Abstract
This review provides a broad overview of my research group's work on social buffering in human development in the context of the field. Much of the focus is on social buffering of the hypothalamic-pituitary-adrenocortical (HPA) system, one of the two major arms of the mammalian stress system. This focus reflects the centrality of the HPA system in research on social buffering in the fields of developmental psychobiology and developmental science. However, buffering of the cardiovascular and autonomic nervous system is also discussed. The central developmental question in this area derives from attachment theory which argues that the infant's experience of stress and arousal regulation in the context of her early attachment relationships is not an immature form of social buffering experienced in adulthood, but rather the foundation out of which individual differences in the capacity to gain stress relief from social partners emerge. The emergence of social buffering in infancy, changes in social buffering throughout childhood and adolescence, the influence of early experience on later individual differences in social buffering, and critical gaps in our knowledge are described.
Over 50 years ago, Schachter (1959) published his seminal book on affiliation in which he argued that human beings are motivated to affiliate, in part, because affiliation is stress reducing. Interest in social buffering has increased since then. Stress reduction is believed to be a major mechanism linking social relationships to health (Kaplan, Cassal, & Gore, 1977). Impaired stress buffering is viewed as a primary pathway through which adverse childhood experiences get under the skin to affect development (Hostinar, Sullivan, & Gunnar, 2014). In this essay I will discuss research on social buffering, highlighting on my own research journey of the last 35 or so years, while also placing my journey in the context of the broader field. I discuss where we have been, what we have learned, and, of course, where we need to be going.
Social buffering is a key concept in the stress field. It describes a phenomenon in which the presence and availability of one or more social partners during times of threat reduces activity of stress-mediating physiological systems, including the sympathetic-adrenomedullary (SAM) and hypothalamic-pituitary-adrenocortical (HPA) systems. Social buffering is a subset of social support. Social support covers both the expectation and actual occurrence of being nurtured, assisted or being a part of a supportive social network. Not all forms of social support are instances of social buffering. For example, some instances of perceived social support may reduce stress reactions because the person expects that support would be available if they asked (Uchino, 2009). No one needs to be present, even symbolically; thus, this is not social buffering, albeit these expectations likely contribute to our ability to use the presence of partners to buffer stress. Using Uchino's (2009) perspective on social support, in addition to perceived support, there is also received support which can be instrumental or emotional I argue that when received support is instrumental and removes or alters the actual nature of the threat, stress reduction cannot be credited to social buffering with any confidence because the change in the stressor might have been the cause of stress reduction. Social buffering, thus, occurs when the presence (actual or symbolic) of a social partner reduces perceptions, reactions, and physiological responses to a potential threat, or when experienced after the threat, helps to speed the return to baseline stress levels without altering the objective nature of the threat.
Social Buffering and Attachment
My interests in social buffering emerged in attempts to understand how infants regulate stress when they cannot cope on their own. This question soon led me to the role that attachment plays in the development of stress reactivity and regulation. Forty years ago, Bowlby's theory was gaining high visibility with the publication of the first two of his 3-volume set on attachment (Bowlby, 1969, 1973). In the 1970's his ideas were discussed intensely in developmental programs around the country, including in the one I attended at Stanford (Maccoby & Masters, 1970). By the mid-1970's Masters and Sroufe (Sroufe & Waters, 1977) were debating its merits at the Institute of Child Development in Minnesota, the program I would join as an assistant professor in 1979. Bowbly provided a different view of the social regulation of stress from the one in social psychology. It was not that the attachment figure reduced stress because she or he altered the infant's perception of threat, although that might happen. Rather, he argued that the attachment figure was embedded as part of the infant's stress and emotion regulatory system. Furthermore, the infant's experience of stress and emotion regulation within the attachment relationship shaped these developing systems and resulted in inner working models of how relationships would provide support (or not) in time of threat. The inner working model forged in the earliest relationships played out through later relationships, affecting the aspects of relationships critical for social buffering effectiveness, potentially, throughout life. Thus, social buffering of stress in the attachment relationship was not just an immature version of adult social buffering, but the foundation out of which the adult capacity would develop. This is a large idea worthy of the decades of research which have supported some of its key tenets. Of course, when I first got involved in research on attachment and social buffering as a postdoctoral student in Seymour (Gig) Levine's lab, I did not have the big picture of where this work was going.
