Abstract
Objective
To compare the psychopathology and longitudinal course of attention deficit hyperactivity disorder (ADHD) symptomatology and global functioning between the offspring with ADHD of bipolar disorder (BP) parents and the offspring with ADHD of community control parents.
Method
One-hundred and twenty-two offspring with ADHD of BP parents and 48 offspring with ADHD of control parents from the Pittsburgh Bipolar Offspring Study (BIOS) were included. DSM-IV lifetime psychiatric disorders were ascertained through the K-SADS-PL. The outcome measures of ADHD symptoms were ascertained at intake and every other year for a period of 6 years using the ADHD section of the K-SADS-PL and the Disruptive Behavior Disorder rating scale (DBD). Global functioning was assessed using the Children’s Global Assessment Scale (CGAS).
Results
The offspring with ADHD of BP parents showed higher lifetime prevalence of mood and anxiety disorders relative to the offspring with ADHD of control parents (p-values ≤0.05). For both groups of offspring with ADHD, the hyperactivity, impulsivity, and total K-SADS-PL ADHD scores decreased over time (p-values <0.001) without differences between the two groups. There were no between- or within-group differences in the inattention scores over time. The DBD ADHD scores decreased with age in both groups (p-values <0.002) without differences between the two groups. For both groups of offspring with ADHD, the global functioning did not improve over time.
Conclusion
ADHD in offspring of BP parents have more psychopathology relative to offspring with ADHD of control parents. However, there were no differences in the developmental courses of ADHD symptomatology between these two groups of ADHD youth.
Keywords: ADHD, bipolar disorder, longitudinal course, psychopathology, functioning
INTRODUCTION
Epidemiological and clinical data consistently show that bipolar disorder (BP) and attention deficit hyperactivity disorder (ADHD) are frequently comorbid in youth.1–4 ADHD and BP have in common several symptoms including distractibility, talkativeness and increased motor activity, and mood symptoms such as irritability and mood lability.3,5,6 Whether the relationship between ADHD and BP is driven purely by the overlap of these symptoms or if there is a significant etiological relationship between the diagnostic entities is not clear. Recent studies show that each disorder increases risk for the other, supporting the hypothesis that these disorders may have some underlying biological features in common.1,7 For example, in a recent meta-analysis of family genetic studies of ADHD and BP, Faraone and colleagues reported high prevalence of ADHD among relatives of BP probands and high prevalence of BP among relatives of ADHD probands.1 However, above-noted studies need to be taken with caution because they used a broad definition of BP, which may have exaggerated the relationship between ADHD and BP and they were cross-sectional.
Longitudinal studies of children with ADHD consistently show that ADHD symptoms decrease with age. More specifically these studies demonstrated that the symptoms of hyperactivity and impulsivity decline over time, particularly during the transition from childhood to adolescence.8–12 However, it is unknown whether the same developmental trajectories of ADHD symptomatology also will be evident in youth with BP and ADHD. If ADHD and BP are two separate disorders, it is expected that the ADHD symptoms in youth with BP will follow similar developmental trajectories as in the children with only ADHD. Also, youth with ADHD show poor psychosocial functioning over time,11 but it is unknown whether the longitudinal course of global functioning will be the same for youth with BP and ADHD.
Some studies indicate offspring of parents with BP are at high risk for both ADHD and BP. Thus, high-risk family studies of offspring of individuals with BP are useful for studying the association between these two disorders.5,7 The majority of the existing high risk studies for BP report an elevated rate of ADHD among offspring of parents with BP compared to the children of parents with other psychiatric disorders or to those of healthy controls.13–15 For instance, in a longitudinal study of adolescent offspring of parents with BP and healthy parents, Duffy and colleagues recently reported an increased rate of ADHD in the offspring of parents with BP (10.2%) relative to the offspring of control parents (1.6%).16 Petresco and colleagues demonstrated that the offspring of bipolar mothers had higher prevalence of ADHD (11.6%) compared to the offspring of control mothers (4.7%).17 We also found high rates of ADHD in offspring of parents with BP and offspring with BP in the Pittsburgh Bipolar Offspring Study (BIOS).13,18 However, these studies were limited by one or more of the following limitations: lack of longitudinal prospective evaluation of the ADHD symptomatology, small sample sizes, not taking into account the effects of several factors that may have affected the outcome (e.g., socioeconomic status, pubertal status, and children’s comorbid disorders), and control groups consisting of offspring of healthy parents only.16,19–21 This last issue is important because we do not know whether the increased risk for ADHD in offspring of parents with BP is due to the parental BP or the parents’ non-BP psychopathology. In fact, in BIOS, the effects of ADHD were no longer significant after adjusting for both biological parents’ non-BP psychopathology.
Most of the above studies were cross-sectional and did not compare the longitudinal course of ADHD in large samples of offspring of parents with BP in comparison with offspring with ADHD of healthy or non-BP parents. Moreover, there is no evidence whether the phenomenology of ADHD in offspring of BP parents is similar to or different from that of the offspring of non-BP or healthy parents. Thus, in this paper we sought to compare these two large groups of offspring based on the following three characteristics: 1) lifetime prevalence of comorbid psychiatric disorders; 2) the severity and developmental course of ADHD symptomatology; and 3) changes in global functioning over time. Based on the literature, we first hypothesized that offspring with ADHD of BP parents would show higher lifetime prevalence of axis-I psychiatric disorders, particularly bipolar spectrum disorders, than offspring with ADHD of community control parents. Second, we hypothesized that the severity of symptoms of ADHD would decrease with age and that the symptom trajectories of the offspring with ADHD of BP parents would be different from the offspring with ADHD of control parents. Finally, considering the expected rates of psychiatric comorbidity, we hypothesized that offspring with ADHD of BP parents would show significantly more impairment in global functioning over time than offspring with ADHD of control parents.
