Abstract
Background: Public health concerns with regard to both low and high folate status exist in the United States. Recent publications have questioned the utility of self-reported dietary intake data in research and monitoring.
Objectives: The purpose of this analysis was to examine the relation between self-reported folate intakes and folate status biomarkers and to evaluate their usefulness for several types of applications.
Design: We examined usual dietary intakes of folate by using the National Cancer Institute method to adjust two 24-h dietary recalls (including dietary supplements) for within-person variation and then compared these intakes with serum and red blood cell (RBC) folate among 4878 men and nonpregnant, nonlactating women aged ≥19 y in NHANES 2011–2012, a nationally representative, cross-sectional survey, with respect to consistency across prevalence estimates and rank order comparisons.
Results: There was a very low prevalence (<1%) of folate deficiency when serum (<7 nmol/L) and RBC (<305 nmol/L) folate were considered, whereas a higher proportion of the population reported inadequate total dietary folate intakes (6%). Similar patterns of change occurred between intakes and biomarkers of folate status when distributions were examined (i.e., dose response), particularly when diet was expressed in μg. Intakes greater than the Tolerable Upper Intake Level greatly increased the odds of having high serum folate (OR: 17.6; 95% CI: 5.5, 56.0).
Conclusions: When assessing folate status in the United States, where fortification and supplement use are common, similar patterns in the distributions of diet and biomarkers suggest that these 2 types of status indicators reflect the same underlying folate status; however, the higher prevalence estimates for inadequate intakes compared with biomarkers suggest, among other factors, a systematic underestimation bias in intake data. Caution is needed in the use of dietary folate data to estimate the prevalence of inadequacy among population groups. The use of dietary data for rank order comparisons or to estimate the potential for dietary excess is likely more reliable.
Keywords: NHANES, folate, folic acid, dietary assessment, measurement error, prevalence
INTRODUCTION
Assessing the folate status of the US population is particularly salient because of the folic acid fortification of the food supply coupled with high dietary supplement use (1). Thus, public health monitoring is needed to ensure that intakes are sufficient to prevent deficiencies, to maintain adequate levels for reproductive-aged women for the prevention of neural tube defects, and to ensure that intakes are safe (2, 3). Intake assessments are also an important component of ongoing epidemiologic research on diet and health relations (4). Some publications have questioned the utility of self-reported dietary data for any use in nutrition research and monitoring (5, 6). Certainly, a well-documented limitation of dietary assessment is a systematic bias in self-reported energy-related intakes (7, 8). Measurement error in dietary self-reporting has been shown to attenuate diet and disease relations, but little is known on the impact for monitoring and surveillance. Therefore, although caution is needed when considering the use of self-reported intake data for some applications, the utility of dietary intake data should not be overlooked (4).
For folate-monitoring applications, we would expect the population prevalence of inadequate intakes to correspond relatively closely to the population prevalence of inadequate serum and red blood cell (RBC)9 folate, because the estimated distribution for human requirements used to develop the Dietary Reference Intakes (DRIs) for folate, and notably the Estimated Average Requirement (EAR), was derived from biomarker data. These studies were conducted before the dietary folate equivalent (DFE) metric was developed (9). The EAR values for intakes for younger adults were based on results from controlled metabolic studies that quantitatively determined the intakes required to maintain normal folate blood concentrations; their relevance for older adults was confirmed with observational studies (9). Thus, whereas serum and RBC folate are not true recovery biomarkers, they function as concentration biomarkers because they have been used as such in carefully controlled feeding studies to quantify the dietary depletion needed to observe changes in the biomarkers (10). However, the dietary Tolerable Upper Intake Level (UL) for “high” folate intakes is based on a potential adverse interaction with vitamin B-12 status (9). The relation between determinations of “high” folate status with the use of intake measures compared with clinical measures was not developed in the same way, so we have no such expectations about agreement at the high end of the distribution. The goals of this analysis were as follows: 1) to compare the prevalence of folate inadequacy among adults on the cutoffs used for serum and RBC concentrations and the EAR and UL cutoffs used for dietary intake data (aim 1), 2) to compare self-reported diet and measured biomarkers of folate status with respect to their distributions and rank order comparisons (aim 2), and 3) to provide insights on the interpretability of self-reported folate dietary intakes (aim 3).
METHODS
Participants and data collection
NHANES is a nationally representative, cross-sectional study of nutrition and health for individuals residing in the United States. All NHANES data are collected by the CDC, National Center for Health Statistics. Participants are first interviewed in their home; 3–10 d after the initial interview participants undergo a health examination in a mobile examination center, and anthropometric measurements and collection of biological specimens for laboratory assessment are carried out. Written informed consent was obtained for all participants or proxies; the survey protocol was approved by the Research Ethics Review Board at the National Center for Health Statistics. The unweighted response rates for participants in this study were 72% for the interview component and 69% for the examination component.
Data from 4878 men and nonpregnant, nonlactating women aged ≥19 y who had complete 24-h dietary intake from NHANES 2011–2012 were used for these analyses. During the home interview, demographic data were collected via computer-assisted software. The self-identified race/ethnic groups defined by NHANES included non-Hispanic white, non-Hispanic black, Hispanic and Mexican American, non-Hispanic Asian American, and “other.” Age was categorized to be representative of the following Dietary Reference Intake (DRI) groupings: 19–30, 31–50, 51–70, and ≥71 y.
