Abstract
Immunotherapy for metastatic melanoma has a decades-long history, and the relatively recent use of checkpoint inhibitors has revolutionized treatment. Durable, and sometimes complete, remission of metastatic melanoma is now achievable in some patients receiving checkpoint-blocking therapy. However, it is unclear why some patients fare better than others. This review highlights several molecular indicators of response to checkpoint inhibition in metastatic melanoma, focusing on tumor PDL1 expression, MHC I expression, mutational load in the tumor, and T cell infiltration in the tumor. Additionally, clinical correlates of response, notably vitiligo and other immune-related adverse events, can potentially shed light on the mechanisms by which checkpoint blockade may achieve such great success, particularly in melanoma. We propose that MITF – a key regulator of melanocyte survival, melanin production, and melanoma transformation – produces a molecular landscape in melanocytes and melanoma cells that can make melanomas particularly susceptible to checkpoint blockade and also that can also result in immune attack on normal melanocytes.
Concise abstract
Several molecular and cellular correlates of melanoma response to checkpoint inhibition have been described, notably tumor PDL1 expression, MHC I expression, mutational load and T cell infiltration. Further considering the clinical correlation to vitiligo suggests a potential mechanistic link to MITF, a transcription factor important in the development of the melanocyte lineage and in survival of melanocytes.
I. Introduction
Once one of the most lethal types of cancer, metastatic melanoma can now be controlled, with long term major responses that are hopefully cures, in significant subsets of patients, using novel immunotherapies. It has been known for more than half a century that melanoma can sometimes regress spontaneously. Even at the time of these early reports, it was clear that the regression was immune-mediated1 but unclear why regression could happen spontaneously in some patients but not others. Starting decades ago, attempts were made to manipulate the immune system in order to achieve more immune-mediated regressions. Original approaches achieved only marginal success and were sometimes toxic to the patient. For example, in a decades-old strategy, bacille Calmette-Guérin (BCG) was injected as an adjuvant at a melanoma region to induce an immune response2. Later, high-dose IL-2 was used as a general immune activator to induce tumor killing3,4. The IL2-elicited immune response achieved a cancer response in a minority of patients, some of whom had durable responses. More recent advances have made it possible to induce a similar immune-mediated disappearance of melanoma metastases in higher fractions of patients. A more targeted approach, rather than inciting a pro-inflammatory milieu at the site of a tumor, takes advantage of endogenous adaptive immunity against transformed cells: the immune system is capable of carrying out cancer surveillance. Immune checkpoint blockade has proven to be an incredibly powerful technique of co-opting the adaptive immune system to attack tumor cells. Many metastatic melanoma patients can be saved, but the problem remains that many other patients’ cancers relapse or remain refractory to immunotherapy. Just as in the early studies of immune-induced clearance of melanoma, it is largely unclear what mechanistically determines response and resistance, though some significant clues are beginning to emerge. In this review, we summarize findings on the biological and clinical patterns underlying response to immune checkpoint blockade.
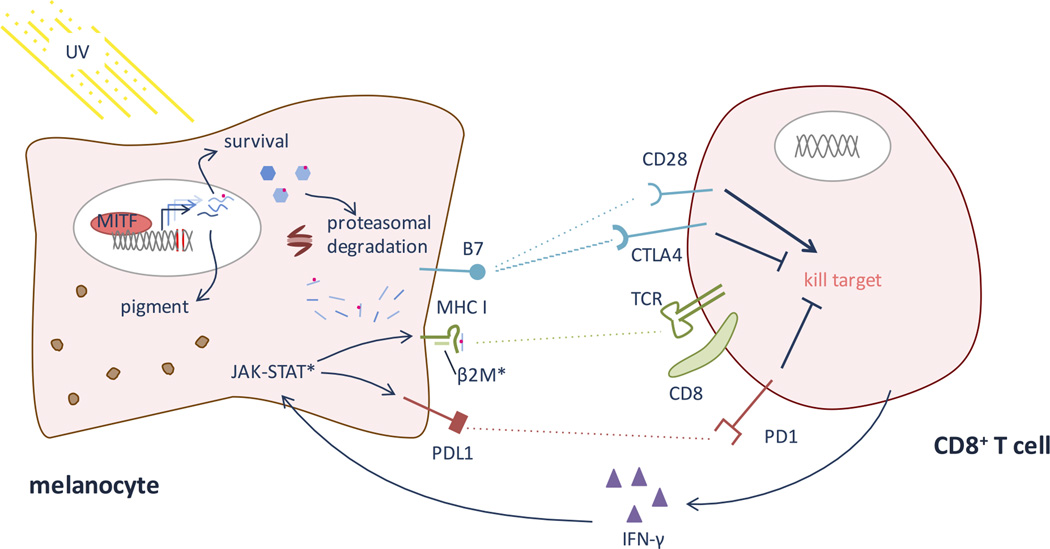
Immune checkpoints play an important role in a healthy immune response by suppressing aberrant responses against normal cells and by down-regulating responses to chronic antigens in order to limit collateral tissue damage. In cancer, co-stimulatory pathways, notably the PD1/PDL1 and the CTLA4/B7 pathways (summarized in Figure 2), are thought to play an important role in suppressing or limiting the native immune response to tumor cells. The biology of these co-stimulatory pathways and their roles as immune checkpoints in cancer have been explained in depth elsewhere5–8. Briefly, in order for a T cell to become active against a tumor cell, it must be educated both to recognize a foreign epitope on that cell – such as a neoantigen peptide loaded on MHC class I of a tumor cell – through its T cell receptor (TCR) and to recognize that this foreign epitope represents danger. The danger signal is conveyed through an array of co-stimulatory signals, two of which (PD1/PDL1 and CTLA4/B7) are particularly important for current immunotherapy. A T cell’s primary signal comes from its TCR binding peptide-loaded MHC on a target cell or an antigen presenting cell (APC). If the T cell expresses PD1 and PD1 binds its ligand PDL1 or PDL2 either on the APC or potential target cell, the interaction suppresses the T cell’s inflammatory response. The T cell can also express CD28 (activating) or CTLA4 (CD157; repressing), both of which compete for binding with B7 on the APC or target cell; engagement of B7 and CD28 sends an activation signal, while B7-CTLA4 binding represses T cell activity. CTLA4’s binding affinity is greater than CD28’s for B7, so the repressive signal generally outweighs the activation signal when both CTLA4 and CD28 are expressed on the T cell and competing for engagement with B7.
Figure 2. T cell recognition of melanocyte antigens and neo-epitopes.