Levine, social buffering and rat pups
Levine's research arguably was seminal to all subsequent neurodevelopmental studies of the social regulation of stress. He is best known for his seminal work on early handling which showed that brief separations of the dam from her pups resulted in the pups developing a better regulated stress response system and behaving less anxiously throughout life (Levine, 1957; Levine, Chevalier, & Korchin, 1956). Levine's group traced the brief separation effect to enhanced maternal stimulation following perturbation of her pups (Smotherman, Brown & Levine, 1977). Today, through the work of Michael Meaney and his students (Zhang, Labonte, Wen, Turecki, & Meaney, 2013), we know that maternal stimulation during a critical early period has epigenetic effects on the pup's developing neural and endocrine systems.
In the 1970's, the Levine group made a transition from focusing on rat psychobiology to including research on non-human primates. First they obtained several colonies of squirrel monkeys and then several groups of rhesus macaques. With the addition of work on non-human primates, Levine’ group encountered mother-infant relationships that more closely resembled human parent-infant relationships. They also began dealing with a mammal who was much more mature neurologically at birth and in this way more closely approximated human brain and neuroendocrine maturity than is the case for laboratory rats and mice. Thus, while the group had a great deal of knowledge about maternal-infant stress regulation in the rat pup, they had to start with the basics in studying primate infants and mothers. The very first question was whether the HPA axis was stress responsive or whether, as in rats, there was a stress hypo-responsive period (SHRP) that was maintained by maternal stimuli (Suchecki, Rosenfeld, & Levine, 1993). To address this they took advantage of the fact that separations appeared to be behaviorally stressful for monkey infants who protested vocally and became physically very agitated when removed from their mothers (Kaufman & Rosenblum, 1969). Did they show elevations in cortisol, the hormonal product of the HPA axis in primates? Indeed, they did, and they were quite large and immediate, whether examined in squirrel monkeys (Mendoza, Smotherman, Miner, Kaplan, & Levine, 1978) or rhesus macaques (Smotherman, Hunt, McGinnis, & Levine, 1979). No SHRP.
Importantly, the first evidence of social buffering by the mother also came from these early separation studies. When we separate human infants from their parents, the parent quietly leaves or waves good-bye and says they will be back soon. When a monkey infant is separated from its mother, the mother aggressively resists separation and there is intense behavioral distress on the part of the infant. Thus, the process of separating mother and infant could be the stressor with the period of separation itself not adding much. To control for this Levine's group always included a sham separation condition called separation-reunion. In the sham separation mother and infant were captured, briefly pulled apart, and then immediately reunited. It takes the HPA axis about 30 minutes to reach peak cortisol concentrations in circulation following activation of the system. Thus if the sham separation activated the system, 30 minutes later cortisol levels should be as high as in infant monkeys who were captured, handled and then remained separated for those 30 minutes. This was not what happened. In study after study, either the infants’ cortisol levels in the sham separation condition were no different from basal levels or they were only slightly elevated relative to the separated infants (Gunnar, Gonzales, Goodlin, & Levine, 1981; Mendoza et al., 1978; Smotherman et al., 1979). For those of us conducting the separations, this finding was profound. Mother and infant were markedly emotionally distressed by our attempts to separate them. Yet, something about immediately reuniting them was blocking or terminating the infant's HPA axis response.
As interest in social buffering increased in Levine's group the question of who beyond the mother could serve as a buffer became critical. Was there something special about the attachment relationship or was this a more general reflection of the effect of a familiar social partner? In squirrel monkeys, other adult females alloparent or “aunt” infants. If the mother was removed, the infant would often spend the separation time in contact with one of these alloparents. Behavioral distress was reduced in these cases, but was the HPA response to separation also buffered? In one of his early studies in the Levine laboratory, Christopher Coe (Coe, Mendoza, Smotherman, & Levine, 1978) separated squirrel monkeys who were living in small social groups of several adult females, their babies and a pregnant female. In one condition the infant remained in the social group while in the other the infant was removed. If the infant remained in the group, the pregnant female attempted to provide comfort. Infants carried by these aunts during separation did not exhibit distressed behavior; however, their cortisol levels were as high as if they had been removed from the group and housed alone during the separation period. The alloparent was not able to buffer the infant's neuroendocrine stress response. While familiar to the infant, she was not the attachment figure.