Dietary methods
During the mobile examination center visit, an in-person 24-h dietary recall (24-HR) is collected as part of the USDA’s What We Eat in America component of NHANES. A second 24-HR is collected via telephone 3–10 d after the first, with emphasis placed on getting both weekday and weekend reports. Both 24-HRs were collected by using the USDA’s Automated Multiple-Pass Method and included dietary supplements (11, 12). Dietary supplements were also assessed by using the Dietary Supplement Questionnaire, administered in the home interview which collects information on the participant’s use of vitamins, minerals, herbs, and other supplements over the previous 30 d. Detailed information about type, consumption frequency, duration, and amount taken is also collected for each reported supplement and used to calculate average daily intakes. Alcohol consumption in NHANES is defined as the average number of drinks per day in the previous year; 1 drink contains 10 g ethanol and is equivalent to 12 ounces of beer (360 mL), 4 ounces of wine (120 mL), or 1 ounce (30 mL) of distilled spirits.
DRIs
The bioavailability of food folate is thought to be lower than that of folic acid present in fortified foods and dietary supplements. For this reason, the DFE conversion was developed to reflect the differential bioavailability (9). We used the EAR to estimate the prevalence of the risk of inadequacy; the DFE is used to account for folate and folic acid in meeting this requirement (9). We used the UL to assess the risk of excessive consumption, and only folic acid is considered for this DRI (9).
Laboratory methods
A questionnaire was used to assess fasting and the use of dietary supplements around the time of the blood draw; fasting before the blood draw was classified as ≥3 to <8 h and ≥8 h. Serum and whole-blood samples were analyzed at the CDC’s Laboratory for Nutritional Biomarkers (13). The microbiological assay was used to estimate RBC folate; serum folate was determined by use of HPLC–tandem mass spectrometry (MS/MS) (14). According to the DRI summary of folate, the cutoffs for assessing the adequacy of folate status are <7 nmol/L for serum folate and <305 nmol/L for RBC folate (9). High serum folate was operationalized as >45 nmol/L (15). Serum cotinine, a marker of tobacco exposure, was assayed via isotope dilution with liquid chromatography and MS/MS. Serum creatinine was measured by a Roche/Hitachi 737 analyzer (Roche Diagnostics) by using the kinetic alkaline picrate reaction and calibrated to the Cleveland Clinic Research Laboratory standard (13). Estimated glomerular filtration rate (eGFR) was calculated for each individual on the basis of serum creatinine concentration, sex, age, and race (16).
Anthropometric measurements and physical activity
Measured height and weight were used to calculate BMI as weight divided by squared height (kg/m2); standard BMI categories were used [obese (BMI ≥30), overweight (BMI of 25–29.9), healthy weight (BMI of 18.5–24.9), and underweight (BMI <18.5)]. Physical activity was categorized as moderate-intensity (any activity that requires moderate physical effort and causes a small increase in breathing or heart rate) and vigorous-intensity (any activity that requires hard physical effort and causes large increases in breathing or heart rate, such as carrying or lifting heavy loads, digging or construction work for ≥10 min continuously) activities.
Statistical methods
Statistical analyses were performed by using SAS (version 9.3; SAS Institute, Inc.) and SUDAAN software (version 11.1; RTI), adjusted for survey design and sampling weights to account for differential nonresponse and noncoverage and to adjust for planned oversampling of some groups. Significance was set at a Bonferroni-adjusted P value <0.01 for main effects; all P values are provided in Tables 1–4.
TABLE 1.
Usual total self-reported dietary intake estimates for folate and folic acid and the prevalence of intakes with regard to the DRI guidelines among US adults by sex and age group, 2011–20121
| Folate |
Folic acid |
||||
| Sex/age groups | n | Value, DFEs | Less than the EAR, % | Value, μg | Above the UL, % |
| All participants, y | |||||
| ≥19 | 4878 | 763 ± 7 | 6.8 ± 0.9 | 314 ± 4 | 1.6 ± 0.2 |
| 19–30 | 1083 | 707 ± 19 | 6.5 ± 1.1 | 292 ± 9 | 1.2 ± 0.4 |
| 31–50 | 1587 | 745 ± 12 | 7.0 ± 0.9 | 302 ± 8 | 1.3 ± 0.4 |
| 51–70 | 1559 | 797 ± 16 | 6.1 ± 1.1 | 322 ± 6 | 1.7 ± 0.4 |
| ≥71 | 649 | 847 ± 20 | 7.7 ± 1.3 | 375 ± 12 | 2.7 ± 0.5 |
| P-trend (age) | 0.0002 | 0.2537 | 0.005 | <0.0001 | |
| Men, y | |||||
| ≥19 | 2469 | 817 ± 132 | 3.3 ± 0.52 | 326 ± 73 | 1.4 ± 0.4 |
| 19–30 | 575 | 767 ± 212 | 3.2 ± 0.93 | 308 ± 112 | 0.9 ± 0.5 |
| 31–50 | 798 | 789 ± 172 | 3.1 ± 0.63 | 305 ± 92 | 0.6 ± 0.2 |
| 51–70 | 769 | 857 ± 382 | 3.4 ± 0.63 | 337 ± 192 | 2.1 ± 0.9 |
| ≥71 | 327 | 943 ± 522 | 4.4 ± 1.03 | 422 ± 323 | 3.5 ± 0.93 |
| P-trend (age) | 0.0015 | 0.3025 | 0.0018 | <0.0001 | |
| Women, y | |||||
| ≥19 | 2409 | 711 ± 13 | 10.1 ± 1.7 | 302 ± 6 | 1.6 ± 0.2 |
| 19–30 | 508 | 635 ± 35 | 10.8 ± 2.0 | 274 ± 17 | 1.5 ± 0.5 |
| 31–50 | 789 | 702 ± 24 | 11.1 ± 1.7 | 299 ± 14 | 2.1 ± 0.6 |
| 51–70 | 790 | 747 ± 21 | 8.7 ± 1.9 | 308 ± 12 | 1.3 ± 0.4 |
| ≥71 | 322 | 770 ± 21 | 10.0 ± 2.1 | 341 ± 12 | 1.9 ± 0.7 |
| P-trend (age) | 0.0006 | 0.1398 | 0.0065 | 0.2531 | |
Values are means or percentages ± SEs unless otherwise indicated. The National Cancer Institute method was used to estimate the self-reported total usual folate and folic acid intake distributions and compliance with the DRI guidelines. SEs were estimated with the Taylor series linearization. Trends by age group and sex comparisons were tested with linear contrasts. DFE, dietary folate equivalent; DRI, Dietary Reference Intake; EAR, Estimated Average Requirement; UL, Tolerable Upper Intake Level.