METHOD
Subjects
The methods for BIOS have been described in detail elsewhere.13 Briefly, parents (probands) with BP were recruited through advertisement (53%), adult BP studies (31%), and outpatient clinics (16%) from November 2001 to July 2007. Parents were required to fulfill the DSM-IV criteria for bipolar I disorder (BP-I) or bipolar II disorder (BP-II). Exclusion criteria included current or lifetime diagnoses of schizophrenia, mental retardation, mood disorders secondary to substance abuse, medical conditions, or medications, and living more than 200 miles away from Pittsburgh. BIOS recruited 233 parents with BP and their 388 offspring aged 6–18 years. Among these offspring, 122 subjects (31.4%) were diagnosed with ADHD via semi-structured interview (see Measures, below).
Control parents consisted of healthy parents or parents with non-BP psychopathology from the community, group matched by age, sex, and neighborhood using the area code and first 3 digits of the telephone number and zip code of the parents with BP. The control parents had the same exclusion criteria as the parents with BP, but, in addition, they could not have BP or have a first degree relative with BP. BIOS recruited 143 control parents and their 251 offspring. Among these offspring, 48 subjects (19.1%) were diagnosed with ADHD.
For this paper, subjects were followed on average 6 years. The overall rate of retention was approximately 90%, with no significant differences between the two groups.
Measures
After Institutional Review Board (IRB) approval, consent was obtained from parents and assent from children. Parents were assessed for psychiatric disorders, family psychiatric history, and other demographic and clinical variables. Only instruments directly related to this paper will be discussed. DSM-IV lifetime psychiatric disorders for parents were ascertained through the Structured Clinical Interview-DSM-IV (SCID)22 plus the ADHD, disruptive behavior disorders (DBD) and separation anxiety disorder (SAD) sections from the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children, Present and Lifetime Version (K-SADS-PL).23 Socioeconomic status (SES) was ascertained using the Hollingshead scale.24
Parents were interviewed about their children and the children were directly interviewed for the presence of lifetime psychiatric disorders using the K-SADS-PL at intake and every other year.23 As per the K-SADS-PL instructions, mood symptoms that were also in common with other psychiatric disorders (e.g., hyperactivity) were not rated as present in the mood sections unless they intensified with the onset of abnormal mood. Comorbid diagnoses were not assigned if they occurred exclusively during a mood episode. All diagnoses were made using the DSM-IV criteria. However, since the DSM does not clearly define BP not otherwise specified (NOS), operationalized criteria for BP-NOS were utilized.6,25 BP-NOS was defined as the presence of clinically relevant BP symptoms that did not fulfill the DSM-IV criteria for BP-I or BP-II. In addition, subjects were required to have a minimum of elated mood plus 2 associated DSM-IV symptoms or irritable mood plus 3 DSM-IV associated symptoms, along with a change in the level of functioning, duration of a minimum of 4 hours within a 24-hour period, and at least 4 cumulative lifetime days meeting the criteria.25 Youth with BP-NOS show less severe clinical picture, but similar longitudinal course than youth with BP-I.4,25 Also, about 50% of youth with BP-NOS convert into BP-I or BP-II, supporting the hypothesis that BP-NOS is on a continuum with BP-I.4
Bachelors- or masters-level interviewers completed all assessments after intensive training for all instruments and after ≥80% agreement with a certified rater. The overall SCID and K-SADS-PL kappas for psychiatric disorders were ≥0.8.
The ADHD symptoms were ascertained at intake and every other year using the ADHD section of the K-SADS-PL and the Disruptive Behavior Disorder rating scale (DBD) parent version. The reliability and validity of the DBD have been established.26 In contrast to the K-SADS-PL, the DBD does not take into account other disorders and it is rated unfiltered (“rate what you see”).
In the K-SADS-PL, individual symptom items are rated on the Likert scales of 0 (no information), 1 (not present), 2 (subthreshold), and 3 (threshold). As per the K-SADS-PL instructions, children and their parents were first interviewed using the ADHD screening interview. This screen includes four ADHD items: “Difficulty sustaining attention on tasks or play activities”, “Easily distracted”, “Difficulty remaining seated”, and “Impulsivity”. If a child received a score of 3 on any of the above screen items, the K-SADS-PL supplement for ADHD was completed. There were significant correlations between the scores of the parent alone and the summary ratings of K-SADS-PL in each domain: inattention, hyperactivity, impulsivity and total ADHD scores (rs = 0.76–0.86, p-values ≤0.01). Therefore, for this paper, we used the current summary ratings of 18 DSM-IV ADHD symptoms for the analysis: 9-items for the inattention symptoms, 6-items for the hyperactivity symptoms, and 3-items for the impulsivity symptoms. The inattention, hyperactivity, and impulsivity scores were generated from the sums of inattention, hyperactivity, and impulsivity items, respectively, and the total score was generated from the sum of inattention, hyperactivity, and impulsivity items.