What about peers? Infant monkeys spend increasing amounts of time in play with age-mates over the first years of life. Despite this, Harlow argued that the mother-infant and peer affectional systems in monkeys were independent (Harlow, 1966) and this was also consistent with Bowlby's views regarding humans (Bowlby, 1969). Early in the 1970's, Patterson (Patterson, Bonvillian, Reynolds, & Maccoby, 1975) examined the social preferences of 7- to 10-month-old infant rhesus macaques when confronted with a fear stimulus. She placed the target infant in a cage bordered by two other cages. On some trials the mother was in one of the other cages and a same sex and age playmate was in the other. On some trials it was the playmate on one side and an empty cage on the other. When confronted with a fear stimulus the infants overwhelming chose to sit near mother and not their peer. Critically, when the choice was an empty cage versus peer they showed no preference. This certainly supported the independence of the two affiliative systems; however, what would the adrenal reveal? We addressed this question using year-old infants separated from their social groups and housed with their mother, a familiar same-sex peer or a peer from another social group (Gunnar, Gonzalez, & Levine, 1980). Unlike the Patterson study, the threat was a novel environment and separation from the social group, and the monkeys had full access to their social partners. We found evidence that under these conditions, yearlings did seem to gain some stress-reduction benefit from being housed with a familiar peer, although being housed with an unfamiliar peer produced the highest cortisol levels.
While I was a post-doctoral fellow in Levine's lab, a fellow post-doctoral student, Michael Hennessy, began his work on social buffering. In the years since, he has become one of the world's experts in the comparative study of this phenomenon. In a recent insightful review (Hennessy, Kaiser, & Sachser, 2009), he and his colleagues pointed out the importance of the animal's developmental and social ecology as it influences who can buffer stress and the conditions under which different partners are successful stress buffers. Attachment figures are the most common and potent stress buffers early in the development of many mammals; however, who and when other conspecifics become social buffers differs by species, by sex within species, and by developmental period.
Neurobiology of Stress
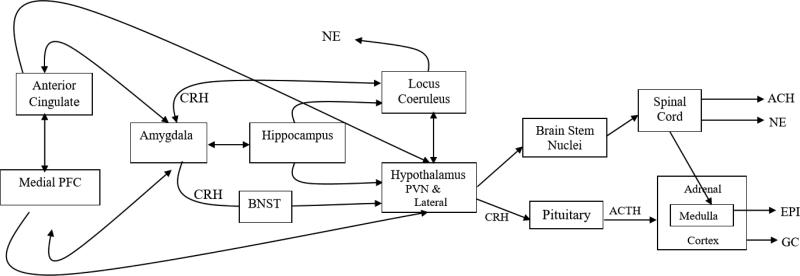
Before turning to the human developmental research, a brief description of the neurobiology of stress and its development is needed. [For more see (Herman & Cullinan, 1997; Herman, McKlveen, Solomon, Carvalho-Netto, & Myers, 2012; Hermans, Henckens, Joëls, & Fernández, 2014; Joëls & Baram, 2009; McKlveen, Myers, & Herman, 2015; Myers, Scheimann, Franco-Villanueva, & Herman, 2016)]. The stress response system in mammals integrates physical and psychological stimuli to allow defense against both actual and perceived or anticipated harm. The core of the stress system is organized in the hypothalamus and involves regulation of the hypothalamic-pituitary-adrenocortical (HPA) axis by cells in the paraventricular nuclei (PVN) and regulation of the sympathetic and parasympathetic systems in lateral hypothalamic projections to the lateral medulla (Figure 1). Notably, in addition to these key outputs from the hypothalamus, neurons in the PVN also s ecrete oxytocin which is released into the periphery via the posterior pituitary and into several sites in the brain. Oxytocin has anti-stress effects on the cardiovascular system and, when administered nasally, inhibits elevations in cortisol to social evaluative stressors (Heinrichs, Baumgartner, Kirschbaum, & Ehlert, 2003). Because contact with attachment figures increases oxytocin, it is considered one pathway through which social buffering may operate
Figure 1.