Different from women: 2P < 0.001, 3P < 0.01.
TABLE 4.
ORs (95% CIs) of suboptimal self-reported dietary intakes and abnormal biomarkers of folate status in US adults, 2011–20121
| Intakes less than the EAR | Intakes above the UL | |
| RBC folate <305 nmol/L | 2.8 (0.28, 28.0) | — |
| P | 0.38 | — |
| Serum folate >45 nmol/L | — | 17.6 (5.5, 56.0) |
| P | — | 1.2 × 10−5 |
n = 4878. All estimates are for fully adjusted models including age, sex, race/ethnicity, physical activity, estimated glomerular filtration rate, duration of fasting before blood draw, serum cotinine concentrations, alcohol consumption, poverty-income ratio, and BMI. EAR, Estimated Average Requirement; RBC, red blood cell; UL, Tolerable Upper Intake Level.
Aim 1: compare the prevalence of folate inadequacy for diet and biomarkers
The DRI report established serum folate <7 nmol/L and RBC folate <305 nmol/L as the appropriate cutoffs that correspond with dietary intakes less than the EAR. When examining the “tails” of dietary intake distributions, usual intake procedures should be used to correct for measurement error and within-person variation (18). The National Cancer Institute method was used to estimate usual intake estimation for all units of dietary folate, including means and percentiles of intake and probabilities of meeting or exceeding the DRI (18, 19). Covariates in the National Cancer Institute usual intake models included age group, day of the week of the 24-HR (weekend or weekday), interview sequence of the 24-HR, and race/ethnicity. To estimate total usual nutrient intake, nutrient intakes from dietary supplements were added to the usual nutrient intake from foods, as recommended (20). SEs were estimated with the Taylor series linearization, a design-based method.
Aim 2: compare diet and biomarkers with respect to their distributions and rank order
Standardized z scores were estimated for the 5th through the 95th percentiles for all age groups combined. Each respective percentile z score was compared with t tests. Plots of the z scores were summarized for the corresponding units in which the variable was measured for ease of interpretation. This analysis used the survey design features and sampling weights.
We also examined contingency tables of quartiles of total folate and folic acid intakes relative to serum and RBC folate; this analysis did not include any covariates because no survey procedures exist to incorporate covariates. Concordance was measured by calculating the percentage of perfect agreement between quartiles of dietary intakes compared with quartiles of biomarkers; we also present the percentage of perfect disagreement in quartiles and the percentage of agreement 1 and 2 quartiles away. As proposed by Cichetti and Allison (17), a C-statistic was used to assess the quartile agreement representing the weighted proximity of quartile observations relative to each other; the C-statistic gives more weight to observations closer to concordance. The C-statistic permits us to assess the degree of misclassification and can interpreted like a correlation coefficient; this was assessed for all adults and by dietary supplement use category. The test of marginal homogeneity compares our “observed” agreement with that of the “expected” agreement on the basis of chance. It assumes under the null hypothesis that the probabilities of the outcome are the same (i.e., quartile agreement based on chance); a chi-square test with 3 df was used to test the observed and expected quartile distributions under the test of homogeneity.
Aim 3: provide insights on self-reported folate intakes
Regression models were used to examine total usual dietary intakes of folate and folic acid relative to biomarkers and to evaluate the impact of covariates on the relation of diet and biomarkers, as well as to investigate the covariates’ role on biomarkers. Logistic regression was used to determine ORs of meeting or exceeding the DRI relative to cutoffs established for biomarkers of folate status. All of the regression models used the following covariates: age, sex, race-ethnicity, eGFR, BMI, fasting, alcohol, physical activity, and cotinine.
