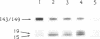Abstract
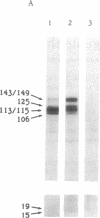
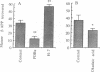
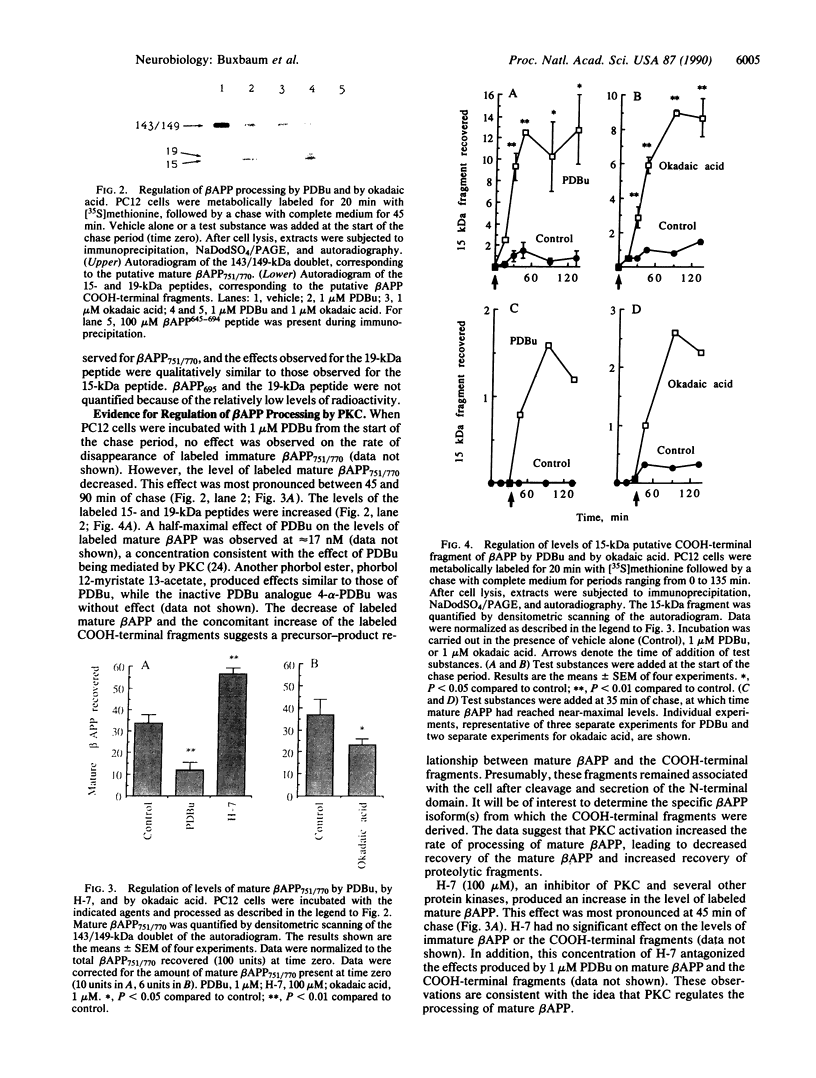
The turnover and processing of the Alzheimer beta/A4 amyloid precursor protein (beta APP) has been studied in PC12 cells after treatment with agents that regulate protein phosphorylation. Phorbol 12,13-dibutyrate, an agent that stimulates protein kinase C, decreased the levels of mature beta APP and increased the levels of 15- and 19-kDa peptides. These peptides appeared to be COOH-terminal fragments of beta APP, which arose when phorbol 12,13-dibutyrate increased the rate of proteolytic processing of mature forms of beta APP. Okadaic acid, an inhibitor of protein phosphatases 1 and 2A, also led to decreased levels of mature beta APP and increased levels of the 15- and 19-kDa peptides. H-7, an inhibitor of protein kinase C and of several other protein kinases, apparently decreased the rate of proteolytic processing of mature beta APP. The sizes of the putative COOH-terminal fragments observed after treatment with either phorbol 12,13-dibutyrate or okadaic acid suggest that one or both may contain the entire beta/A4 region of beta APP and thus be amyloidogenic. Our results support the hypothesis that abnormal protein phosphorylation may play a role in the development of the cerebral amyloidosis that accompanies Alzheimer disease.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baudier J., Cole R. D. Phosphorylation of tau proteins to a state like that in Alzheimer's brain is catalyzed by a calcium/calmodulin-dependent kinase and modulated by phospholipids. J Biol Chem. 1987 Dec 25;262(36):17577–17583. [PubMed] [Google Scholar]
- Beguinot L., Hanover J. A., Ito S., Richert N. D., Willingham M. C., Pastan I. Phorbol esters induce transient internalization without degradation of unoccupied epidermal growth factor receptors. Proc Natl Acad Sci U S A. 1985 May;82(9):2774–2778. doi: 10.1073/pnas.82.9.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P., Holmes C. F., Tsukitani Y. Okadaic acid: a new probe for the study of cellular regulation. Trends Biochem Sci. 1990 Mar;15(3):98–102. doi: 10.1016/0968-0004(90)90192-e. [DOI] [PubMed] [Google Scholar]
- Cole G., Dobkins K. R., Hansen L. A., Terry R. D., Saitoh T. Decreased levels of protein kinase C in Alzheimer brain. Brain Res. 1988 Jun 14;452(1-2):165–174. doi: 10.1016/0006-8993(88)90021-2. [DOI] [PubMed] [Google Scholar]
- Esch F. S., Keim P. S., Beattie E. C., Blacher R. W., Culwell A. R., Oltersdorf T., McClure D., Ward P. J. Cleavage of amyloid beta peptide during constitutive processing of its precursor. Science. 1990 Jun 1;248(4959):1122–1124. doi: 10.1126/science.2111583. [DOI] [PubMed] [Google Scholar]