The anterior cingulate cortex (ACC) and the medial prefrontal cortex (mPFC) relay information to subcortical structures involved in the stress response. There are regions of the ACC and mPFC that activate and others that inhibit stress responding, in part through reciprocal connections with the amygdala. Both the hippocampus and the amygdala also maintain connections to the locus coeruleus (LC) which releases NE to brain areas involved in alerting. The ACC, mPFC, and amygdala, as well as the hippocampus, all have inputs into the hypothalamus, although these inputs are multisynaptic. Nuclei in the lateral hypothalamus activate highly interconnected nuclei in the brainstem, including the parabrachial nuclei, that regulate the sympathetic (norepinephrine, NE and Epinephrine, Epi) and parasympathetic (acetylcholine, Ach) nervous systems via pathways traveling through the spinal cord to preganglionic nuclei or to target organs (e.g. the adrenal medulla). In the paraventricular region of the hypothalamus, corticotropin-releasing hormone is produced; it then travelsthrough the hypophysial portal system to the anterior pituitary gland and stimulates the production and release of adrenocorticotropic hormone (ACTH). ACTH stimulates cells in the adrenal cortex to produce glucocorticoids (cortisol in humans). There are complex feedback loops from the periphery to the CNS that help to regulate the stress system. (Reprinted with Permission from Gunnar & Davis, 2003).
Brainstem nuclei receive output from the hypothalamus, and also provide information about the state of the body back to the hypothalamus and other brain regions. Threats to homeostasis including blood volume loss, immune responses to pathogen invasion, and cardiorespiratory distress will provoke strong stress responses of both the sympathetic and HPA systems, even in an unconscious person. Perception of threat and safety through the processing of information at the cortical and limbic levels of the brain is a primary factor in stress activation and inhibition in most human research. At these levels the study of stress becomes intertwined with the study of fear perception and response. Thus research on social buffering and research on fear learning and extinction have much to offer one another (Gunnar & Sullivan, 2016)
The brainstem and hypothalamic levels of stress regulation are relatively mature at birth in humans and other primates but do still continue to develop over the first years of life (Gunnar & Vazquez, 2006; Porges & Furman, 2011). Parasympathetic tone improves in the first year and increasingly balances sympathetic activity. Cortisol binding globulin increases resulting in a gradual increase in total cortisol to maintain needed levels of unbound or biologically-active cortisol; although there is some evidence that the free-fraction may actually decrease slightly. The fetal adrenal involutes and the adrenal cortex assumes a more mature morphology. More importantly, both the autonomic and HPA systems increasingly come under social regulation as limbic regions of the brain mature and increasingly come to regulate hypothalamic and brainstem functions. A major development over childhood and adolescence involves the increasing maturity of the prefrontal cortex and its connections with limbic brain regions. Thus, as with other aspects of development, the neurodevelopmental picture for stress regulation is one of increasing capacity for planful, executive coping and stress regulation. Social buffering by attachment figures can be viewed as scaffolding and supporting the child's developing cortico-limbic stress regulatory system until the brain is mature enough for the child to take over the executive regulation of stress.
While this is a very plausible way of viewing social buffering during development, it is notable that one function of activation of the stress system is to take the executive control network of the brain off-line and shift functioning to what has been termed the salience network (Hermans et al., 2014). The hormones and neuromodulators that are increased as part of the stress response shift the mature brain from a reflective, executive mode that supports working memory, inhibitory control, and flexible problem solving to a mode that supports vigilance, fear, and habitual responses (Hermans et al., 2014). The shift in functioning from executive control to salience plays out over time during a stress response and is influenced at different points in the response first by catecholamines and later by corticosteroids. Corresponding to this shift from executive to salience neural dominance is a shift of metabolic activity from fostering growth and repair to supporting immediate action (Dallman et al., 2007).