When MITF is induced (see Figure 1), it causes up-regulation of many genes relevant for pigmentation and cell survival. Because of accumulated UV-induced DNA damage, some of these genes likely harbor mutations. The proteasome system degrades proteins in the cell, allowing presentation of endogenous antigens on MHC class I. Thus, MHC will present some neo-epitopes. In addition to melanocyte-intrinsic survival factors, the melanocyte must survive CD8 T cell recognition of these neo-epitopes, via TCR binding to peptide-MHC, and a cytotoxic response. Co-stimulatory molecules, notably the B7-CD28/CTLA4 and PDL1-PD1 pathways, help dictate whether the CD8 T cell will respond by killing the melanocyte or becoming tolerant upon TCR engagement of a foreign epitope. PD1 and CTLA4 send suppressive, tolerizing signals, while CD28 engagement signals CD8 activation of a cytotoxic response. Checkpoint inhibitors – notably anti-PD1, anti-PDL1, and anti-CTLA antibodies – encourage the CD8 T cell to kill a target cell when its TCR engages a peptide-MHC complex perceived as foreign. Once an immunogenic epitope is recognized, epitope spreading can occur, leading to activation against other antigens co-expressed on the melanocyte. (*) Represent main sites of known resistance to immunotherapy. Melanomas can evolve to evade immune recognition of their neo-epitopes by down-regulating MHC I, often via mutations in β2 microglobulin (β2M). Additionally, mutations in the JAK-STAT pathway prevent IFN-γ mediated up-regulation of PDL1 and MHC class I.
Antibodies against PD1, PDL1 and CTLA4 can block these checkpoints, lifting a set of brakes that govern immune tolerance and thereby releasing an immune response to melanoma cells that was otherwise suppressed. When these immune checkpoint inhibitors are successful, they can achieve remission or even cure, with ~25–50% of metastatic malignant melanoma patients achieving progression free survival9. It remains largely unclear why some patients respond but others do not. The long tail of survivors on the Kaplan-Meier curves are even more intriguing; why do some patients not only respond but undergo complete, durable remissions, whereas others exhibit only partial responses? To date, multiple molecular and clinical biomarkers have been proposed as correlates of immunotherapy success, but the biological and molecular mechanisms underlying why some melanomas respond to treatment and others do not remain incompletely understood. Molecular, cellular and clinical correlates of response have been described and together reflect an intertwined system of immune regulation of cell killing. Moreover, melanocytes and melanoma cells are subject to a delicate balance between immune recognition of tumor cells and the ingrained transcriptional program that encourages melanocyte survival even after DNA damage.
II. Molecular correlates of melanoma response to checkpoint blockade
Multiple molecular correlates of melanoma response to immune checkpoint inhibition have been proposed. These correlates are not only useful as biomarkers but also as harbingers of the underlying biological mechanisms that make some melanomas susceptible to immune checkpoint blocking therapy, potentially providing clues for co-treatment options that might enhance the efficacy of checkpoint blockade.
a. Tumor PDL1 expression
Based on early knowledge of the PD1/PDL1 inhibitory pathway, it was hypothesized that successful anti-PD1/anti-PDL1 therapy would mechanistically rely on expression of PDL1 on tumor cells or APCs. In support of this hypothesis, it was observed that mouse tumors expressing PDL1 resisted immune detection and destruction; conversely, lack of PD1 or treatment with an anti-PDL1 antibody prevented tumor growth10. Blocking this pathway would only work, in theory, if the tumor were already evading immune surveillance using the PD1/PDL1 signal. An early clinical trial of anti-PD1 therapy measured PDL1 expression on tumor cells and suggested that PDL1 expression in tumor biopsies correlated with response to therapy11. Since this relatively early study, multiple groups have followed up with varying results on the correlation of PDL1 expression for the success of PD1—PDL1 blocking immunotherapy. Some analyses have found PDL1 not to be a biomarker of response to melanoma12. These discrepant conclusions may stem from differences in experimental protocol, for example: the threshold of PDL1 expression in determining whether a tumor is PDL1-positive; distinguishing between PDL1 expression on tumor cells, stromal cells, and infiltrating immune cells (by co-staining for PDL1 and cell type markers); or the number of different tumor locations examined for PDL1 expression in each patient.
A recent meta-analysis of anti-PD1 and anti-PDL1 therapy (nivolumab and pembrolizumab) examined twenty trials, seven in melanoma patients, and reported that overall response was significantly higher in tumors positive for PDL1. Notably, this study found that the threshold for calling tumor PDL1 positivity had an impact on study conclusion (i.e. tumor response correlated with PDL1 expression called at a 5% cutoff but not a 1% cutoff), providing one possible explanation for differing conclusions across studies13.
Although T cells can receive the PDL1 signal from multiple cell types, notably both APCs and tumor cells, the particular PDL1-positive cells within the tumor microenvironment may influence the biology of checkpoint blockade. Some have found that PDL1 expression only on infiltrating immune cells, and not tumor cells, correlates with response in melanoma (and other cancers)14. In some patients who initially respond to anti-PD1 therapy, melanoma cells pre-therapy express PDL1, but upon relapse, melanoma cells are PDL1-negative; in contrast, in the same study, macrophages and stromal cells did express PDL1 during relapse15. Thus, while cell type may influence the PD1-PDL1 interactions and the impact of checkpoint blockade, the precise relationship between cell type specific PDL1 expression and response to checkpoint blockade remains unclear.
Apart from these experimental considerations in the link between PDL1 expression and response to checkpoint blockade, there may be molecular considerations that complicate the direct correlation between PDL1 and response. The immunological tumor microenvironment may play a role in regulating tumor PDL1 expression. IFN-γ, secreted by infiltrating lymphocytes, is associated with up-regulation of PDL1 in melanoma cells16. Melanomas can evolve to be refractory to this IFN-γ signaling, reducing the efficacy of PD1 or PDL1 blockade. In melanomas that initially responded to anti-PD1 therapy, JAK1 and JAK2 loss-of-function mutations were found to arise in subsequently recalcitrant melanomas15. Loss of functional JAK2 prevented signaling from IFN-γ, including failure to induce pSTAT1 and PDL1, resulting in unchecked growth15. Even if the tumor cells have evolved away from the PDL1 immune evasion mechanism, it is still feasible that anti-PDL1/anti-PD1 treatment could work by blocking T cell exhaustion elsewhere, such as in a lymph node, allowing increased anti-tumor immunity. The problem with this model is that tumors negative for PDL1 have probably evolved an alternative mechanism for evading immune surveillance, so T cells activated elsewhere with PD1/PDL1 blockade would need to surpass the other mechanism(s) of immune evasion upon entering the tumor. This model and the IFN-γ signaling observations suggest that PDL1 expression may be considered a biomarker of many processes in the tumor, which may help explain the frequent but non-ubiquitous correlation between tumor PDL1 positivity and response to checkpoint blockade.
PDL1 expression is not only relevant to anti-PD1 and anti-PDL1 therapy. In anti-CTLA4 therapy (combined with radiation), resistance is associated with PDL1 up-regulation, suggesting that tumors circumvent CTLA4 blockade by evolving to use the PD1-PDL1 immune evasion pathway. In a mouse model, concomitant use of anti-CTLA4, radiation and anti-PDL1 or anti-PD1 therapy improved survival substantially17, further supporting the model that tumors evolve to use whatever immune evasion techniques are available to them, sometimes involving PDL1 up-regulation and other times requiring PDL1 down-regulation, with up-regulation of alternative immune evasion pathways.
b. Tumor MHC expression
T cells recognize and kill infected or foreign cells when they recognize peptide bound to the major histocompatibility complex (MHC). CD8+ T cells recognize MHC class I, normally present on all cells, which presents peptides derived from intracellular protein processing (Figures 1–2). CD4+ T cells recognize MHC class II, normally present on APCs of the immune system, which sample antigens from their environment, cleave them, and present these extracellular peptides.