RESULTS
Mean intakes of folate and folic acid showed a strong linear increase with age (Table 1), likely due to the increased use of folic acid–containing dietary supplements (Supplemental Table 1). Depending on the sex and/or age group, 3–11% of US adults have usual total dietary intakes of folate less than the EAR. Men have a lower prevalence of intakes less than the EAR (3.1–4.4%) than do women (8.7–11.1%) across all age groups. Approximately 2% of adults exceeded the UL for folic acid; no sex differences were observed in exceeding the UL when age groups were combined, but among those aged ≥71 y, men had a higher prevalence than women. Among men, but not women, exceeding the UL increased with increasing age.
Aim 1
A very low prevalence of folate deficiency exists on the basis of serum and RBC folate (Table 2); given the low overall prevalence, differences in sex and age group were not evident. Men had lower means and prevalence below cutoffs of adequacy for RBC folate and serum folate than did women when all age groups were combined, with notable sex differences in age group observed mainly for serum folate. A rather high percentage of adults had “high” serum folate concentrations of >45 nmol/L (43.1%); women had a higher prevalence of “high” serum folate concentrations >45 nmol/L (women compared with men: 48% compared with 38.3%; P < 0.001). In combined and separate analyses by sex, the prevalence of high serum folate and folic acid increased with age.
TABLE 2.
Low and high serum and RBC folate status stratified by sex and age group among US adults, 2011–20121
| Serum folate |
RBC folate |
|||||
| Sex/age groups | n | Value, nmol/L | <7 nmol/L, % | >45 nmol/L, % | Value, nmol/L | <305 nmol/L, % |
| All participants, y | ||||||
| ≥19 | 4878 | 47.2 ± 0.7 | 0.02 ± 0.022 | 43.1 ± 1.5 | 1162 ± 23 | 0.3 ± 0.1 |
| 19–30 | 1083 | 39.4 ± 0.9 | 0 | 32.5 ± 2.4 | 957 ± 22 | 0.25 ± 0.12 |
| 31–50 | 1587 | 41.6 ± 0.7 | 0.03 ± 0.032 | 35 ± 2.3 | 1096 ± 22 | 0.2 ± 0.1 |
| 51–70 | 1559 | 51.8 ± 2.1 | 0.04 ± 0.052 | 51.8 ± 2.9 | 1255 ± 46 | 0.41 ± 0.22 |
| ≥71 | 649 | 68.6 ± 2.1 | 0 | 67 ± 2.8 | 1531 ± 43 | 0.3 ± 0.2 |
| P-trend (age) | <0.0001 | 0.3801 | <0.0001 | <0.0001 | 0.3270 | |
| Men, y | ||||||
| ≥19 | 2469 | 44.0 ± 0.83 | 0.02 ± 0.022 | 38.3 ± 23 | 1119 ± 284 | 0.23 ± 0.15 |
| 19–30 | 575 | 37.6 ± 1.13 | 0 | 28.5 ± 3.7 | 950 ± 30 | 0 |
| 31–50 | 798 | 39.1 ± 0.93 | 0.06 ± 0.062 | 31.2 ± 2.7 | 1068 ± 27 | 0.16 ± 0.12 |
| 51–70 | 769 | 48.3 ± 1.54 | 0 | 46.5 ± 3.4 | 1202 ± 44 | 0.54 ± 0.52 |
| ≥71 | 327 | 65.2 ± 3.2 | 0 | 63.8 ± 3.7 | 1478 ± 63 | 0 |
| P-trend (age) | <0.0001 | 0.3614 | <0.0001 | <0.0001 | 0.2550 | |
| Women, y | ||||||
| ≥19 | 2409 | 50.4 ± 1.1 | 0.03 ± 0.032 | 48 ± 1.6 | 1204 ± 20 | 0.4 ± 0.12 |
| 19–30 | 508 | 41.5 ± 1.0 | 0 | 37.2 ± 3.7 | 965 ± 22 | 0.5 ± 0.32 |
| 31–50 | 789 | 44.1 ± 1.1 | 0 | 38.7 ± 3 | 1125 ± 26 | 0.24 ± 0.12 |
| 51–70 | 790 | 55.1 ± 3.1 | 0.08 ± 0.092 | 56.8 ± 4.7 | 1303 ± 51 | 0.3 ± 0.22 |
| ≥71 | 322 | 71.3 ± 2.0 | 0 | 69.6 ± 2.9 | 1571 ± 43 | 0.6 ± 0.32 |
| P-trend (age) | <0.0001 | 0.3707 | 0.0001 | <0.0001 | 0.9517 | |
Values are means or percentages ± SEs unless otherwise indicated. The National Cancer Institute method was used to estimate the self-reported total usual folate and folic acid intake distributions. The microbiological assay was used to estimate RBC folate; serum folate was determined by use of HPLC–tandem mass spectrometry. SEs were estimated with the Taylor series linearization. Trends by age group and sex comparisons were tested with linear contrasts. RBC, red blood cell.
Indicates an estimate with a high relative SE; may not be statistically reliable.
Different from women: 3P < 0.001, 4P < 0.01.