The DBD consists of 41 DSM-IV items (ADHD = 18, ODD = 8, CD = 15). Each item is rated on a four-point scale (0 = not at all, 1 = just a little, 2 = pretty much, 3 = very much). For this paper we only used the ADHD symptoms which are composed of 9 inattention, 6 hyperactivity, and 3 impulsivity items. The inattention, hyperactivity, impulsivity, and total scores were generated using the same method described above with respect to the K-SADS-PL ADHD scores. Parents of children aged 6 to 18 years completed the DBD at baseline and each follow-up assessment.
Global overall functioning was assessed by interviewers using the Children’s Global Assessment Scale (CGAS) at intake and every other year. The CGAS is measured in 3 domains: current (prior month before the interview), most severe past (lifetime) and highest past year. For this paper, we used the current functioning measure. The reliability and validity of the CGAS have been established.27
Statistical Analyses
Between-group demographic and clinical characteristics were evaluated using t, chi-square, and Fisher exact tests as appropriate. Effect sizes for continuous and categorical variables (d and h, respectively) were calculated as described by Cohen.28
Linear growth curve models were used to compare the K-SADS-PL and DBD ADHD symptoms between the two offspring groups. We also estimated the effect of age on the change of ADHD symptoms and whether these age effects differed across the groups. Because the response variables are not normally distributed, a generalized linear mixed model was used. Group, age and age by group interactions were kept in the model as fixed effects while intercept was set as a random effect. These analyses were repeated adjusting for between-group significant demographic characteristics and whether the offspring met criteria for lifetime bipolar spectrum disorder. All of the p-values are based on two-tailed tests. Statistical analyses were performed in SAS 9.3 and SPSS 20.
RESULTS
Demographic and Clinical Characteristics
A total of 122 offspring with ADHD of BP parents and 48 offspring with ADHD of control parents (38 from parents with non-bipolar psychopathology and 10 from healthy parents) were included for the analyses (Table 1). Subjects were followed on average 5.9±2.9 years. There was no between-group difference in the length of the follow-up time.
Table 1.
Demographic and clinical characteristics of ADHD offspring of parents with BP vs. ADHD offspring of control parents
| Characteristic | ADHD offspring of parents with BP (n=122) | ADHD offspring of control parents (n=48) | Statistic | P value (Effect Size) |
|---|---|---|---|---|
|
| ||||
| Demographic | ||||
| Age at intake, mean (SD) years | 11.0 (3.4) | 11.2 (3.5) | t=0.29 | 0.78 (0.06) |
| Age at the last assessment, mean (SD) | 16.8 (4.4) | 16.8 (4.4) | t=0.01 | >0.9 (0.01) |
| Had at least 1 F/U assessment, n (%) | 109 (89.3%) | 43 (89.6%) | χ2= 0.05 | >0.9 (0.01) |
| Number of F/U assessments, mean (SD) | 3.6 (1.4) | 3.4 (1.2) | t=0.65 | 0.50 (0.15) |
| Years of F/U, mean (SD) | 6.0 (2.8) | 5.5 (2.8) | t=1.04 | 0.30 (0.18) |
| Female, n (%) | 52 (42.6%) | 18 (37.5%) | χ2= 0.37 | 0.54 (0.10) |
| Caucasian, n (%) | 93 (76.2%) | 29 (60.4%) | χ2= 4.25 | 0.04 (0.34) |
| Living with both biological parents, n (%) | 43 (35.2%) | 19 (39.6%) | χ2=0.28 | 0.60 (0.09) |
| SES, mean (SD) | 31.4 (13.9) | 31.1 (12.2) | t=0.14 | 0.89 (0.02) |
| Lifetime ADHD diagnosis of parents, n (%) | 38 (31.1%) | 2 (4.2%) | FET | <0.001 (0.77) |
| Lifetime stimulant exposure, n (%) | 73 (59.8%) | 23 (47.9%) | χ2=1.54 | 0.16 (0.24) |
| ADHD symptoms at intake, mean (SD) | ||||
| DBD | ||||
| Inattention | 17.0 (7.6) | 13.1 (6.4) | t=3.02 | 0.003 (0.54) |
| Hyperactivity | 8.6 (4.9) | 5.9 (4.4) | t=3.29 | 0.001 (0.57) |
| Impulsivity | 4.4 (2.9) | 3.6 (2.5) | t=1.68 | 0.09 (0.29) |
| Total | 30.1 (13.3) | 22.6 (11.4) | t=3.32 | 0.001 (0.59) |
| K-SADS-PL | ||||
| Inattention | 22.5 (4.6) | 22.1 (3.8) | t=0.52 | 0.60 (0.09) |
| Hyperactivity | 13.1 (3.6) | 12.4 (3.9) | t=0.98 | 0.33 (0.19) |
| Impulsivity | 6.4 (2.2) | 6.6 (2.1) | t=0.30 | 0.77 (0.09) |
| Total | 42.0 (8.2) | 41.0 (6.5) | t=0.68 | 0.50 (0.13) |
ADHD, attention deficit hyperactivity disorder; BP, bipolar disorder; DBD, disruptive behavior disorder rating scale; FET, Fisher exact test; F/U, follow-up; K-SADS-PL, Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version; SES, socioeconomic status
The offspring with ADHD of BP parents were more frequently Caucasian (p=0.04) and their parents showed greater lifetime (intake and during the follow-up) prevalence of ADHD relative to the offspring with ADHD of control parents (31.1% vs. 4.2%, p<0.001). There were no other between-group demographic differences including socioeconomic status and lifetime stimulant exposure.