- Gallis B., Lewis A., Wignall J., Alpert A., Mochizuki D. Y., Cosman D., Hopp T., Urdal D. Phosphorylation of the human interleukin-2 receptor and a synthetic peptide identical to its C-terminal, cytoplasmic domain. J Biol Chem. 1986 Apr 15;261(11):5075–5080. [PubMed] [Google Scholar]
- Gandy S., Czernik A. J., Greengard P. Phosphorylation of Alzheimer disease amyloid precursor peptide by protein kinase C and Ca2+/calmodulin-dependent protein kinase II. Proc Natl Acad Sci U S A. 1988 Aug;85(16):6218–6221. doi: 10.1073/pnas.85.16.6218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldgaber D., Lerman M. I., McBride O. W., Saffiotti U., Gajdusek D. C. Characterization and chromosomal localization of a cDNA encoding brain amyloid of Alzheimer's disease. Science. 1987 Feb 20;235(4791):877–880. doi: 10.1126/science.3810169. [DOI] [PubMed] [Google Scholar]
- Huttner W. B., Greengard P. Multiple phosphorylation sites in protein I and their differential regulation by cyclic AMP and calcium. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5402–5406. doi: 10.1073/pnas.76.10.5402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J., Lemaire H. G., Unterbeck A., Salbaum J. M., Masters C. L., Grzeschik K. H., Multhaup G., Beyreuther K., Müller-Hill B. The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987 Feb 19;325(6106):733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- Kitaguchi N., Takahashi Y., Tokushima Y., Shiojiri S., Ito H. Novel precursor of Alzheimer's disease amyloid protein shows protease inhibitory activity. Nature. 1988 Feb 11;331(6156):530–532. doi: 10.1038/331530a0. [DOI] [PubMed] [Google Scholar]
- Lin C. R., Chen W. S., Lazar C. S., Carpenter C. D., Gill G. N., Evans R. M., Rosenfeld M. G. Protein kinase C phosphorylation at Thr 654 of the unoccupied EGF receptor and EGF binding regulate functional receptor loss by independent mechanisms. Cell. 1986 Mar 28;44(6):839–848. doi: 10.1016/0092-8674(86)90006-1. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Oltersdorf T., Ward P. J., Henriksson T., Beattie E. C., Neve R., Lieberburg I., Fritz L. C. The Alzheimer amyloid precursor protein. Identification of a stable intermediate in the biosynthetic/degradative pathway. J Biol Chem. 1990 Mar 15;265(8):4492–4497. [PubMed] [Google Scholar]
- Pang D. T., Wang J. K., Valtorta F., Benfenati F., Greengard P. Protein tyrosine phosphorylation in synaptic vesicles. Proc Natl Acad Sci U S A. 1988 Feb;85(3):762–766. doi: 10.1073/pnas.85.3.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponte P., Gonzalez-DeWhitt P., Schilling J., Miller J., Hsu D., Greenberg B., Davis K., Wallace W., Lieberburg I., Fuller F. A new A4 amyloid mRNA contains a domain homologous to serine proteinase inhibitors. Nature. 1988 Feb 11;331(6156):525–527. doi: 10.1038/331525a0. [DOI] [PubMed] [Google Scholar]