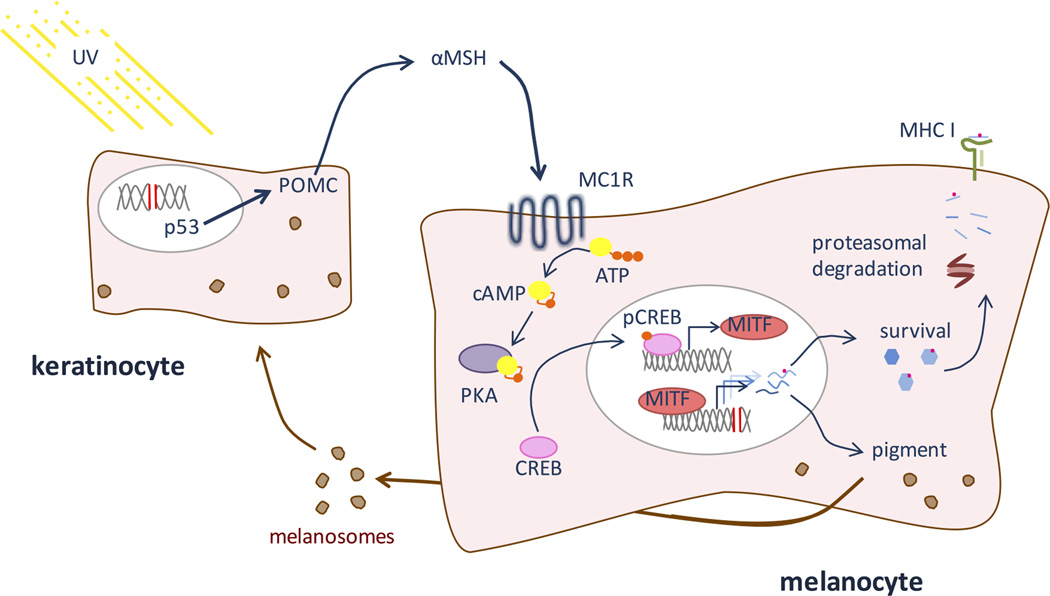
Figure 1. The MITF pathway.
The sun’s ultraviolet light damages DNA in cells in the skin, particularly keratinocytes and melanocytes. In keratinocytes, DNA damage triggers p53, which induces expression of POMC. POMC is cleaved to produce αMSH, a hormone that is released from the keratinocyte. MC1R, a receptor on melanocytes, binds αMSH, causing activation of PKA through production of cAMP. PKA then phosphorylates CREB, activating it as a transcription factor that up-regulates the expression of MITF, a melanocyte lineage specific transcription factor. MITF in turn activates multiple genes involved in pigmentation and cell survival, allowing continued melanin production despite UV-induced DNA damage. Because the melanocyte has sustained DNA damage, some proteins produced downstream of MITF may harbor mutations. Proteins expressed downstream of MITF are degraded by the proteasome and epitopes, including those with mutations, are presented on MHC class I.
Down-regulating MHC I would be one tumor survival strategy that could feasibly detract from the efficacy of checkpoint blockade. Without MHC I, neoantigens from the tumor cells could not be presented, so tumor-specific T cells would have difficulty recognizing a target epitope, let alone bind a co-stimulatory ligand. In an early study of immunotherapy, using tumor-infiltrating CD8+ T cells, some melanoma biopsies showed a lack of β2 microglobulin (β2M), a necessary molecular component of MHC I. Patients with these MHC I-deficient melanomas did not respond to T cell-based immunotherapy, but CD8+ T cells were capable of killing them in culture once β2M was restored18. Since then, others have validated that melanomas can evade tumor-infiltrating lymphocytes through loss of MHC I expression15,19,20. In particular, β2M mutations, resulting in loss of MHC I expression, have been correlated with acquired resistance to anti-PD1 therapy after initial response15. It remains to be seen how abundant these mechanisms are, and whether heterozygous loss of function mutations can dominantly affect downstream biological functions. In contrast, some analyses have failed to find MHC I down-regulation on tumor cells and have found no correlation with response12.
Some form of MHC I dysregulation is relatively common in melanoma cell lines, but the phenotype is often reversible in culture, with IFN-γ treatment21. Molecularly, IFN-γ induces MHC I expression through phosphorylation of STAT1. Melanoma cell lines have been found to lose the capability to signal downstream of IFN-γ, thus losing IFN-γ mediated MHC I induction22.
These results suggest that down-regulation of MHC I, potentially due to loss of IFN-γ signaling machinery, could be one mechanism, but unlikely the sole mechanism, of immune evasion. The mechanism highlights particular resistance to checkpoint blockade, which relies on TCR binding of peptide-MHC complexes on tumor cells. Without proper peptide presentation, T cell mediated cytotoxicity is difficult, but natural killer cells have the ability to kill cells lacking MHC I; in culture, natural killer cells can kill melanoma cell lines lacking MHC I23, suggesting future potential synergies between T cell and NK cell based immunotherapy approaches.
Of note, some melanomas aberrantly express MHC II – some express HLA-DR, HLA-DP and HLA-DQ, while some only express a subset of the three21. Even in some melanoma cell lines without constitutive MHC II expression, MHC II could be induced with IFN-γ treatment21. Aberrant expression of the Class II Transactivator (CIITA), through a mechanism normally active in B cells, induces MHC II expression in melanomas24. HLA-DR expression has been found to correlate with response to anti-PD-1 or anti-PD-L1 therapy12.
c. Mutational load in the tumor
Cutaneous melanocytes are unusually long-lived, considering the large amount of radiation they withstand and concomitant mutations they accumulate. Melanocytes are subject to a large amount of UV radiation from sunlight, which causes signature dipyrimidine mutations that accumulate in the melanocyte genome25,26. Melanomas have, on average, a greater mutational load than other cancers, but the mutational load is also highly variable among melanomas27. High mutational load has been found to be correlated with melanoma response to anti-CTLA4 therapy28. In line with this observation, several types of tumors with mismatch-repair defects, including melanoma, respond better to anti-PD1 therapy29 presumably due to their weakened ability to repair DNA damage. It has also been observed that cancer classes associated with high mutational load (e.g. melanomas or lung cancers) tend to respond better to immune checkpoint inhibition than other tumor-types (e.g. colorectal cancer)29.