Aim 2
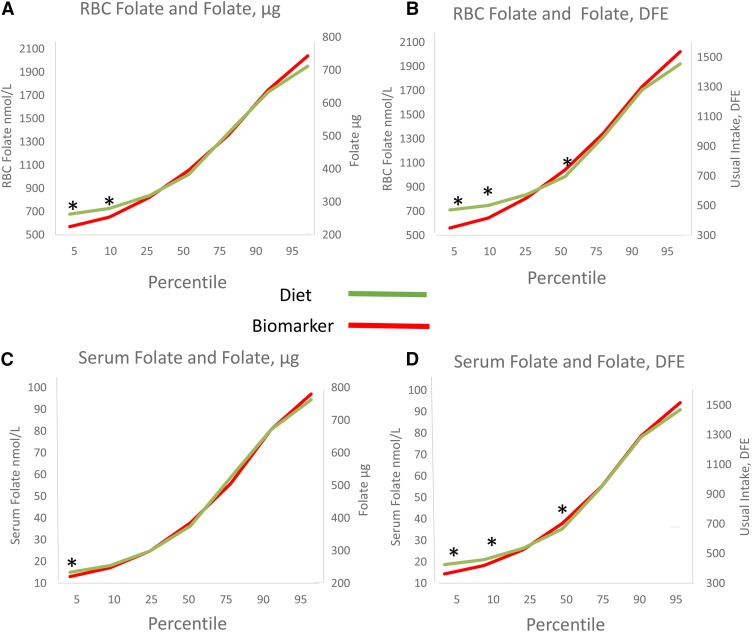
The distribution patterns of dietary folate (in DFEs and micrograms) and both biomarkers of folate status are plotted in Figure 1. In general, the lower percentiles (i.e., 5th and 10th) differed significantly for diet when compared with both RBC folate (for both micrograms and DFEs) and serum folate (DFEs only). However, when folate intake was quantified as DFEs, significant differences at the 50th percentile also occurred for both serum and RBC folate. No other significant differences occurred across the distributions of either biomarker when compared with folate intake measured in micrograms.
FIGURE 1.
Standardized z scores of percentiles of self-reported dietary folate intake by percentiles of RBCs and serum folate in US adults: NHANES 2011–2012. n = 4878. Standardized z scores at each percentile were compared with t tests. *P < 0.01. DFE, dietary folate equivalent; RBC, red blood cell.
We also examined the distributions by quartiles of diet and biomarkers. When supplement users and nonusers were combined, misclassification was more pronounced at lower intakes than at higher intakes (data not shown). Overall, the concordance between diet (DFEs) and RBC folate was 37%; however, concordance was higher for supplement users (45%) than for nonusers (34%) (Table 3). Concordance overall was slightly higher overall (40%) for diet (DFEs) and serum folate, with the same trend of higher concordance observed for users (51%) compared with nonusers (36%) of dietary supplements. Thus, supplement users appear to have less misclassification than do supplement nonusers. When comparing the fourth quartile for diet (both forms) and biomarkers (both serum and RBC) among users of supplements, >86% of adults were correctly classified (data not shown). Higher concordance was observed for serum folate for non-Hispanic whites and non-Hispanic Asians, both of whom are also the highest users of folic acid supplements (Supplemental Table 2).
TABLE 3.
Comparison of quartile distributions for self-reported dietary folate with serum and RBC folate: an unweighted exploratory use of NHANES, 2011–20121
| All (n = 4878) | Nonusers (n = 3828) | Users (n = 1050) | |
| Quartiles of diet (DFE) compared with RBC folate (nmol/L) | |||
| Perfect agreement, % | 36.8 | 33.6 | 45.0 |
| Disagree by 1 quartile, % | 38.9 | 41.9 | 31.3 |
| Disagree by 2 quartiles, % | 18.7 | 19.1 | 17.7 |
| Perfect disagreement, % | 5.6 | 5.4 | 5.1 |
| Agreement, C-statistic (95% CI) | 0.690 (0.681, 0.698) | 0.678 (0.669, 0.689) | 0.7172 (0.701, 0.734) |
| Homogeneity (chi-square) | 1.07 | 334.64 | 524.51 |
| P | 0.78 | <0.0001 | <0.0001 |
| Quartiles of diet (DFE) compared with serum folate (nmol/L) | |||
| Perfect agreement, % | 40.2 | 35.8 | 51.2 |
| Disagree by 1 quartile, % | 38.4 | 42.0 | 29.4 |
| Disagree by 2 quartiles, % | 16.5 | 18.0 | 13.3 |
| Perfect disagreement, % | 4.7 | 4.2 | 6.0 |
| Agreement, C-statistic (95% CI) | 0.714 (0.705, 0.722) | 0.698 (0.688, 0.708) | 0.7533 (0.737, 0.770) |
| Homogeneity (chi-square) | 7.66 | 337.96 | 393.53 |
| P | 0.054 | <0.0001 | <0.0001 |
The microbiological assay was used to estimate RBC folate; serum folate was determined by the use of HPLC–tandem mass spectrometry. SEs were estimated with the Taylor series linearization. The analyses in this table do not include the NHANES survey design features or sampling weights. Quartile agreement was estimated by a C-statistic, a weighted κ approach proposed by Cicchetti and Allison (17). A nonparametric test of marginal homogeneity compared the observed and expected quartile distributions. DFE, dietary folate equivalent; RBC, red blood cell.
Different from nonusers: 2z score = 7.58, P < 0.0001; 3z score = 5.64, P <0.0001.
