were not different (Table 2). Furthermore, there were no improvements and no trends for improvements in adipose tissue markers of inflammation, including senescent cells; total, pro- or anti-inflammatory macrophages; and crown-like structures (Table 2).
DISCUSSION
Metabolic syndrome, specifically systemic insulin resistance, may be a consequence of adipose tissue abnormalities. Failure of insulin to normally inhibit adipose tissue lipolysis can result in elevated FFAs, a known contributor to insulin resistance (4, 37). It has been proposed that proinflammatory cytokines produced by senescent cells and macrophages infiltrating adipose tissue contribute to local and systemic inflammation (38). Therefore, interventions targeting adipose tissue inflammation, such as supplements of the ω-3 polyunsaturated fatty acids EPA and DHA (25, 39), could improve systemic metabolism. However, the insulin-sensitizing effects of ω-3 fatty acids are still in debate. As part of this double-blind, placebo-controlled, 6-mo intervention using maximal FDA-approved doses of EPA and DHA given to insulin-resistant adults, we studied the insulin regulation of adipose tissue lipolysis and adipose inflammation. We found significant increases in EPA and DHA in plasma and subcutaneous adipose tissue in the group supplemented with very-long-chain ω-3 fatty acids but no concurrent changes in adipose tissue insulin sensitivity or inflammation.
Increased EPA and DHA intake is well known to improve hypertriglyceridemia (25, 26, 40). However, other features of metabolic syndrome, such as insulin sensitivity, do not consistently improve with increased very-long-chain ω-3 intake (25, 41, 42). We previously reported that, although skeletal muscle insulin sensitivity and pancreatic β cell insulin secretion did not change, hepatic insulin sensitivity improved with ω-3 supplements (26). By measuring FFA kinetics before and during the pancreatic clamp, we also could determine insulin sensitivity of adipose tissue lipolysis. Our finding that in vivo regulation of adipose tissue lipolysis by insulin was unchanged helps put into perspective previous work showing no change in gene expression of lipolysis enzymes in adipose tissue after ω-3 supplementation (41). Together, these findings suggest that even an extended duration of high-dose, very-long-chain ω-3 fatty acids will not improve adipose tissue insulin sensitivity with regard to FFA metabolism.
In addition to FFAs, adipose tissue is the major tissue involved in release of adipokines that are linked to inflammation and insulin resistance. DHA and EPA are thought to induce anti-inflammatory actions by reducing proinflammatory cytokines in adipose tissue. Several investigators have reported decreased gene expression of proteins in the proinflammatory pathway (e.g., monocyte chemoattractant protein-1, IL-6) in cultured adipocytes (43) and animal models (44). In humans, however, neither expression of inflammation-related genes in subcutaneous adipose tissue (25, 41) nor circulating markers of inflammation (25, 41) consistently improve with ω-3 fatty acid treatment. However, Spencer et al. (25) found decreases in total macrophage (pro- and anti-inflammatory) and crown-like structure content in subcutaneous adipose tissue after EPA and DHA supplementation. The baseline abdominal adipose tissue EPA and DHA content we observed is consistent with those previously reported in femoral subcutaneous adipose tissue (45), and the changes in adipose EPA and DHA content were in line with those of Spencer et al. (25). Although we confirmed no changes in the circulating inflammatory markers leptin, adiponectin, C-reactive protein, or IL-6 (26), we could not replicate the improvements in adipose tissue inflammatory markers with a higher dose of DHA and EPA supplementation for a longer duration (Table 2). The conflicting results may be due to recruitment criteria; Spencer et al. (25) recruited participants with features of metabolic syndrome and found those with the greatest number of adipose tissue macrophages at baseline had the greatest decrease with treatment. Our participants were also insulin resistant and carried excess weight but may have had less marked metabolic abnormalities and were ∼10 y younger.