Mechanistically, higher mutational load in melanomas may increase the efficacy of checkpoint inhibition through the production and presentation of immunogenic neoantigens, allowing T cell recognition. Indeed, a case study of successful melanoma treatment with anti-CTLA4 revealed that expanded T cell populations existed whose TCRs were specific for neoantigens generated through somatic mutation in melanoma30. Some have speculated that mutational load itself is not a driver of successful immune recognition; instead, mutational load may simply be correlated with the generation of specific epitopes, which serve as the key flags for immune attack when checkpoint inhibitors are administered. For example, in one study, although response to anti-CTLA4 therapy correlated with mutational load, it correlated even more closely with the presence of a set of signature tetrapeptides. These tetrapeptides were themselves associated with a high mutational load. The tetrapeptides were found to be similar to microbial antigens, potentially explaining their ability to predict immune destruction of melanoma after anti-CTLA4 therapy31. Although neoantigens’ similarity to microbial epitopes driving effective anti-CTLA4 response is an intriguing and elegantly intuitive hypothesis, it has not been replicated in subsequent studies28. For example, in another study, while the load of potentially immunogenic neoantigens correlated with response to anti-CTLA4, there were no clear patterns of repeated neoantigens across responders that distinguished them from non-responders28. Taken together, the correlation between mutational load and response to checkpoint blockade is fairly well established, while it is still unclear whether these somatic mutations produce a set of signature neo-epitopes that causally drive response to therapy. It is also uncertain whether there may exist recurrent (eg microbial-related) neoantigens/epitopes that function for tumor recognition and killing across different patients.
The correlation between mutational load and response to checkpoint inhibition is complicated by the dependency on proper protein processing and presentation on MHC I in order for mutational burden to have any immunological significance. Further, if a neo-epitope is presented, it is possible for tumors to evade immune attack by presenting tolerance signals – for example PDL1 upregulation after anti-CTLA4 therapy. Thus, each of these molecular correlates of response to checkpoint blockade is linked to the others.
If increased mutational load does indeed cause greater response to checkpoint blockade, the concomitant use of radiation may serve as a potential enhancer of immunotherapy efficacy32. The phenomenon of systemic tumor clearance that can be induced by local radiation is termed the “abscopal” effect. The abscopal effect has been documented following radiation in several cancers; a melanoma case report documented that combination of anti-CTLA4 therapy followed by radiation of one melanoma lesion resulted in regression of lesions that were not irradiated, probably due to immune activation against antigens in the irradiated tumor that were shared with unirradiated metastases33. The underlying biological mechanism for the abscopal effect was further investigated in a mouse model with bilateral tumors. A combination anti-CTLA4 therapy with unilateral radiation was more effective in causing regression of the unirradiated tumor than anti-CTLA4 therapy alone17. Radiation and checkpoint blockade appear to work synergistically, with anti-CTLA4 decreasing regulatory T cell infiltration in the tumor and radiation increasing TCR diversity in the infiltrating T cells. Addition of anti-PDL1 increased the number of infiltrating CD8+ T cells; triple therapy of radiation, anti-CTLA4 and anti-PD1/anti-PDL1 causes even more profound tumor regression and survival17. Although the immunological mechanism and case reports are intriguing, evidence does not yet conclusively establish that the abscopal effect, and subsequent tumor regression, can be induced by a combination of checkpoint blockade and radiation. In one recent study, radiation was found to be tolerated with ipilimumab, although the benefit of dual therapy is not yet well defined34.
d. T cell infiltration in the lesion
If response to checkpoint blockade depends on melanoma tumor expression of PDL1 and MHC I, with neo-epitopes loaded on MHC, it follows that T cells must be present in the tumor to recognize these molecular features. Thus, T cell infiltration into a tumor has been measured as a correlate of success for checkpoint blockade. In biopsies of melanoma patients before treatment with anti-PD1, during response, and during relapse, CD8+ T cells were observed inside the tumor only during response to therapy, whereas before therapy and during relapse, CD8+ T cells were relegated to the tumor margins15.
Assessments of T cell infiltration are complicated by the interplay between T cells and other correlates of response, such as MHC and the PD1/PDL1 interaction. In a mouse experiment testing vaccination with irradiated melanoma cells plus anti-PD1/anti-PDL1 and/or anti-CTLA4 checkpoint blockade, treatment with only one checkpoint inhibitor induced T cell infiltration early on but resulted in subsequent up-regulation of the other checkpoint pathway, preventing further immune attack of the tumor35. Measuring T cell infiltration is also complicated by varying T cell roles and the cytokines that shape these roles. In the mouse model, a combined treatment strategy enhanced T cell infiltration into the tumor over a prolonged period of time, with a notable increase in the effector T cell to regulatory T cell ratio inside the tumor35. Cytokines in the tumor microenvironment may help shape this response, including IFN-γ and TNF-α secreted by infiltrating T cells35.
This study illustrates the complexity of assessing any single molecular correlate of melanoma response, because checkpoint ligand/receptor expression, MHC expression, epitope presentation, and T cell infiltration are all inter-related. Although there is a compelling mechanistic explanation for T cell infiltration to correlate with response to checkpoint blockade, in some studies, CD8+ T cell infiltration does not correlate with response29. It is difficult to determine which of these conclusions reflects a meaningful biological correlate or is instead an artifact of an underlying network of cellular interactions. For example, infiltrating CD4+ T cells are strong TNF producers, which, in an environment high in IFN-γ, suppresses CD8+ T cell reactivity to melanoma36. Measurements of “T cell infiltration” are very likely sensitive to the types and activity of T cells assessed. Similarly, infiltrating T cells produce IFN-γ, which causes up-regulation of PDL1, so measuring PDL1 and T cell infiltration are not independent biological variables16. As another example, one study found PDL1 expression, mismatch repair deficiency, and mutation load to be correlated with each other, but in that case PDL1 expression did not correlate with response to checkpoint inhibition29.
The microenvironment produced by the tumor – by infiltrating T cells, by innate immune cells, or by sentinel cells at the site of the tumor – likely plays an enormous role in the efficacy of T cells locating and killing tumor cells. Checkpoint inhibition can surely alter the tumor microenvironment by changing the balance of tumor tolerance and rejection. Many more factors also contribute to this environment. Clinically, for example, the combination of intra-tumoral injection of IL-2 with anti-CTLA4 appears to produce responses in a phase 1 trial37. In summary, each of these molecular and cellular markers is linked to the others, and all have been found to correlate, to some extent, with checkpoint inhibition response. While PDL1 expression, MHC expression and mutational load all certainly play a part in the molecular mechanism underlying the success or failure of checkpoint blockade, they are linked in a network that is not yet fully understood and likely involves many other components of immune signaling. Further work is required to disentangle these, and many other, molecular and cellular variables to elucidate the pathways that particularly predict response to therapy.
III. Clinical correlates of response to checkpoint blockade: autoimmunity and vitiligo
While the above cellular and molecular correlates of response to checkpoint blockade suggest important biological mechanisms for immune recognition and destruction of tumor cells after checkpoint blocking therapy, clinical observations in patients receiving these treatments may also offer clues to successful therapy. In particular, autoimmunity has been well documented in patients treated with checkpoint inhibitors. Of particular interest, vitiligo often accompanies immunotherapy in melanoma patients and even correlates with tumor regression after checkpoint blockade.