With respect to the ADHD symptoms at intake, there were no between-group differences in the K-SADS-PL ADHD scores. The DBD inattention, hyperactivity, and total ADHD scores were significantly higher in the offspring with ADHD of BP parents relative to the offspring with ADHD of control parents (p-values ≤0.003).
Lifetime Comorbid Axis I Disorders
As shown in Table 2, the offspring with ADHD of BP parents showed significantly higher lifetime (intake and during the follow-up) prevalence of bipolar spectrum disorders, any depression, any anxiety disorders, and elimination disorders relative to the offspring with ADHD of control parents (p values, ≤0.05; effect sizes, 0.39–0.74). The increased rates of bipolar spectrum disorders and any depression in the offspring with ADHD of BP parents were accounted for by significantly higher rate of BP-NOS (p=0.003; effect size, 0.61; odds ratio [OR] = 10.9; 95% confidence interval [CI] = 1.4–83.3) and major depressive disorder (p=0.02; effect size, 0.42; OR = 2.8; 95% CI = 1.1–6.7), respectively. The offspring with ADHD of BP parents also showed greater lifetime prevalence of enuresis than the offspring with ADHD of control parents (p=0.03; effect size, 0.42; OR = 3.1; 95% CI = 1.1–8.4).
Table 2.
Lifetime axis I psychiatric disorders of ADHD offspring of parents with BP vs. ADHD offspring of community control parents
| Lifetime axis I psychiatric disorder, n (%) | ADHD offspring of parents with BP (n=122) | ADHD offspring of control parents (n=48) | Statistic | P value (Effect Size) |
|---|---|---|---|---|
|
| ||||
| Bipolar spectrum disorders | 36 (29.5%) | 2 (4.2%) | FET | <0.001 (0.74) |
| BP-I | 9 (7.4%) | 0 (0.0%) | FET | 0.06 (0.55) |
| BP-II | 4 (3.3%) | 1 (2.1%) | FET | >0.9 (0.07) |
| BP-NOS | 23 (18.9%) | 1 (2.1%) | FET | 0.003 (0.61) |
| Any depression | 69 (56.6%) | 13 (27.1%) | χ2= 11.99 | 0.001 (0.61) |
| Dysthymic disorder | 6 (4.9%) | 0 (0.0%) | FET | 0.19 (0.45) |
| Major depressive disorder | 39 (32.0%) | 7 (14.6%) | χ2=5.27 | 0.02 (0.42) |
| Any anxiety disorders | 61 (50.0%) | 15 (31.2%) | χ2=4.90 | 0.03 (0.39) |
| Generalized anxiety disorder | 24 (19.7%) | 4 (8.3%) | FET | 0.11 (0.34) |
| Separation anxiety disorder | 28 (23.0%) | 8 (16.7%) | χ2=0.82 | 0.37 (0.16) |
| Social phobia | 15 (12.3%) | 7 (14.6%) | χ2=0.16 | 0.69 (0.07) |
| Specific phobia | 28 (23.0%) | 7 (14.6%) | χ2=1.48 | 0.23 (0.22) |
| Panic disorder | 11 (9.0%) | 1 (2.1%) | FET | 0.18 (0.32) |
| Obsessive-compulsive disorder | 8 (6.6%) | 2 (4.2%) | FET | 0.73 (0.11) |
| Posttraumatic stress disorder | 10 (8.2%) | 5 (10.4%) | χ2=0.21 | 0.65 (0.08) |
| Disruptive behavior disorders | 63 (51.6%) | 18 (37.5%) | χ2=2.76 | 0.10 (0.28) |
| Oppositional defiant disorder | 60 (49.2%) | 17 (35.4%) | χ2=2.63 | 0.11 (0.28) |
| Conduct disorder | 23 (18.9%) | 7 (14.6%) | χ2=0.43 | 0.51 (0.12) |
| Tic disorders | 5 (4.1%) | 2 (4.2%) | FET | >0.9 (0.01) |
| Transient tic disorder | 1 (0.8%) | 1 (2.1%) | FET | 0.49 (0.11) |
| Chronic tic disorder | 2 (1.6%) | 2 (4.2%) | FET | 0.32 (0.16) |
| Tourette’s disorder | 2 (1.6%) | 0 (0.0%) | FET | >0.9 (0.25) |
| Elimination disorders | 34 (27.9%) | 6 (12.5%) | χ2=4.52 | 0.03 (0.39) |
| Enuresis | 32 (26.2%) | 5 (10.4%) | χ2=5.06 | 0.03 (0.42) |
| Encopresis | 6 (4.9%) | 1 (2.1%) | FET | 0.68 (0.16) |
| Substance use disorders | 21 (17.2%) | 6 (12.5%) | χ2=0.57 | 0.45 (0.13) |
ADHD, attention deficit hyperactivity disorder; BP, bipolar disorder; FET, Fisher exact test; NOS, not otherwise specified
Severity and Developmental Course of ADHD Symptomatology
K-SADS-PL (Figure 1). For both groups of offspring, the hyperactivity, impulsivity, and total ADHD scores significantly decreased over time (all p-values <0.001). There were no significant between-group differences in any of these scores. Similar results were obtained after adjusting for offspring’s race and lifetime bipolar spectrum disorders, and parents’ lifetime ADHD. There were no between- or within-group statistical differences in the ADHD inattention scores over time.