Our study is limited to the effects of very-long-chain ω-3 supplements on adipose tissue insulin resistance and abdominal subcutaneous adipose tissue inflammation. For practical reasons we did not measure inflammatory responses in visceral fat, a depot known to be inherently more immune-cell infiltrated than subcutaneous fat. Some investigators have reported positive effects of ω-3 fatty acid supplements on visceral fat (46, 47), and others have noted that greater self-reported ω-3 fatty acid intakes are negatively associated with visceral fat and percentage of body fat in healthy children (48). We cannot know whether there might have been a beneficial effect of ω-3 fatty acids on visceral fat inflammation in our participants, but if there was it did not improve inflammatory markers (26) or insulin sensitivity. We previously reported the results with regard to skeletal muscle and liver insulin sensitivity (26), but we did not measure inflammatory markers in these tissues. Proinflammatory macrophage infiltration and/or cytokine production in skeletal muscle and the liver could contribute to systemic insulin resistance and inflammation (49). Effects of ω-3 FFA supplementation may have varied effects on features of metabolic syndrome depending on disease severity. For example, adults with type 2 diabetes (41) and those with ≥3 features of metabolic syndrome (25) are reported to have improvements in adipose tissue health and function, whereas those with 1–2 components of metabolic syndrome, such as the participants in the current study, do not. To date, there is insufficient evidence from clinical trials to clearly define which stage in the progression of metabolic syndrome might benefit from ω-3 supplement therapies. However, we note that although 33% of US adults have ≥3 features of metabolic syndrome (50), this would indicate that an even larger portion of US adults have ≤2 metabolic syndrome features, and our findings more likely apply to this group.
In summary, high-dose ω-3 supplementation for 6 mo, sufficient to raise plasma and adipose tissue ω-3 FFAs, had no beneficial effects on insulin-mediated suppression of lipolysis or adipose tissue inflammation in insulin-resistant, overweight and obese adults. Our study does not support the hypothesis that very-long-chain ω-3 fatty acids can improve the features of metabolic syndrome via actions on regulation of adipose tissue insulin sensitivity and reducing inflammation, which is thought to be a potential underlying cause of this disorder.
Acknowledgments
We thank Christy Allred, Barbara Norby, and Carley Vrieze for technical assistance and help with data collection.
The authors’ responsibilities were as follows—IRL and MDJ: designed the research; IRL and KCH: conducted the research; KCH and MDJ: wrote the manuscript; MDJ: had primary responsibility for the final content; and all authors: analyzed the data or performed the statistical analysis, and read and approved the final manuscript. None of the authors reported a conflict of interest related to the study.
REFERENCES
- 1.Boden G. Role of fatty acids in the pathogenesis of insulin resistance and NIDDM. Diabetes 1997;46:3–10. [PubMed] [Google Scholar]
- 2.Basu A, Basu R, Shah P, Vella A, Rizza RA, Jensen MD. Systemic and regional free fatty acid metabolism in type 2 diabetes. Am J Physiol Endocrinol Metab 2001;280:E1000–6. [DOI] [PubMed] [Google Scholar]
- 3.Guo Z, Hensrud DD, Johnson CM, Jensen MD. Regional postprandial fatty acid metabolism in different obesity phenotypes. Diabetes 1999;48:1586–92. [DOI] [PubMed] [Google Scholar]