Immune-related adverse events may occur during either anti-CTLA4 or anti-PD1/anti-PDL1 therapy38,39. In multiple types of cancers, the occurrence of immune-related adverse events correlates with response to checkpoint blockade40. In melanoma, patients responding well to checkpoint blockade and other types of immunotherapy often experience vitiligo41–43. Vitiligo is a much more common adverse event in melanoma immunotherapy compared to other solid tumor immunotherapy44,45. Other immune-mediated cutaneous complications have also been described during checkpoint inhibitor therapy45. Anti-PD1 not only causes vitiligo but can also cause cutaneous autoimmune destruction similar to toxic epidermal necrolysis46. Bullous pemphigoid has been reported, in the absence of melanoma response to anti-PD1 therapy47. Most provocatively, in a clinical trial for anti-PD1, rash and vitiligo, but not other immune-related adverse events, correlated with survival of patients with metastatic melanoma48. Previously, vitiligo was correlated with metastatic melanoma response to IL2 and other types of immunotherapy44,49.
In addition to vitiligo secondary to checkpoint inhibition, hapten-associated depigmentation has been described clinically. Imiquimod, a TLR-7 agonist, is used topically to treat human papilloma virus (HPV) and basal cell carcinoma but local depigmentation has been observed following treatment50,51. Monobenzone causes haptenization of melanocyte proteins, inducing an immune response against melanocytes that both causes vitiligo-like depigmentation and attack on melanoma cells52. These observations suggest that prompting an immunological attack on skin epitopes, either through checkpoint blockade or through these other topically applied immune activators, prompts a dual attack on cancer cells and healthy melanocytes.
When vitiligo occurs in the absence of cancer immunotherapy, it is most commonly mediated by an autoimmune attack on melanocytes, presenting clinically as progressively spreading depigmented lesions. Immunostaining of vitiligo lesions has shown a CD8+ T cell, Th 17 cell, and dendritic cell infiltrate, with a loss of regulatory T cells (reviewed in53). Genome-wide association studies have revealed several genes that may be linked to vitiligo, including MHC class I and class II, part of the inflammasome, and genes involved in regulatory T cells (reviewed in53). Unsurprisingly, many of these pathways are similar to those highlighted in the molecular correlates of melanoma response to checkpoint blockade. It might be argued that these pathways are simply fundamental tenets of any immune response. Alternatively, the dual clinical and mechanistic correlation between vitiligo and response to checkpoint blockade may shed light on the mechanisms of successful immune attack of melanoma cells, and incidental attack of normal melanocytes.
A mouse model has been developed that in some ways mimics the dual attack on melanoma cells and healthy melanocytes in the context of immune checkpoint blockade or topical hapten treatment. The pmel-1 CTLA-4−/− mouse model is deficient in CTLA-4 and has T cells with a transgenic TCR that is reactive to melanoma54,55. While these mice survive longer than CTLA-4−/− mice without a manipulated T cell repertoire, they do develop autoimmune vitiligo55. These results suggest a skin-targeted autoimmune attack when loss of an immune checkpoint is combined with melanoma-specific T cells. The results show a compelling mouse model parallel to anti-PD1, anti-PDL1 or anti-CTLA4 treatment in humans: effective treatment is thought to rely on the presence of tumor-specific T cells, and efficacy against melanoma is correlated with the development of vitiligo. In parallel, in a melanoma clinical trial of adoptive transfer of MART-1 specific CD8+ T cells, inflammation was induced at pigmented lesions, with infiltration of anti-MART-1 T cells; loss of melanocytes and vitiligo followed56.
Immune attack on healthy melanocytes upon melanoma sensitization appears to occur in humans as well. Two observations have been made in humans, where topical treatment using contact sensitizers on a melanoma metastasis, combined with anti-PD1, resulted in systemic tumor responses as well as vitiligo symptoms57,58.
IV. Checkpoint blockade response and vitiligo in the context of the melanocyte lineage transcriptional landscape
The downstream transcriptional effects of MITF, an oncogene and a master transcriptional regulator of the melanocyte lineage, may contribute to the connection between melanoma response to immune checkpoint inhibition and the vitiligo phenotype. Normal melanocytes survive a large accumulation of DNA damage, a necessary adaptation in order to prevent melanocyte loss after UV exposure and therefore retain tanning capability after DNA damage. Melanocyte survival hinges on the downstream effects of MITF, the M isoform of which (MITF-M) is a melanocyte lineage specific transcription factor (reviewed in59). MITF is required for melanocyte development, as evidenced by MITF-mutant mice exhibiting phenotypes that include white fur, due to lack of melanocyte development or survival59.
Melanocyte survival after UV and DNA damage is hard-wired in the melanocyte lineage. In the UV stimulated pigmentation response, epidermal melanocytes respond to signals from overlaying keratinocytes. When keratinocytes experience DNA damage due to ultraviolet radiation, p53 activates expression of proopiomelanocortin (POMC)60, from which α-melanocyte stimulating hormone (α-MSH), a short peptide hormone, is proteolytically cleaved and secreted by the keratinocyte. Secreted α-MSH subsequently docks on a melanocortin 1 receptor (MC1R) of a nearby melanocyte. MC1R, a G protein coupled receptor, stimulates adenylate cyclase to catalyze the synthesis of cAMP from ATP, allowing cAMP response element binding protein (CREB) to be phosphorylated and activate MITF-M expression (Figure 1). MITF-M then acts as a wide-ranging transcription factor (reviewed in61). MITF-M has multiple target genes important in both the tanning response and the survival or proliferation of melanocytes. For example, BCL2, known for its roles in regulation of apoptosis, is a downstream target of MITF that is necessary for melanocyte survival62.
MITF has also been characterized as an oncogene driving melanoma-genesis (reviewed in63). MITF is also mutated at a recurrent position in certain familial melanomas64. It is amplified in 5-20% of sporadic melanomas but not in benign nevi65. MITF amplification in the context of melanoma is associated with decreased survival65 but has not been assessed for correlation with response to checkpoint blockade. The MITF-pigmentation pathway is also linked to melanoma risk in red-headed individuals. The phenotype of red hair and an inability to tan is caused by loss of function polymorphic variants in MC1R, the receptor for α-MSH that allows signaling from keratinocytes to melanocytes and eventually activation of MITF expression (Figure 1). In addition to the lack of dark UV-shielding eumelanin, these individuals have an increased risk of melanoma independent of UV exposure thought to be related to pro-oxidant roles of red/blond pheomelanin pigment66, and their melanomas have higher numbers of both UV and non-UV associated mutational loads67. The finding of UV-independent melanoma risk among MC1R-variants (redheads) has been recently corroborated in humans68.
Importantly, MITF’s regulatory role involves inducing transcription of many genes important in pigment production and the maintenance of the melanocyte, both in melanoma and in normal cells. Following immune checkpoint therapy, the immune system likely targets epitopes within genes downstream of MITF (Figure 2). Some of these MITF-regulated genes may contain tumor-specific neo-antigens, and others of which may represent melanocyte lineage-specific “wildtype” antigens. The immune system’s activation against MITF-associated epitopes, some recognized as foreign due to somatic mutation and others recognized due to epitope spreading, results in destruction of melanoma cells, sometimes with collateral destruction of melanocytes in the process. Consistent with this mechanism, antibodies to similar antigens in melanoma and normal melanocytes have been found to account for melanoma-associated vitiligo69.