Viewed from a developmental perspective, frequent bouts of acute stress should enhance the development of the salience network (fear, vigilance, impulsive and habitual responses) and impair the development of the executive control network. This is what we see in children who have grown up under adverse conditions (Cowell, Cicchetti, Rogosch, & Toth, 2015; Wiik et al., 2011). With regards to physical growth, frequent or prolonged periods of stress may slow growth and serve as an index of allostatic load (Johnson, Bruce, Tarullo, & Gunnar, 2011). However, when the child has an adequate social buffering system in place, it should support the child's ability to maintain developmentally-expected levels of cognitive, social and physical development even in the face of poverty and other forms of adversity. This argument points to the critical importance of having a potent stress buffering system in place, particularly for children growing up in harsh and threatening circumstance.
Attachment and the Emergence of Social Buffering in Children
At birth the HPA axis is responsive and it activates in a graded fashion to aversive stimulation (Gunnar et al., 1989)). The newborn shows reductions in behavioral distress to social-related stimuli (i.e., sucking, rocking). However, there is no evidence that any of these forms of stimulation block or reduce activation of the HPA axis; newborns who suck on a pacifier while being given a physical exam cry less but produce elevations in cortisol similar to those not give a pacifier (Gunnar, 1992). Sucrose analgesia, which presumably reflects the impact of sugars and fats on the natural opiate system (Blass & Ciaramitaro, 1994), also reduces behavioral distress but has no effect on the HPA response a pain stressor (Stang et al., 1997).
The HPA system remains highly reactive to stimulation through the first three months after birth (Gunnar, Talge, & Herrera, 2009). During this time events as minor as a physical exam, being taken from the bath or even playing for 15 minutes with an intrusive mother will elevate cortisol (Spangler, Schieche, Ilg, Maier, & Ackermann, 1994); but by four months of age babies seem to be able to tolerate such stimulation even though it can still trigger crying and fussing (Gunnar et al., 2009). It is also during the first three months that we begin to see evidence that sensitive maternal behavior influences HPA axis regulation. In one study, 3-month-old infants were studied being bathed by their mothers and maternal sensitivity and responsiveness was scored (Albers, Riksen-Walraven, Sweep, & de Weerth, 2008). Removal from the bath increased cortisol levels significantly 25 minutes later, with levels returning to baseline by 40-minutes post bath. There was no association between the sensitivity of the mother's behavior and the elevation in cortisol to bath removal. However, more sensitive mothers had babies who returned to baseline faster. We would have to be assured, however, that faster return to baseline was not because the more sensitive mothers got their babies warmed up more quickly (i.e., instrumental support) to be confident that this finding reflected social buffering of stress.
Infant reactivity to painful stimulation such as childhood inoculations changes markedly over the first two years in ways that suggest the emergence of social buffering. Cortisol reactivity goes from large increases at two months to smaller ones at four and six months to no significant increases in the second year (Gunnar, Brodersen, Krueger, & Rigatuso, 1996; Gunnar, Brodersen, Nachmias, Buss, & Rigatuso, 1996; Jacobson, Bihun, Chiodo, & Berube, 1994; Lewis & Ramsay, 1995). A key question is whether this is this social buffering or whether the HPA axis is becoming hyporesponsive. In humans it is difficult and often unethical to use methods developed in animal studies to differentiate a period of hyporesponsivity from the emerge of social buffering. That is, we cannot require that the mother or father leave the room when the infant receives a shot versus remain present. For this reason, we decided instead to examine whether the security of the attachment relationship reflected the extent to which the parent was able to buffer the infant's stress responses. This idea was inspired by earlier work demonstrating using the Strange Situation that in secure attachment relationships heart rate returned to baseline quickly after the mother's return in the strange situation test (Sroufe & Waters, 1979). In insecure relationships, even avoidant ones where the infant does not seem upset by separation, heart rate elevated and remained high long after the mother's return. Because the mother's behavior is constrained in the Strange Situation task, this seemed evidence of social buffering of the response to separation and reunion.