- 4.Boden G, Chen X, Ruiz J, White JV, Rossetti L. Mechanisms of fatty acid-induced inhibition of glucose uptake. J Clin Invest 1994;93:2438–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kelley DE, Mokan M, Simoneau JA, Mandarino LJ. Interaction between glucose and free fatty acid metabolism in human skeletal muscle. J Clin Invest 1993;92:91–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rebrin K, Steil GM, Getty L, Bergman RN. Free fatty acids as a link in the regulation of hepatic glucose output by peripheral insulin. Diabetes 1995;44:1038–45. [DOI] [PubMed] [Google Scholar]
- 7.Unger RH. Lipotoxicity in the pathogenesis of obesity-dependent NIDDM. Genetic and clinical implications. Diabetes 1995;44:863–70. [DOI] [PubMed] [Google Scholar]
- 8.Cusi K, Kashyap S, Gastaldelli A, Bajaj M, Cersosimo E. Effects on insulin secretion and insulin action of a 48-h reduction of plasma free fatty acids with acipimox in nondiabetic subjects genetically predisposed to type 2 diabetes. Am J Physiol Endocrinol Metab 2007;292:E1775–81. [DOI] [PubMed] [Google Scholar]
- 9.Tchkonia T, Zhu Y, van Deursen J, Campisi J, Kirkland JL. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J Clin Invest 2013;123:966–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hotamisligil GS. Inflammation and metabolic disorders. Nature 2006;444:860–7. [DOI] [PubMed] [Google Scholar]
- 11.Harman-Boehm I, Bluher M, Redel H, Sion-Vardy N, Ovadia S, Avinoach E, Shai I, Kloting N, Stumvoll M, Bashan N, et al. Macrophage infiltration into omental versus subcutaneous fat across different populations: effect of regional adiposity and the comorbidities of obesity. J Clin Endocrinol Metab 2007;92:2240–7. [DOI] [PubMed] [Google Scholar]
- 12.Blüher M. The distinction of metabolically ‘healthy’ from ‘unhealthy’ obese individuals. Curr Opin Lipidol 2010;21:38–43. [DOI] [PubMed] [Google Scholar]
- 13.Garland M, Sacks FM, Colditz GA, Rimm EB, Sampson LA, Willett WC, Hunter DJ. The relation between dietary intake and adipose tissue composition of selected fatty acids in US women. Am J Clin Nutr 1998;67:25–30. [DOI] [PubMed] [Google Scholar]
- 14.London SJ, Sacks FM, Caesar J, Stampfer MJ, Siguel E, Willett WC. Fatty acid composition of subcutaneous adipose tissue and diet in postmenopausal US women. Am J Clin Nutr 1991;54:340–5. [DOI] [PubMed] [Google Scholar]
- 15.Lee JS, Pinnamaneni SK, Eo SJ, Cho IH, Pyo JH, Kim CK, Sinclair AJ, Febbraio MA, Watt MJ. Saturated, but not n-6 polyunsaturated, fatty acids induce insulin resistance: role of intramuscular accumulation of lipid metabolites. J Appl Physiol 2006;100:1467–74. [DOI] [PubMed] [Google Scholar]
- 16.Lombardo YB, Hein G, Chicco A. Metabolic syndrome: effects of n-3 PUFAs on a model of dyslipidemia, insulin resistance and adiposity. Lipids 2007;42:427–37. [DOI] [PubMed] [Google Scholar]
- 17.Neschen S, Morino K, Dong J, Wang-Fischer Y, Cline GW, Romanelli AJ, Rossbacher JC, Moore IK, Regittnig W, Munoz DS, et al. n-3 Fatty acids preserve insulin sensitivity in vivo in a peroxisome proliferator-activated receptor-alpha-dependent manner. Diabetes 2007;56:1034–41. [DOI] [PubMed] [Google Scholar]
- 18.Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W, Li P, Lu WJ, Watkins SM, Olefsky JM. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 2010;142:687–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Storlien LH, Kraegen EW, Chisholm DJ, Ford GL, Bruce DG, Pascoe WS. Fish oil prevents insulin resistance induced by high-fat feeding in rats. Science 1987;237:885–8. [DOI] [PubMed] [Google Scholar]
- 20.Browning LM, Krebs JD, Moore CS, Mishra GD, O’Connell MA, Jebb SA. The impact of long chain n-3 polyunsaturated fatty acid supplementation on inflammation, insulin sensitivity and CVD risk in a group of overweight women with an inflammatory phenotype. Diabetes Obes Metab 2007;9:70–80. [DOI] [PubMed] [Google Scholar]