V. Conclusions: lineage-specific pathways modulating immunotherapy responses
The necessary role of MITF in the normal melanocyte lineage and in melanomas is likely not incidental to the observation that melanoma tends to respond well to checkpoint blockade. MITF has dual complementary and critical roles in melanocytes: driving production of melanin and promoting survival after skin UV exposure. Indeed, the original signal that drives MITF is UV-induced DNA damage via p53 (Figure 1). MITF’s role in maintaining a functional, melanin producing melanocyte population after UV radiation likely overrides immunological and cell-intrinsic factors that, in other cells, could lead to cell death when DNA damage and mutations are sensed. Thus, when immunotherapy is successful, immune cells override MITF control of melanocyte survival. The intersections between MITF and immune regulation are as yet incompletely characterized. There must be a delicately balanced regulation between MITF and the immune system that allows MITF to function as a melanocyte lineage promoter, even in the context of large mutational burden70, protected from immune surveillance, but somehow allowing the immune system to limit cancerous transformation (Figure 2). When this balance is interrupted, melanoma results. When the immune system is prompted to recognize melanoma antigens, in the context of checkpoint blockade, the balance is disrupted in the opposite direction. The immune attack sometimes destroys normal melanocytes, which, like melanoma, present melanocyte-specific epitopes – many of them likely in genes downstream of MITF – possibly including neoantigens resulting from accumulated UV damage. We hypothesize that, under this mechanism, checkpoint blockade unleashes the immune system on previously protected melanocytes, producing vitiligo.
The molecular and cellular correlates of melanoma response to checkpoint blockade likely reflect players in the immune balance with melanocyte survival. PDL1 expression, MHC expression, mutational load, and T cell infiltration are highlighted here, but each of these markers is intertwined with the others. The immune system’s interconnected pathways of regulation, combined with MITF’s regulation of melanocyte survival, should allow for immune tolerance and survival of normal melanocytes, as they accumulate DNA damage, and potentially also for immune attack of transformed melanocytes. The observations that some patients spontaneously clear melanoma and that some patients are cured after broad immune activation with high-dose IL-2 therapy, suggest that these immune-melanocyte relationships are exceedingly finely tuned. Checkpoint inhibition pushes the balance in the direction of immune activation, which allows tumor killing, as well as normal tissue killing, more in the skin than elsewhere in the body. Hence, melanoma is particularly susceptible to checkpoint inhibition, and vitiligo sometimes results. Further work is required to understand the regulatory network between melanocytes and the immune system that, in most cases, succeeds in protecting against melanoma while retaining melanocytes for the tanning response. Clinical entities such as metastatic melanoma of unknown primary, in which a presumed cutaneous primary lesion has been spontaneously destroyed by immune attack, may represent an example of this phenomenon in patients. Understanding how immune regulation intersects with the MITF pathway may further elucidate the determinants of a melanoma that portend susceptibility versus resistance to checkpoint inhibitor therapy.
Acknowledgments
The authors thank Professor Shiv Pillai for useful immunology discussions. The authors also gratefully acknowledge NIH grants P01 CA163222 and R01 AR043369-19, and funding from the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation, and the Melanoma Research Alliance. The review was also supported by award Number T32GM007753 from the National Institute of General Medical Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of General Medical Sciences or the National Institutes of Health.
Bibliography
- 1.Sumner WC. Spontaneous regression of melanoma. Report of a case. Cancer. 1953;6(5):1040–1043. doi: 10.1002/1097-0142(195309)6:5<1040::aid-cncr2820060525>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 2.Morton DL, Eilber FR, Holmes EC, et al. BCG immunotherapy of malignant melanoma: summary of a seven-year experience. Ann Surg. 1974;180(4):635–643. doi: 10.1097/00000658-197410000-00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atkins MB, Lotze MT, Dutcher JP, et al. High-Dose Recombinant Interleukin 2 Therapy for Patients With Metastatic Melanoma: Analysis of 270 Patients Treated Between 1985 and 1993. Journal of Clinical Oncology. 1999;17(7):2105–2105. doi: 10.1200/JCO.1999.17.7.2105. [DOI] [PubMed] [Google Scholar]
- 4.Rosenberg SA, Yang JC, Topalian SL, et al. Treatment of 283 Consecutive Patients With Metastatic Melanoma or Renal Cell Cancer Using High-Dose Bolus Interleukin 2. JAMA. 1994;271(12):907–913. [PubMed] [Google Scholar]
- 5.Ott PA, Hodi FS, Robert C. CTLA-4 and PD-1/PD-L1 Blockade: New Immunotherapeutic Modalities with Durable Clinical Benefit in Melanoma Patients. Clinical Cancer Research. 2013;19(19):5300–5309. doi: 10.1158/1078-0432.CCR-13-0143. [DOI] [PubMed] [Google Scholar]
- 6.O'Day SJ, Hamid O, Urba WJ. Targeting cytotoxic T-lymphocyte antigen-4 (CTLA-4): a novel strategy for the treatment of melanoma and other malignancies. Cancer. 2007;110(12):2614–2627. doi: 10.1002/cncr.23086. [DOI] [PubMed] [Google Scholar]
- 7.Merelli B, Massi D, Cattaneo L, Mandalà M. Targeting the PD1/PD-L1 axis in melanoma: biological rationale, clinical challenges and opportunities. Crit Rev Oncol Hematol. 2014;89(1):140–165. doi: 10.1016/j.critrevonc.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 8.Kyi C, Postow MA. Checkpoint blocking antibodies in cancer immunotherapy. FEBS letters. 2014 doi: 10.1016/j.febslet.2013.10.015. [DOI] [PubMed] [Google Scholar]