An exploratory analysis that used NHANES without the survey design and weights examined the proximity of quartile agreement with the C-statistic. This analysis suggested that agreement between biomarkers and diet in terms of quartiles was ∼70% (Table 3). Agreement was significantly higher in dietary supplement users for both biomarkers. In addition, the test of marginal homogeneity suggested that the quartiles agreed beyond what was expected by chance for both biomarkers when supplement users and nonusers were examined separately; however, this was not the case at the group level (users and nonusers combined).
Aim 3
Total usual dietary folate (DFE) intake had a significant influence on RBC folate (β = 0.16, P = 0.002) and for serum folate (β = 0.02, P = 0.003) in fully adjusted models (Supplemental Tables 3 and 4). For both serum and RBC folate, several other factors were strong, significant predictors of these biomarkers: age, sex, race, eGFR, duration of fasting before blood draw, serum cotinine, weight status, and alcohol (serum folate only). Intakes above the UL greatly increased the odds of having high serum folate (OR: 17.6; 95% CI: 5.5, 56.0) (Table 4).
DISCUSSION
The DRI report on folate and folic acid directly derived its intake reference values from average concentrations of serum and RBC folate known to reflect deficiency (9). Therefore, the population prevalence of those considered at risk of folate inadequacy by biomarkers and diet should theoretically be similar. In actuality, a higher prevalence of inadequacy was estimated by self-reported diet when compared with biomarkers (aim 1). However, better agreement was observed with rank order comparisons of the distributions of diet and biomarkers, and within sex and age both mean intakes and mean biomarkers increased linearly, suggesting a strong dose-response relation (aim 2). Although usual dietary folate intake is a significant predictor of folate biomarkers in regression models, so were many other factors unrelated to diet (i.e., age, body size, kidney function). Therefore, the cutoffs for biomarkers of folate, although directly related to EAR intakes, from small controlled feeding trials in young, healthy adults may not translate similarly on the population level. However, dietary intakes above the UL based on the relation of high folate intakes to vitamin B-12 status have high agreement with cutoffs of high serum folate (aim 3). Taken collectively, self-reported dietary data serve as a good proxy for high exposures of folate status as well as a good representation of the distribution pattern of biomarkers (i.e., rank order); however, characterizing inadequacy on the basis of dietary intakes alone may overestimate the actual prevalence.
In interpreting our data, several points should be addressed. First, dietary assessment methods, including 24-HRs used in the current analysis, have a well-documented limitation of energy underreporting (8). This is particularly salient for folic acid because it is added to the food supply through the fortification of cereal grains and other calorie-containing foods. Therefore, dietary folate underreporting would be expected to be affected by energy underreporting. We know that women have a greater magnitude of energy underreporting than men (8, 21), perhaps one of the potential reasons that a higher prevalence of intakes less than the EAR was observed for women than for men, but this could also be due to lower caloric intakes overall among women. Similarly, there is a likelihood for the folic acid content of dietary supplements to be underestimated by current databases that rely exclusively on label declarations. Documented overages range from +13% to +16% more folic acid in the supplement than in labeled amounts (22).
Some issues related to the estimation of biomarkers are also germane. First, the analytic method used to determine serum and RBC folate greatly influenced the estimates (23); this analysis included information provided through the use of the microbiological assay for RBC folate and liquid chromatography–MS/MS for serum folate. We have no way of determining the comparability of the current NHANES analytic procedures to the results from the studies relied on in the DRIs to derive intake EARs from biomarker data. In addition, little is known about the influence of within-person variability in folate biomarkers and how this variability exerts an effect, if any, on population prevalence estimates. It is unfortunate that multiple samples of biomarkers were not available in the survey years presented in this report or for the microbiological assay during the fortification era to assess the impact, if any, of within-person variation on prevalence estimates; other analytic methods suggest little within-person variation for folate biomarkers (24). Finally, discrepancy between self-reported diet and biomarkers of folate status can also be greatly influenced by genetic polymorphisms, most notably the 5,10-methylenetetrahydrofolate reductase (MTHFR) C677T (25). Again, we lack NHANES data to make adjustments for these confounders.
Accurate characterization of biomarker status depends on the cutoff that is applied. The methods used in our report were assay-matched to the cutoffs applied in the National Academy of Medicine report, which strengthens the interpretability of their use (26). As with deriving cutoffs for intake data, the cutoffs for determining biomarker-based status of sufficiency derive from a distribution that reflects variability in requirements among individuals (27). The study given the most weight in determining the EAR was a small study in nonpregnant women aged 21–27 y (n = 18) who were randomly assigned to receive 200, 300, or 400 μg folate/d for 70 d; this study was conducted before the DFE metric was established. The estimated values corresponding to the 3 groups in the DFE metric were 319, 489, and 659, respectively. The EAR was set based in part on the fact that 3 of 6 women in the 200- and 300-μg groups had serum folate <6.8 nmol/L and 4 of 6 women in the 200-μg and 1 of 6 in the 300-μg groups had RBC folate <362 nmol/L, as determined by the microbiological assay. Cutoffs from the DRI report were based on folate derived through food sources only. The accuracy of the bioavailability adjustments of added sources of folate (i.e., folic acid) was also based on small, short-term studies.