Figure 1.
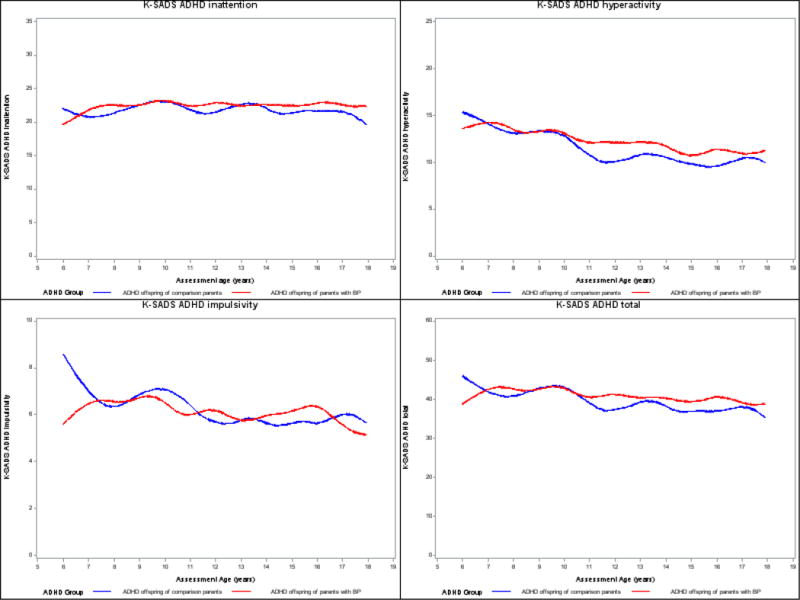
Developmental trajectories of ADHD symptoms based on K-SADS-PL. Y-axis: Lines represent observed means on ADHD symptoms over time.
ADHD, attention deficit hyperactivity disorder; BP, bipolar disorder; K-SADS-PL, Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children, Present and Lifetime Version
DBD (Figure 2). For both groups of offspring, the inattention, hyperactivity, impulsivity, and total ADHD scores significantly decreased with age (all p-values <0.002). There were no between-group differences in any of these scores. Similar results were obtained after adjusting for offspring’s race and lifetime bipolar spectrum disorders, and parents’ lifetime ADHD.
Figure 2.
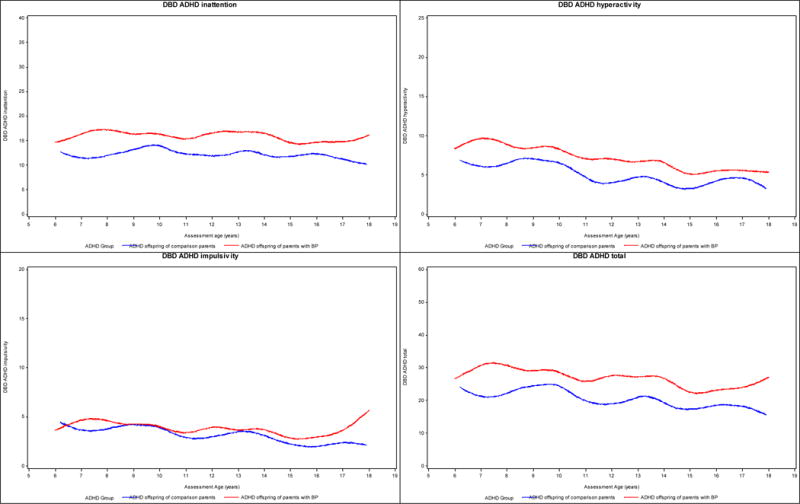
Developmental trajectories of ADHD symptoms based on DBD. Y-axis: Lines represent observed means on ADHD symptoms over time.
ADHD, attention deficit hyperactivity disorder; BP, bipolar disorder; DBD, disruptive behavior disorder rating scale
Longitudinal Course of Global Functioning
CGAS (Figure 3). For both groups of offspring, there were no between- or within-group statistical differences in the CGAS scores over time. Although not statistically significant, the CGAS scores were consistently lower over time in the offspring with ADHD of BP parents relative to the offspring with ADHD of control parents.
Figure 3.
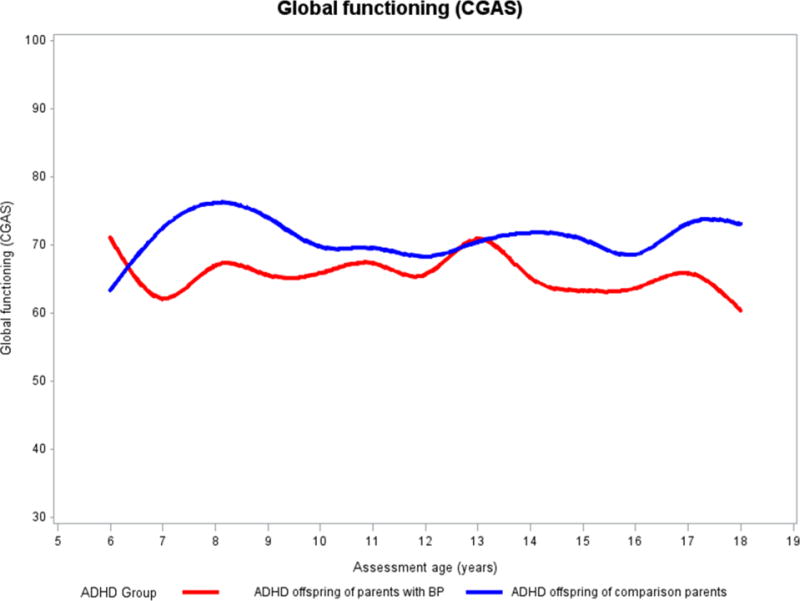
Developmental trajectories of global functioning based on CGAS. Y-axis: Lines represent observed means on CGAS scores over time.