- 9.Robert C, Schachter J, Long GV, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med. 2015;372(26):2521–2532. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 10.Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proceedings of the National Academy of Sciences. 2002;99(19):12293–12297. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Estrada MV, Salgado R, Sanchez V, et al. Melanoma-specific MHC-II expression represents a tumour-autonomous phenotype and predicts response to anti-PD-1/PD-L1 therapy. Nature Communications. 2016;7:1–10. doi: 10.1038/ncomms10582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carbognin L, Pilotto S, Milella M, et al. Differential Activity of Nivolumab, Pembrolizumab and MPDL3280A according to the Tumor Expression of Programmed Death-Ligand-1 (PD-L1): Sensitivity Analysis of Trials in Melanoma, Lung and Genitourinary Cancers. In: Santini D, editor. PLoS ONE. 6. Vol. 10. 2015. p. e0130142–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herbst RS, Soria J-C, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515(7528):563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zaretsky JM, Garcia-Diaz A, Shin DS, et al. Mutations Associated with Acquired Resistance to PD-1 Blockade in Melanoma. N Engl J Med. 2016 Jul; doi: 10.1056/NEJMoa1604958. NEJMoa1604958–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taube JM, Anders RA, Young GD, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012;4(127):127ra37–127ra37. doi: 10.1126/scitranslmed.3003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Twyman-Saint Victor C, Rech AJ, Maity A, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520(7547):373–377. doi: 10.1038/nature14292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Restifo NP, Marincola FM, Kawakami Y, Taubenberger J, Yannelli JR, Rosenberg SA. Loss of Functional Beta2-Microglobulin in Metastatic Melanomas From Five Patients Receiving Immunotherapy. JNCI J Natl Cancer Inst. 1996;88(2):100–108. doi: 10.1093/jnci/88.2.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khong HT, Wang QJ, Rosenberg SA. Identification of multiple antigens recognized by tumor-infiltrating lymphocytes from a single patient: tumor escape by antigen loss and loss of MHC expression. J Immunother. 2004;27(3):184–190. doi: 10.1097/00002371-200405000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bradley SD, Chen Z, Melendez B, et al. BRAFV600E Co-opts a Conserved MHC Class I Internalization Pathway to Diminish Antigen Presentation and CD8+ T-cell Recognition of Melanoma. Cancer Immunology Research. 2015;3(6):602–609. doi: 10.1158/2326-6066.CIR-15-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mendez R, Aptsiauri N, Del Campo A, et al. HLA and melanoma: multiple alterations in HLA class I and II expression in human melanoma cell lines from ESTDAB cell bank. Cancer Immunol Immunother. 2009;58(9):1507–1515. doi: 10.1007/s00262-009-0701-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodríguez T, Mendez R, Del Campo A, et al. Distinct mechanisms of loss of IFN-gamma mediated HLA class I inducibility in two melanoma cell lines. BMC Cancer. 2007;7(1):1415–1411. doi: 10.1186/1471-2407-7-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Porgador A, Mandelboim O, Restifo NP, Strominger JL. Natural killer cell lines kill autologous beta2-microglobulin-deficient melanoma cells: implications for cancer immunotherapy. Proceedings of the National Academy of Sciences. 1997;94(24):13140–13145. doi: 10.1073/pnas.94.24.13140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deffrennes V, Vedrenne J, Stolzenberg MC, et al. Constitutive Expression of MHC Class II Genes in Melanoma Cell Lines Results from the Transcription of Class II Transactivator Abnormally Initiated from Its B Cell-Specific Promoter. The Journal of Immunology. 2001;167(1):98–106. doi: 10.4049/jimmunol.167.1.98. [DOI] [PubMed] [Google Scholar]
- 25.Pleasance ED, Cheetham RK, Stephens PJ, et al. A comprehensive catalogue of somatic mutations from a human cancer genome. Nature. 2009;463(7278):191–196. doi: 10.1038/nature08658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shain AH, Yeh I, Kovalyshyn I, et al. The Genetic Evolution of Melanoma from Precursor Lesions. N Engl J Med. 2015;373(20):1926–1936. doi: 10.1056/NEJMoa1502583. [DOI] [PubMed] [Google Scholar]
- 27.Alexandrov LB, Nik-Zainal S, Wedge DC, et al. Signatures of mutational processes in human cancer. Nature. 2013;500(7463):415–421. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Allen EM, Miao D, Schilling B, et al. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science. 2015;350(6257):207–211. doi: 10.1126/science.aad0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Le DT, Uram JN, Wang H, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372(26):2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Rooij N, van Buuren MM, Philips D, et al. Tumor exome analysis reveals neoantigen-specific T-cell reactivity in an ipilimumab-responsive melanoma. J Clin Oncol. 2013;31(32):e439–e442. doi: 10.1200/JCO.2012.47.7521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Snyder A, Makarov V, Merghoub T, et al. Genetic Basis for Clinical Response to CTLA-4 Blockade in Melanoma. N Engl J Med. 2014;371(23):2189–2199. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salama AKS, Postow MA, Salama JK. Irradiation and immunotherapy: From concept to the clinic. Cancer. 2016;122(11):1659–1671. doi: 10.1002/cncr.29889. [DOI] [PubMed] [Google Scholar]
- 33.Postow MA, Callahan MK, Barker CA, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. 2012;366(10):925–931. doi: 10.1056/NEJMoa1112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qin R, Olson A, Singh B, et al. Safety and Efficacy of Radiation Therapy in Advanced Melanoma Patients Treated With Ipilimumab. Int J Radiat Oncol Biol Phys. 2016;96(1):72–77. doi: 10.1016/j.ijrobp.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 35.Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci USA. 2010;107(9):4275–4280. doi: 10.1073/pnas.0915174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Donia M, Andersen R, Kjeldsen JW, et al. Aberrant Expression of MHC Class II in Melanoma Attracts Inflammatory Tumor-Specific CD4+ T- Cells, Which Dampen CD8+ T-cell Antitumor Reactivity. Cancer Research. 2015;75(18):3747–3759. doi: 10.1158/0008-5472.CAN-14-2956. [DOI] [PubMed] [Google Scholar]
- 37.Ray A, Williams MA, Meek SM, et al. A phase I study of intratumoral ipilimumab and interleukin-2 in patients with advanced melanoma. Oncotarget. 2016 Jul; doi: 10.18632/oncotarget.10453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brahmer JR, Tykodi SS, Chow LQM, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Phan GQ, Yang JC, Sherry RM, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proceedings of the National Academy of Sciences. 2003;100(14):8372–8377. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang JC, Hughes M, Kammula U, et al. Ipilimumab (anti-CTLA4 antibody) causes regression of metastatic renal cell cancer associated with enteritis and hypophysitis. J Immunother. 2007;30(8):825–830. doi: 10.1097/CJI.0b013e318156e47e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hua C, Boussemart L, Mateus C, et al. Association of Vitiligo With Tumor Response in Patients With Metastatic Melanoma Treated With Pembrolizumab. JAMA Dermatol. 2016;152(1):45–47. doi: 10.1001/jamadermatol.2015.2707. [DOI] [PubMed] [Google Scholar]
- 42.Boasberg PD, Hoon DSB, Piro LD, et al. Enhanced Survival Associated with Vitiligo Expression during Maintenance Biotherapy for Metastatic Melanoma. Journal of Investigative Dermatology. 2006;126(12):2658–2663. doi: 10.1038/sj.jid.5700545. [DOI] [PubMed] [Google Scholar]