No established DRI criteria exist for the prevalence of excess or high folate status for biomarkers, and limited biological conclusions can be drawn as a result of our data. The cutoffs of “high” biomarkers (i.e., >45 nmol/L) presented in this article and in other NHANES analyses were developed in population-based studies (15), not controlled studies with carefully measured intakes and biomarker concentrations. Thus, caution must be exercised in interpreting population status with these cutoffs. A mismatch also exists for the prevalence of intakes above the UL and both high serum folate and folic acid. Although the UL threshold yielded a much lower prevalence of excess dietary intakes than did serum folate, caution is needed in evaluating whether these comparisons are a reflection of inaccuracy in the intake data or simply a difference in the meaning of the cutoff values. The UL was established from case reports citing concerns of high folate status “masking” the hematologic diagnosis of vitamin B-12 deficiency (9). Given this concern, as well as others recently cited, more research is needed to establish the appropriate cutoffs for high folate status (28).
Concentration biomarkers are often highly correlated with intakes but cannot be directly linked to intake in the same way that recovery biomarkers can. Both dietary intake assessments and concentration biomarkers are subject to known and unknown confounding. Despite the differences in the prevalence estimates with the application of cutoffs, we observed surprisingly similar patterns of change between dietary intakes and biomarkers of folate status across the distribution, particularly when folate was expressed in micrograms rather than the DFE. When the distributions of diet and biomarkers were compared, serum folate and diet as measured in micrograms had the greatest agreement, deviating only at the fifth percentile. Given the proximity of the blood draw to the dietary assessment in the NHANES protocol this makes biological sense. We would expect more agreement in recent measures of status (serum) than in measures of long-term folate status (RBC folate).
Folate exposure assessment is critical for research and surveillance applications in the United States where folic acid fortification and supplement use are common. This report indicates that the use of self-reported dietary data alone is likely most useful for rank order comparisons (e.g., epidemiologic applications) and screening for individuals at risk of high exposure (i.e., above the UL, biomarkers in the highest end of the population distributions). It should be noted that the biological and clinical implications of high folate biomarkers are largely unknown. For folate, self-reported dietary data should be used in conjunction with biomarkers to strengthen the ability to detect relations with health outcomes for epidemiologic applications. For folate policy and monitoring applications, particularly with regard to inadequacy, we suggest that decisions largely be based on biochemical data. Caution is needed in the use of dietary folate data alone to estimate the prevalence of inadequacy among population groups.
Acknowledgments
The authors’ responsibilities were as follows—RLB, EAY, CMP, and CLT: developed the concept of the project; VLF: completed the usual intake and biomarker statistical analysis of the project; GPM: completed the exploratory, unweighted statistical analysis comparing self-reported diet and biomarkers shown in Table 3; SVT: contributed to the statistical analysis and prepared data tables and sections of the manuscript; and all authors: read and approved the final manuscript. None of the authors reported a conflict of interest related to the study.
Footnotes
Abbreviations used: DFE, dietary folate equivalent; DRI, Dietary Reference Intake; EAR, Estimated Average Requirement; eGFR, estimated glomerular filtration rate; MS/MS, tandem mass spectrometry; RBC, red blood cell; UL, Tolerable Upper Intake Level; 24-HR, 24-h dietary recall.
REFERENCES
- 1.Bailey RL, Dodd KW, Gahche JJ, Dwyer JT, McDowell MA, Yetley EA, Sempos CA, Burt VL, Radimer KL, Picciano MF. Total folate and folic acid intake from foods and dietary supplements in the United States: 2003-2006. Am J Clin Nutr 2010;91:231–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rader JI, Yetley EA. Nationwide folate fortification has complex ramifications and requires careful monitoring over time. Arch Intern Med 2002;162:608–9. [DOI] [PubMed] [Google Scholar]
- 3.Yetley EA, Rader JI. Modeling the level of fortification and post-fortification assessments: U.S. experience. Nutr Rev 2004;62:S50–9; discussion: S60–1. [DOI] [PubMed] [Google Scholar]
- 4.Subar AF, Freedman LS, Tooze JA, Kirkpatrick SI, Boushey C, Neuhouser ML, Thompson FE, Potischman N, Guenther PM, Tarasuk V, et al. . Addressing current criticism regarding the value of self-report dietary data. J Nutr 2015;145:2639–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dhurandhar NV, Schoeller DA, Brown AW, Heymsfield SB, Thomas D, Sorensen TI, Speakman JR, Jeansonne M, Allison DB; Energy Balance Measurement Working Group. Response to ‘energy balance measurement: when something is not better than nothing’. Int J Obes (Lond) 2015;39:1175–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Archer E, Hand GA, Blair SN. Validity of U.S. nutritional surveillance: National Health and Nutrition Examination Survey caloric energy intake data, 1971-2010. PLoS One 2013;8:e76632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schoeller DA, Bandini LG, Dietz WH. Inaccuracies in self-reported intake identified by comparison with the doubly labelled water method. Can J Physiol Pharmacol 1990;68:941–9. [DOI] [PubMed] [Google Scholar]
- 8.Subar AF, Kipnis V, Troiano RP, Midthune D, Schoeller DA, Bingham S, Sharbaugh CO, Trabulsi J, Runswick S, Ballard-Barbash R, et al. . Using intake biomarkers to evaluate the extent of dietary misreporting in a large sample of adults: the OPEN study. Am J Epidemiol 2003;158:1–13. [DOI] [PubMed] [Google Scholar]
- 9.Food and Nutrition Board, National Academy of Medicine. Dietary Reference Intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline. Washington (DC): National Academies Press; 1998. [PubMed] [Google Scholar]
- 10.Freedman LS, Kipnis V, Schatzkin A, Tasevska N, Potischman N. Can we use biomarkers in combination with self-reports to strengthen the analysis of nutritional epidemiologic studies? Epidemiol Perspect Innov 2010;7:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blanton CA, Moshfegh AJ, Baer DJ, Kretsch MJ. The USDA automated multiple-pass method accurately estimates group total energy and nutrient intake. J Nutr 2006;136:2594–9. [DOI] [PubMed] [Google Scholar]
- 12.Moshfegh AJ, Rhodes DG, Baer DJ, Murayi T, Clemens JC, Rumpler WV, Paul DR, Sebastian RS, Kuczynski KJ, Ingwersen LA, et al. . The US Department of Agriculture automated multiple-pass method reduces bias in the collection of energy intakes. Am J Clin Nutr 2008;88:324–32. [DOI] [PubMed] [Google Scholar]
- 13.CDC [Internet]. NHANES lab methods [cited 2016 Mar 19]. Available from: https://www.cdc.gov/nchs/nhanes/nhanes2011-2012/lab_methods_11_12.htm.