ADHD, attention deficit hyperactivity disorder; BP, bipolar disorder; CGAS, Children’s Global Assessment Scale
DISCUSSION
To our knowledge, the present study is the first to compare lifetime psychiatric comorbidity rates and longitudinal clinical course of ADHD in offspring of BP parents with offspring with ADHD of community control parents. As compared with the offspring with ADHD of control parents, the offspring with ADHD of BP parents demonstrated more severe ADHD symptomatology at intake and higher lifetime prevalence of bipolar spectrum disorders, depression, anxiety disorders, and elimination disorders. For both groups of offspring, the hyperactivity, impulsivity, and total ADHD scores ascertained through the K-SADS-PL and DBD equally decreased over time. Also, there were no differences in the global functioning over time.
Before discussing the above results in detail, it is important to consider the limitations of this study. Similar to other studies, the main informants were the mothers. In addition, the psychopathology in the biological co-parents was mostly ascertained by interviewing the main informant. However, there were no differences between the bipolar and control parent groups in the rate of mothers serving as main informants and in the proportion of direct and indirect interviews of biological co-parents in BIOS.13 Given that this is a high-risk study for BP, the results may not be generalizable to other populations. Finally, collateral information (e.g., teachers) regarding ADHD symptomatology were not measured.
As expected and consistent with previous findings,2,29,30 our analyses showed that ADHD was associated with a broad range of lifetime axis I psychiatric disorders in both groups of offspring. For example, in large longitudinal studies, Biederman and colleagues showed that youth with ADHD had elevated risk of lifetime mood and anxiety disorders, and disruptive behavior disorders as compared with controls.29,30
The offspring with ADHD of BP parents did not show significantly higher lifetime prevalence of BP-I relative to the offspring with ADHD of control parents. This finding is in line with a recent review of prospective high-risk studies of the offspring of parents with BP, which showed that ADHD does not appear to increase the risk for BP.31 However, using a strict definition of BP-NOS, we found a significantly higher rate of this subtype of BP in the offspring with ADHD of BP parents than in the offspring with ADHD of control parents, suggesting the possibility of a significant relationship between ADHD and BP.
It is important to note that the DBD ADHD scores at intake were higher in the offspring with ADHD of BP parents relative to the offspring with ADHD of control parents. However, there were no between-group differences in the K-SADS-PL ADHD scores. The discrepancy in these results might be accounted for by the way ADHD symptoms were ascertained. The K-SADS-PL symptoms were based on summary ratings that include information by parents, children, the interviewers, and the child psychiatrist who supervised the case. In contrast, the DBD questionnaire was only based on information by parents.
With respect to continuity and change in ADHD symptomatology over time, our results are consistent with the findings of previous studies that symptoms of ADHD decrease with age. In Hart and colleagues’ study,9 hyperactive and impulsive symptoms of ADHD significantly declined with age, whereas inattentive symptoms did not. Biederman and colleagues10 demonstrated that hyperactivity, impulsivity, and inattention symptoms decreased with age. It is worth noting that above-noted studies were based on clinic samples, and symptom trajectory studies of ADHD using non-referred samples are limited.
Contrary to our hypothesis, ADHD symptoms ascertained through the K-SADS-PL and DBD did not differ over time between the offspring with ADHD of BP parents and the offspring with ADHD of control parents. Similar results were obtained after controlling for a range of possible confounding variables such as offspring’s race and lifetime bipolar spectrum disorders, and parents’ lifetime ADHD. There are no other studies of this nature in the literature to compare our results. Thus, further replication studies are necessary.
Finally, with respect to the longitudinal course of global functioning, although there were no statistically significant differences between the offspring with ADHD of BP parents relative to the offspring with ADHD of control parents, the CGAS scores were consistently lower over time in the offspring with ADHD of BP parents. This may be accounted for by the higher lifetime prevalence of comorbid psychiatric disorders in the latter offspring group. Of note, the CGAS scores were generally between 60 and 70 in both groups of offspring over time, which indicates that both groups experienced functional impairments in one or more areas at baseline and follow-up assessments, although the severity of ADHD symptoms decreased over time.
In summary, the results of this study indicate that ADHD in offspring of BP parents is associated with more severe ADHD symptomatology at intake and higher lifetime prevalence of a broad range of axis I psychiatric disorders, including mood and anxiety disorders, relative to offspring with ADHD of community control parents. However, after taking into account confounding factors, our results do not suggest that there are differences in the developmental course of ADHD symptomatology between these two groups of offspring. Further longitudinal studies are warranted to evaluate whether there are neurobiological, cognitive, and treatment response differences between offspring with ADHD of parents with and without BP.
Clinical points.