- 43.Gogas H, Ioannovich J, Dafni U, et al. Prognostic significance of autoimmunity during treatment of melanoma with interferon. N Engl J Med. 2006;354(7):709–718. doi: 10.1056/NEJMoa053007. [DOI] [PubMed] [Google Scholar]
- 44.Rosenberg SA, White DE. Vitiligo in patients with melanoma: normal tissue antigens can be targets for cancer immunotherapy. J Immunother Emphasis Tumor Immunol. 1996;19(1):81–84. [PubMed] [Google Scholar]
- 45.Sibaud V, David I, Lamant L, et al. Acute skin reaction suggestive of pembrolizumab-induced radiosensitization. Melanoma Research. 2015;25(6):555–558. doi: 10.1097/CMR.0000000000000191. [DOI] [PubMed] [Google Scholar]
- 46.Goldinger SM, Stieger P, Meier B, et al. Cytotoxic Cutaneous Adverse Drug Reactions during Anti-PD-1 Therapy. Clinical Cancer Research. 2016 Mar;:1–7. doi: 10.1158/1078-0432.CCR-15-2872. [DOI] [PubMed] [Google Scholar]
- 47.Carlos G, Anforth R, Chou S, Clements A, Fernandez-Peñas P. A case of bullous pemphigoid in a patient with metastatic melanoma treated with pembrolizumab. Melanoma Research. 2015;25(3):265–268. doi: 10.1097/CMR.0000000000000155. [DOI] [PubMed] [Google Scholar]
- 48.Freeman-Keller M, Kim Y, Cronin H, Richards A, Gibney G, Weber JS. Nivolumab in Resected and Unresectable Metastatic Melanoma: Characteristics of Immune-Related Adverse Events and Association with Outcomes. Clinical Cancer Research. 2016;22(4):886–894. doi: 10.1158/1078-0432.CCR-15-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Teulings H-E, Limpens J, Jansen SN, et al. Vitiligo-like depigmentation in patients with stage III–IV melanoma receiving immunotherapy and its association with survival: a systematic review and meta-analysis. J Clin Oncol. 2015;33(7):773–781. doi: 10.1200/JCO.2014.57.4756. [DOI] [PubMed] [Google Scholar]
- 50.Li W, Xin H, Ge L, Song H, Cao W. Induction of vitiligo after imiquimod treatment of condylomata acuminata. BMC Infect Dis. 2014;14(1):329. doi: 10.1186/1471-2334-14-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Burnett CT, Kouba DJ. Imiquimod-induced depigmentation: report of two cases and review of the literature. Dermatol Surg. 2012;38(11):1872–1875. doi: 10.1111/j.1524-4725.2012.02512.x. [DOI] [PubMed] [Google Scholar]
- 52.van den Boorn JG, Picavet DI, van Swieten PF, et al. Skin-depigmenting agent monobenzone induces potent T-cell autoimmunity toward pigmented cells by tyrosinase haptenation and melanosome autophagy. J Invest Dermatol. 2011;131(6):1240–1251. doi: 10.1038/jid.2011.16. [DOI] [PubMed] [Google Scholar]
- 53.Oiso N, Suzuki T, Fukai K, Katayama I, Kawada A. Nonsegmental Vitiligo and Autoimmune Mechanism. Dermatology Research and Practice. 2011;2011(5):1–7. doi: 10.1155/2011/518090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Abad JD, Wrzensinski C, Overwijk W, et al. T-cell receptor gene therapy of established tumors in a murine melanoma model. J Immunother. 2008;31(1):1–6. doi: 10.1097/CJI.0b013e31815c193f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gattinoni L, Ranganathan A, Surman DR, et al. CTLA-4 dysregulation of self/tumor-reactive CD8+ T-cell function is CD4+ T-cell dependent. Blood. 2006;108(12):3818–3823. doi: 10.1182/blood-2006-07-034066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yee C, Thompson JA, Roche P, et al. Melanocyte Destruction after Antigen-Specific Immunotherapy of Melanoma Direct Evidence of T Cell–Mediated Vitiligo. J Exp Med. 2000;192(11):1637–1644. doi: 10.1084/jem.192.11.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gulati N, Carvajal RD, Postow MA, Wolchok JD, Krueger JG. Definite regression of cutaneous melanoma metastases upon addition of topical contact sensitizer diphencyprone to immune checkpoint inhibitor treatment. Exp Dermatol. 2016;25(7):553–554. doi: 10.1111/exd.13030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fujimura T, Furudate S, Kakizaki A, et al. Contact immunotherapy enhances the therapeutic effects of nivolumab in treating in-transit melanoma: Two cases reports. J Dermatol. 2016;43(6):686–689. doi: 10.1111/1346-8138.13229. [DOI] [PubMed] [Google Scholar]
- 59.Hsiao JJ, Fisher DE. The roles of microphthalmia-associated transcription factor and pigmentation in melanoma. Archives of Biochemistry and Biophysics. 2014;563:28–34. doi: 10.1016/j.abb.2014.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cui R, Widlund HR, Feige E, et al. Central role of p53 in the suntan response and pathologic hyperpigmentation. Cell. 2007;128(5):853–864. doi: 10.1016/j.cell.2006.12.045. [DOI] [PubMed] [Google Scholar]
- 61.Lo JA, Fisher DE. The melanoma revolution: From UV carcinogenesis to a new era in therapeutics. Science. 2014;346(6212):945–949. doi: 10.1126/science.1253735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McGill GG, Horstmann M, Widlund HR, et al. Bcl2 regulation by the melanocyte master regulator Mitf modulates lineage survival and melanoma cell viability. Cell. 2002;109(6):707–718. doi: 10.1016/s0092-8674(02)00762-6. [DOI] [PubMed] [Google Scholar]
- 63.Levy C, Khaled M, Fisher DE. MITF: master regulator of melanocyte development and melanoma oncogene. Trends in Molecular Medicine. 2006;12(9):406–414. doi: 10.1016/j.molmed.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 64.Yokoyama S, Woods SL, Boyle GM, et al. A novel recurrent mutation in MITF predisposes to familial and sporadic melanoma. Nature. 2011;480(7375):99–103. doi: 10.1038/nature10630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Garraway LA, Widlund HR, Rubin MA, Getz G. Integrative genomic analyses identify MITF as a lineage survival oncogene amplified in malignant melanoma. Nature. 2005 doi: 10.1038/nature03664. [DOI] [PubMed] [Google Scholar]
- 66.Mitra D, Luo X, Morgan A, Wang J, Hoang MP, Lo J. An ultraviolet-radiation-independent pathway to melanoma carcinogenesis in the red hair/fair skin background. Nature. 2012 doi: 10.1038/nature11624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Robles-Espinoza CD, Roberts ND, Chen S, et al. Germline MC1R status influences somatic mutation burden in melanoma. Nature Communications. 2016;7:12064. doi: 10.1038/ncomms12064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wendt J, Rauscher S, Burgstaller-Muehlbacher S, et al. Human Determinants and the Role of Melanocortin-1 Receptor Variants in Melanoma Risk Independent of UV Radiation Exposure. JAMA Dermatol. 2016;152(7):776–782. doi: 10.1001/jamadermatol.2016.0050. [DOI] [PubMed] [Google Scholar]
- 69.Cui J, Bystryn JC. Melanoma and vitiligo are associated with antibody responses to similar antigens on pigment cells. Arch Dermatol. 1995;131(3):314–318. [PubMed] [Google Scholar]
- 70.Houghton AN, Eisinger M, Albino AP, Cairncross JG, Old LJ. Surface antigens of melanocytes and melanomas. Markers of melanocyte differentiation and melanoma subsets. J Exp Med. 1982;156(6):1755–1766. doi: 10.1084/jem.156.6.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]