- Robakis N. K., Ramakrishna N., Wolfe G., Wisniewski H. M. Molecular cloning and characterization of a cDNA encoding the cerebrovascular and the neuritic plaque amyloid peptides. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4190–4194. doi: 10.1073/pnas.84.12.4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackelford D. A., Trowbridge I. S. Identification of lymphocyte integral membrane proteins as substrates for protein kinase C. Phosphorylation of the interleukin-2 receptor, class I HLA antigens, and T200 glycoprotein. J Biol Chem. 1986 Jun 25;261(18):8334–8341. [PubMed] [Google Scholar]
- Shivers B. D., Hilbich C., Multhaup G., Salbaum M., Beyreuther K., Seeburg P. H. Alzheimer's disease amyloidogenic glycoprotein: expression pattern in rat brain suggests a role in cell contact. EMBO J. 1988 May;7(5):1365–1370. doi: 10.1002/j.1460-2075.1988.tb02952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisodia S. S., Koo E. H., Beyreuther K., Unterbeck A., Price D. L. Evidence that beta-amyloid protein in Alzheimer's disease is not derived by normal processing. Science. 1990 Apr 27;248(4954):492–495. doi: 10.1126/science.1691865. [DOI] [PubMed] [Google Scholar]
- Sternberger N. H., Sternberger L. A., Ulrich J. Aberrant neurofilament phosphorylation in Alzheimer disease. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4274–4276. doi: 10.1073/pnas.82.12.4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzi R. E., Gusella J. F., Watkins P. C., Bruns G. A., St George-Hyslop P., Van Keuren M. L., Patterson D., Pagan S., Kurnit D. M., Neve R. L. Amyloid beta protein gene: cDNA, mRNA distribution, and genetic linkage near the Alzheimer locus. Science. 1987 Feb 20;235(4791):880–884. doi: 10.1126/science.2949367. [DOI] [PubMed] [Google Scholar]
- Tanzi R. E., McClatchey A. I., Lamperti E. D., Villa-Komaroff L., Gusella J. F., Neve R. L. Protease inhibitor domain encoded by an amyloid protein precursor mRNA associated with Alzheimer's disease. Nature. 1988 Feb 11;331(6156):528–530. doi: 10.1038/331528a0. [DOI] [PubMed] [Google Scholar]
- Van Huynh T., Cole G., Katzman R., Huang K. P., Saitoh T. Reduced protein kinase C immunoreactivity and altered protein phosphorylation in Alzheimer's disease fibroblasts. Arch Neurol. 1989 Nov;46(11):1195–1199. doi: 10.1001/archneur.1989.00520470049026. [DOI] [PubMed] [Google Scholar]
- Weidemann A., König G., Bunke D., Fischer P., Salbaum J. M., Masters C. L., Beyreuther K. Identification, biogenesis, and localization of precursors of Alzheimer's disease A4 amyloid protein. Cell. 1989 Apr 7;57(1):115–126. doi: 10.1016/0092-8674(89)90177-3. [DOI] [PubMed] [Google Scholar]