Longitudinal prospective studies of offspring of parents with BP are useful to study the association between ADHD and BP.
ADHD in offspring of BP parents showed more psychopathology relative to offspring with ADHD of control parents, but there were no between-group differences in the developmental courses of ADHD symptomatology over time.
The developmental trajectories of ADHD symptoms in the offspring with ADHD appear not to be influenced by history of parental BP or lifetime prevalence of bipolar spectrum disorders in the offspring.
Acknowledgments
The authors thank Mary Kay Gill, M.S.N., at the Department of Psychiatry, University of Pittsburgh School of Medicine, for the assistance with manuscript preparation.
This research was supported by National Institute of Mental Health (NIMH) grant MH60952 (principal investigator, Dr. Birmaher). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of NIMH or the National Institutes of Health.
Footnotes
Ms. Gill reports no biomedical financial interests or potential conflicts of interest.
This work was presented in part at the 60th Annual Meeting of the American Academy of Child and Adolescent Psychiatry, Orlando, October 2013.
Disclosure: Dr. T. Goldstein has received research support from NIMH, National Institute on Drug Abuse (NIDA), National Institute of Child Health and Human Development (NICHD), The Find Foundation, The Ryan Licht Sang Foundation, and The Pittsburgh Foundation, and royalties from Guilford Press. Dr. Birmaher has received grant or research support from NIMH, and has received or will receive royalties for publications from Random House, Inc., Lippincott Williams and Wilkins, and Up to Date. Drs. Kim, Ryan, Axelson, B. Goldstein, Diler, and Sakolsky, and Mr. Yu, Ms. Monk, Ms. Hickey, and Mr. Merranko report no biomedical financial interests or potential conflicts of interest.
References
- 1.Faraone SV, Biederman J, Wozniak J. Examining the comorbidity between attention deficit hyperactivity disorder and bipolar I disorder: a meta-analysis of family genetic studies. Am J Psychiatry. 2012 Dec 1;169(12):1256–1266. doi: 10.1176/appi.ajp.2012.12010087. [DOI] [PubMed] [Google Scholar]
- 2.Kessler RC, Avenevoli S, McLaughlin KA, et al. Lifetime co-morbidity of DSM-IV disorders in the US National Comorbidity Survey Replication Adolescent Supplement (NCS-A) Psychol Med. 2012 Sep;42(9):1997–2010. doi: 10.1017/S0033291712000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kowatch RA, Youngstrom EA, Danielyan A, Findling RL. Review and meta-analysis of the phenomenology and clinical characteristics of mania in children and adolescents. Bipolar Disord. 2005 Dec;7(6):483–496. doi: 10.1111/j.1399-5618.2005.00261.x. [DOI] [PubMed] [Google Scholar]
- 4.Axelson DA, Birmaher B, Strober MA, et al. Course of subthreshold bipolar disorder in youth: diagnostic progression from bipolar disorder not otherwise specified. J Am Acad Child Adolesc Psychiatry. 2011 Oct;50(10):1001–1016 e1003. doi: 10.1016/j.jaac.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skirrow C, Hosang GM, Farmer AE, Asherson P. An update on the debated association between ADHD and bipolar disorder across the lifespan. J Affect Disord. 2012 Dec 10;141(2–3):143–159. doi: 10.1016/j.jad.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 6.Axelson D, Birmaher B, Strober M, et al. Phenomenology of children and adolescents with bipolar spectrum disorders. Arch Gen Psychiatry. 2006 Oct;63(10):1139–1148. doi: 10.1001/archpsyc.63.10.1139. [DOI] [PubMed] [Google Scholar]
- 7.Singh MK, DelBello MP, Kowatch RA, Strakowski SM. Co-occurrence of bipolar and attention-deficit hyperactivity disorders in children. Bipolar Disord. 2006 Dec;8(6):710–720. doi: 10.1111/j.1399-5618.2006.00391.x. [DOI] [PubMed] [Google Scholar]
- 8.Mannuzza S, Klein RG, Bonagura N, Malloy P, Giampino TL, Addalli KA. Hyperactive boys almost grown up. V. Replication of psychiatric status. Arch Gen Psychiatry. 1991 Jan;48(1):77–83. doi: 10.1001/archpsyc.1991.01810250079012. [DOI] [PubMed] [Google Scholar]
- 9.Hart EL, Lahey BB, Loeber R, Applegate B, Frick PJ. Developmental change in attention-deficit hyperactivity disorder in boys: a four-year longitudinal study. J Abnorm Child Psychol. 1995 Dec;23(6):729–749. doi: 10.1007/BF01447474. [DOI] [PubMed] [Google Scholar]
- 10.Biederman J, Mick E, Faraone SV. Age-dependent decline of symptoms of attention deficit hyperactivity disorder: impact of remission definition and symptom type. Am J Psychiatry. 2000 May;157(5):816–818. doi: 10.1176/appi.ajp.157.5.816. [DOI] [PubMed] [Google Scholar]