- 14.Pfeiffer CM, Sternberg MR, Fazili Z, Lacher DA, Zhang M, Johnson CL, Hamner HC, Bailey RL, Rader JI, Yamini S, et al. . Folate status and concentrations of serum folate forms in the US population: National Health and Nutrition Examination Survey 2011-2. Br J Nutr 2015;113:1965–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.WHO [Internet]. Serum and red blood cell folate concentrations for assessing folate status in populations [cited 2016 April 1]. Available from: http://apps.who.int/iris/bitstream/10665/162114/1/WHO_NMH_NHD_EPG_15.01.pdf?ua=1.
- 16.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, et al. . A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cicchetti DV, Allison T. A new procedure for assessing reliability of scoring EEG sleep recordings. Am J EEG Technol 1971;11:101–9. [Google Scholar]
- 18.Dodd KW, Guenther PM, Freedman LS, Subar AF, Kipnis V, Midthune D, Tooze JA, Krebs-Smith SM. Statistical methods for estimating usual intake of nutrients and foods: a review of the theory. J Am Diet Assoc 2006;106:1640–50. [DOI] [PubMed] [Google Scholar]
- 19.Tooze JA, Midthune D, Dodd KW, Freedman LS, Krebs-Smith SM, Subar AF, Guenther PM, Carroll RJ, Kipnis V. A new statistical method for estimating the usual intake of episodically consumed foods with application to their distribution. J Am Diet Assoc 2006;106:1575–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garriguet D. Combining nutrient intake from food/beverages and vitamin/mineral supplements. Health Rep 2010;21:71–84. [PubMed] [Google Scholar]
- 21.Freedman LS, Commins JM, Moler JE, Arab L, Baer DJ, Kipnis V, Midthune D, Moshfegh AJ, Neuhouser ML, Prentice RL, et al. . Pooled results from 5 validation studies of dietary self-report instruments using recovery biomarkers for energy and protein intake. Am J Epidemiol 2014;180:172–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roseland JM, Holden JM, Andrews KW, Zhao C, Schweitzer A, Harnly J, Wolf WR, Perry CR, Dwyer JT, Picciano MF, et al. . Dietary Supplement Ingredient Database (DSID): preliminary USDA studies on the composition of adult multivitamin/mineral supplements. J Food Compost Anal 2008;21:S69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yetley EA, Pfeiffer CM, Phinney KW, Fazili Z, Lacher DA, Bailey RL, Blackmore S, Bock JL, Brody LC, Carmel R, et al. . Biomarkers of folate status in NHANES: a roundtable summary. Am J Clin Nutr 2011;94:303S–12S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yetley EA, Johnson CL. Folate and vitamin B-12 biomarkers in NHANES: history of their measurement and use. Am J Clin Nutr 2011;94(Suppl):322S–31S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fazili Z, Pfeiffer CM, Zhang M, Jain RB, Koontz D. Influence of 5,10-methylenetetrahydrofolate reductase polymorphism on whole-blood folate concentrations measured by LC-MS/MS, microbiologic assay, and Bio-rad radioassay. Clin Chem 2008;54:197–201. [DOI] [PubMed] [Google Scholar]
- 26.Pfeiffer CM, Sternberg MR, Hamner HC, Crider KS, Lacher DA, Rogers LM, Bailey RL, Yetley EA. Applying inappropriate cutoffs leads to misinterpretation of folate status in the US population. Am J Clin Nutr 2016;104:1607–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sauberlich HE, Kretsch MJ, Skala JH, Johnson HL, Taylor PC. Folate requirement and metabolism in nonpregnant women. Am J Clin Nutr 1987;46:1016–28. [DOI] [PubMed] [Google Scholar]
- 28.Boyles AL, Yetley EA, Thayer KA, Coates PM. Safe use of high intakes of folic acid: research challenges and paths forward. Nutr Rev 2016;74:469–74. [DOI] [PMC free article] [PubMed] [Google Scholar]