- 21.Giacco R, Cuomo V, Vessby B, Uusitupa M, Hermansen K, Meyer BJ, Riccardi G, Rivellese AA. Fish oil, insulin sensitivity, insulin secretion and glucose tolerance in healthy people: is there any effect of fish oil supplementation in relation to the type of background diet and habitual dietary intake of n-6 and n-3 fatty acids? Nutr Metab Cardiovasc Dis 2007;17:572–80. [DOI] [PubMed] [Google Scholar]
- 22.Haugaard SB, Madsbad S, Hoy CE, Vaag A. Dietary intervention increases n-3 long-chain polyunsaturated fatty acids in skeletal muscle membrane phospholipids of obese subjects. Implications for insulin sensitivity. Clin Endocrinol (Oxf) 2006;64:169–78. [DOI] [PubMed] [Google Scholar]
- 23.Itoh M, Suganami T, Satoh N, Tanimoto-Koyama K, Yuan X, Tanaka M, Kawano H, Yano T, Aoe S, Takeya M, et al. Increased adiponectin secretion by highly purified eicosapentaenoic acid in rodent models of obesity and human obese subjects. Arterioscler Thromb Vasc Biol 2007;27:1918–25. [DOI] [PubMed] [Google Scholar]
- 24.Krebs JD, Browning LM, McLean NK, Rothwell JL, Mishra GD, Moore CS, Jebb SA. Additive benefits of long-chain n-3 polyunsaturated fatty acids and weight-loss in the management of cardiovascular disease risk in overweight hyperinsulinaemic women. Int J Obes (Lond) 2006;30:1535–44. [DOI] [PubMed] [Google Scholar]
- 25.Spencer M, Finlin BS, Unal R, Zhu B, Morris AJ, Shipp LR, Lee J, Walton RG, Adu A, Erfani R, et al. Omega-3 fatty acids reduce adipose tissue macrophages in human subjects with insulin resistance. Diabetes 2013;62:1709–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lalia AZ, Johnson ML, Jensen MD, Hames KC, Port JD, Lanza IR. Effects of dietary n-3 fatty acids on hepatic and peripheral insulin sensitivity in insulin-resistant humans. Diabetes Care 2015;38:1228–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jensen MD, Kanaley JA, Reed JE, Sheedy PF. Measurement of abdominal and visceral fat with computed tomography and dual-energy x-ray absorptiometry. Am J Clin Nutr 1995;61:274–8. [DOI] [PubMed] [Google Scholar]
- 28.Triay J, Mundi M, Klein S, Toledo FGS, Smith SR, Abu-Lebdeh H, Jensen M. Does rimonabant independently affect free fatty acid and glucose metabolism? J Clin Endocrinol Metab 2012;97:819–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jensen MD, Heiling VJ. Heated hand vein blood is satisfactory for measurements during free fatty acid kinetic studies. Metabolism 1991;40:406–9. [DOI] [PubMed] [Google Scholar]
- 30.Persson X-MT, Blachnio-Zabielska AU, Jensen MD. Rapid measurement of plasma free fatty acid concentration and isotopic enrichment using LC/MS. J Lipid Res 2010;51:2761–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tchoukalova YD, Harteneck DA, Karwoski RA, Tarara J, Jensen MD. A quick, reliable, and automated method for fat cell sizing. J Lipid Res 2003;44:1795–801. [DOI] [PubMed] [Google Scholar]
- 32.Xu M, Tchkonia T, Ding H, Ogrodnik M, Lubbers ER, Pirtskhalava T, White TA, Johnson KO, Stout MB, Mezera V, et al. JAK inhibition alleviates the cellular senescence-associated secretory phenotype and frailty in old age. Proc Natl Acad Sci USA 2015;112:E6301–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aron-Wisnewsky J, Tordjman J, Poitou C, Darakhshan F, Hugol D, Basdevant A, Aissat A, Guerre-Millo M, Clement K. Human adipose tissue macrophages: m1 and m2 cell surface markers in subcutaneous and omental depots and after weight loss. J Clin Endocrinol Metab 2009;94:4619–23. [DOI] [PubMed] [Google Scholar]
- 34.Jensen MD, Nielsen S. Insulin dose response analysis of free fatty acid kinetics. Metabolism 2007;56:68–76. [DOI] [PubMed] [Google Scholar]
- 35.Shadid S, Jensen MD. Pioglitazone increases non-esterified fatty acid clearance in upper body obesity. Diabetologia 2006;49:149–57. [DOI] [PubMed] [Google Scholar]