- 11.Mick E, Faraone SV, Biederman J. Age-dependent expression of attention-deficit/hyperactivity disorder symptoms. Psychiatr Clin North Am. 2004 Jun;27(2):215–224. doi: 10.1016/j.psc.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 12.Molina BS, Hinshaw SP, Swanson JM, et al. The MTA at 8 years: prospective follow-up of children treated for combined-type ADHD in a multisite study. J Am Acad Child Adolesc Psychiatry. 2009 May;48(5):484–500. doi: 10.1097/CHI.0b013e31819c23d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Birmaher B, Axelson D, Monk K, et al. Lifetime psychiatric disorders in school-aged offspring of parents with bipolar disorder: the Pittsburgh Bipolar Offspring study. Arch Gen Psychiatry. 2009 Mar;66(3):287–296. doi: 10.1001/archgenpsychiatry.2008.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Birmaher B, Axelson D, Goldstein B, et al. Psychiatric disorders in preschool offspring of parents with bipolar disorder: the Pittsburgh Bipolar Offspring Study (BIOS) Am J Psychiatry. 2010 Mar;167(3):321–330. doi: 10.1176/appi.ajp.2009.09070977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duffy A. The early natural history of bipolar disorder: what we have learned from longitudinal high-risk research. Can J Psychiatry. 2010 Aug;55(8):477–485. doi: 10.1177/070674371005500802. [DOI] [PubMed] [Google Scholar]
- 16.Duffy A, Alda M, Crawford L, Milin R, Grof P. The early manifestations of bipolar disorder: a longitudinal prospective study of the offspring of bipolar parents. Bipolar Disord. 2007 Dec;9(8):828–838. doi: 10.1111/j.1399-5618.2007.00421.x. [DOI] [PubMed] [Google Scholar]
- 17.Petresco S, Gutt EK, Krelling R, Lotufo Neto F, Rohde LA, Moreno RA. The prevalence of psychopathology in offspring of bipolar women from a Brazilian tertiary center. Rev Bras Psiquiatr. 2009 Sep;31(3):240–246. doi: 10.1590/s1516-44462009000300009. [DOI] [PubMed] [Google Scholar]
- 18.Goldstein BI, Shamseddeen W, Axelson DA, et al. Clinical, demographic, and familial correlates of bipolar spectrum disorders among offspring of parents with bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2010 Apr;49(4):388–396. [PMC free article] [PubMed] [Google Scholar]
- 19.Duffy A, Grof P, Kutcher S, Robertson C, Alda M. Measures of attention and hyperactivity symptoms in a high-risk sample of children of bipolar parents. J Affect Disord. 2001 Dec;67(1–3):159–165. doi: 10.1016/s0165-0327(01)00391-3. [DOI] [PubMed] [Google Scholar]
- 20.Gotlib IH, Traill SK, Montoya RL, Joormann J, Chang K. Attention and memory biases in the offspring of parents with bipolar disorder: indications from a pilot study. J Child Psychol Psychiatry. 2005 Jan;46(1):84–93. doi: 10.1111/j.1469-7610.2004.00333.x. [DOI] [PubMed] [Google Scholar]
- 21.Garcia-Amador M, de la Serna E, Vila M, et al. Parents with bipolar disorder: Are disease characteristics good predictors of psychopathology in offspring? Eur Psychiatry. 2013 May;28(4):240–246. doi: 10.1016/j.eurpsy.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 22.Spitzer RL, Williams JB, Gibbon M, First MB. The Structured Clinical Interview for DSM-III-R (SCID). I: History, rationale, and description. Arch Gen Psychiatry. 1992 Aug;49(8):624–629. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- 23.Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997 Jul;36(7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 24.Hollingshead AB, editor. Index of social status. Minneapolis: University of Minnesota Press; 1982. [Google Scholar]
- 25.Birmaher B, Axelson D, Strober M, et al. Clinical course of children and adolescents with bipolar spectrum disorders. Arch Gen Psychiatry. 2006 Feb;63(2):175–183. doi: 10.1001/archpsyc.63.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Silva RR, Alpert M, Pouget E, et al. A rating scale for disruptive behavior disorders, based on the DSM-IV item pool. Psychiatr Q. 2005 Winter;76(4):327–339. doi: 10.1007/s11126-005-4966-x. [DOI] [PubMed] [Google Scholar]
- 27.Shaffer D, Gould MS, Brasic J, et al. A children’s global assessment scale (CGAS) Arch Gen Psychiatry. 1983 Nov;40(11):1228–1231. doi: 10.1001/archpsyc.1983.01790100074010. [DOI] [PubMed] [Google Scholar]
- 28.Cohen J, editor. Statistical Power Analysis for the Behavioral Sciences. 2nd. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 29.Biederman J, Petty CR, Woodworth KY, Lomedico A, Hyder LL, Faraone SV. Adult outcome of attention-deficit/hyperactivity disorder: a controlled 16-year follow-up study. J Clin Psychiatry. 2012 Jul;73(7):941–950. doi: 10.4088/JCP.11m07529. [DOI] [PubMed] [Google Scholar]
- 30.Biederman J, Petty CR, Monuteaux MC, et al. Adult psychiatric outcomes of girls with attention deficit hyperactivity disorder: 11-year follow-up in a longitudinal case-control study. Am J Psychiatry. 2010 Apr;167(4):409–417. doi: 10.1176/appi.ajp.2009.09050736. [DOI] [PubMed] [Google Scholar]
- 31.Duffy A. The nature of the association between childhood ADHD and the development of bipolar disorder: a review of prospective high-risk studies. Am J Psychiatry. 2012 Dec 1;169(12):1247–1255. doi: 10.1176/appi.ajp.2012.11111725. [DOI] [PubMed] [Google Scholar]