- 36.Magkos F, Fraterrigo G, Yoshino J, Luecking C, Kirbach K, Kelly SC, de Las Fuentes L, He S, Okunade AL, Patterson BW, et al. Effects of moderate and subsequent progressive weight loss on metabolic function and adipose tissue biology in humans with obesity. Cell Metab 2016;23:591–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Permana PA, Menge C, Reaven PD. Macrophage-secreted factors induce adipocyte inflammation and insulin resistance. Biochem Biophys Res Commun 2006;341:507–14. [DOI] [PubMed] [Google Scholar]
- 38.Harford KA, Reynolds CM, McGillicuddy FC, Roche HM. Fats, inflammation and insulin resistance: insights to the role of macrophage and T-cell accumulation in adipose tissue. Proc Nutr Soc 2011;70:408–17. [DOI] [PubMed] [Google Scholar]
- 39.Kalupahana NS, Claycombe KJ, Moustaid-Moussa N. (n-3) Fatty acids alleviate adipose tissue inflammation and insulin resistance: mechanistic insights. Adv Nutr 2011;2:304–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rivellese AA, Maffettone A, Iovine C, Di Marino L, Annuzzi G, Mancini M, Riccardi G. Long-term effects of fish oil on insulin resistance and plasma lipoproteins in NIDDM patients with hypertriglyceridemia. Diabetes Care 1996;19:1207–13. [DOI] [PubMed] [Google Scholar]
- 41.Kabir M, Skurnik G, Naour N, Pechtner V, Meugnier E, Rome S, Quignard-Boulange A, Vidal H, Slama G, Clement K, et al. Treatment for 2 mo with n 3 polyunsaturated fatty acids reduces adiposity and some atherogenic factors but does not improve insulin sensitivity in women with type 2 diabetes: a randomized controlled study. Am J Clin Nutr 2007;86:1670–9. [DOI] [PubMed] [Google Scholar]
- 42.Veleba J, Kopecky J Jr, Janovska P, Kuda O, Horakova O, Malinska H, Kazdova L, Oliyarnyk O, Skop V, Trnovska J, et al. Combined intervention with pioglitazone and n-3 fatty acids in metformin-treated type 2 diabetic patients: improvement of lipid metabolism. Nutr Metab (Lond) 2015;12:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yeop Han C, Kargi AY, Omer M, Chan CK, Wabitsch M, O’Brien KD, Wight TN, Chait A. Differential effect of saturated and unsaturated free fatty acids on the generation of monocyte adhesion and chemotactic factors by adipocytes: dissociation of adipocyte hypertrophy from inflammation. Diabetes 2010;59:386–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Todoric J, Loffler M, Huber J, Bilban M, Reimers M, Kadl A, Zeyda M, Waldhausl W, Stulnig TM. Adipose tissue inflammation induced by high-fat diet in obese diabetic mice is prevented by n-3 polyunsaturated fatty acids. Diabetologia 2006;49:2109–19. [DOI] [PubMed] [Google Scholar]
- 45.Heskey CE, Jaceldo-Siegl K, Sabate J, Fraser G, Rajaram S. Adipose tissue alpha-linolenic acid is inversely associated with insulin resistance in adults. Am J Clin Nutr 2016;103:1105–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sato T, Kameyama T, Ohori T, Matsuki A, Inoue H. Effects of eicosapentaenoic acid treatment on epicardial and abdominal visceral adipose tissue volumes in patients with coronary artery disease. J Atheroscler Thromb 2014;21:1031–43. [DOI] [PubMed] [Google Scholar]
- 47.D’Archivio M, Scazzocchio B, Giammarioli S, Fiani ML, Vari R, Santangelo C, Veneziani A, Iacovelli A, Giovannini C, Gessani S, et al. omega3-PUFAs exert anti-inflammatory activity in visceral adipocytes from colorectal cancer patients. PLoS One 2013;8:e77432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cardel M, Lemas DJ, Jackson KH, Friedman JE, Fernandez JR. Higher intake of PUFAs is associated with lower total and visceral adiposity and higher lean mass in a racially diverse sample of children. J Nutr 2015;145:2146–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol 2010;72:219–46. [DOI] [PubMed] [Google Scholar]
- 50.Aguilar M, Bhuket T, Torres S, Liu B, Wong RJ. Prevalence of the metabolic syndrome in the United States, 2003-2012. JAMA 2015;313:1973–4. [DOI] [PubMed] [Google Scholar]