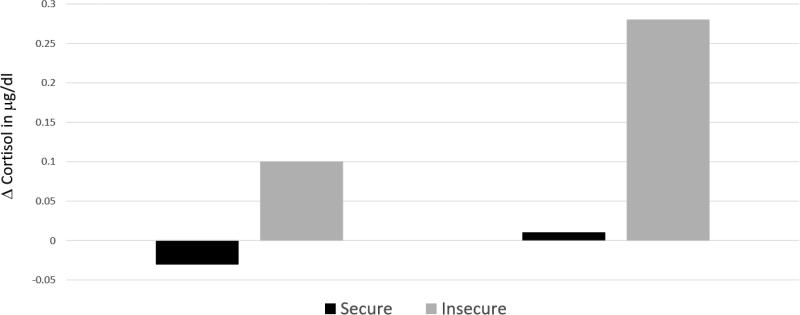
My students and I approached the attachment quality-stress buffering question in two ways. First, in our longitudinal study of infant responses to well-baby examinations and inoculations were examined the role of attachment security in determining cortisol reactions at the 15 month inoculations (Gunnar, Brodersen, Nachmias, et al., 1996). Second, we examined the social buffering-attachment quality question by bringing 18-month-olds to the laboratory and showing them things that are potentially scary and then testing them a week later in the Strange Situation to determine attachment classification (Nachmias, Gunnar, Mangelsdorf, Parritz, & Buss, 1996). In the well-baby exam study, elevations in cortisol at 15 months both reflected attachment security and the child's temperamental fearfulness. Temperamentally fearful infants elevated cortisol to the 15-month inoculations if the attachment relationship was insecure, but not if it was secure. In the scary situation study (Nachmias et al., 1996), toddlers were presented with a live clown who entered the room and asked them to play, two loud puppets who put on a little show and asked the child to come play with them, and a clown robot who moved erratically, lifted his hat and tooted a horn. We scored how readily children approached these stimuli and then reverse-scored this measure to yield a measure of behavioral inhibition or fear. Again, we found that children who were more temperamentally fearful produced elevations in cortisol if they were insecurely attached to the parent who was with them but did not if they were securely attached (figure 2). The findings from the inoculation study and the scary toy study were remarkably similar. Both indicated that in the child's second year the parent in secure relationships was a powerful stress buffering agent and both indicated that the effect was the most readily observed among children who were more temperamentally fearful and anxious.
Figure 2.
Cortisol Responses to Scary Toy Test as a Function of Fearfulness and Attachment Security. (Reprinted with permission from Nachmias et al., 1996).
Social Buffering and Child Care Settings
While these results appear to provide evidence of social buffering in secure attachment relationships, there remained an alternative explanation. Specifically, in secure relationships the parent may have shaped a less reactive stress axis in the child. Thus, even if the parent was not available, the securely attached child might have shown less of a cortisol response to threat. We ruled out this possibility in work by Lilo Ahnert (Ahnert, Gunnar, Lamb, & Barthel, 2004). She collected saliva cortisol samples in Berlin from mothers who were going back to work when their babies were between 12 months and 2 years. She assessed attachment security and sampled saliva before the child was placed in child care. These centers required the mothers to come with their toddlers for an adaptation period prior to leaving them for the first time. Saliva for cortisol was collected on the first and last adaptation day, days 1, 5, and 9 of child care, and after the children had been in these centers for 5 months. Securely attached toddlers had lower cortisol than insecurely attached toddlers when their mothers were adapting them to the center; however, once the mothers were no longer accompanying them, the securely attached toddlers produced elevated cortisol levels comparable to the levels of the insecurely attached toddlers. Even at 5 months all children appeared to average slightly higher cortisol than they had before starting child care. Taken together, all of these studies zeroed in on the attachment figure as a potent stress buffering agent.
As a parent, it was disconcerting to see that even securely attached toddlers were producing high cortisol levels for up to nine days after starting day care. When would this stop? Would infants, toddlers and young children be able to use the child care provider as a surrogate attachment figure to buffer stress? In studies of children five years and older we do not, on average, see increases in cortisol over the day when children are at school and away from home and family (Bernard, Peloso, Laurenceau, Zhang, & Dozier, 2015; Dettling, Gunnar, & Donzella, 1999). However, infants, toddlers and young preschoolers do show morning to afternoon increases in center-based care, suggesting that being away from home and in the company of many children is stressful. Importantly, presence of the providers does not buffer this response (Bernard et al., 2015) and also these meta-analyses, (Geoffroy, Cote, Parent, & Seguin, 2006; Vermeer & Van IJzendoorn, 2006)]. Some studies of family day care have shown similar findings (Gunnar, Kryzer, vanRyzin, & Phillips, 2010). However, elevations are not always seen (Groeneveld, Vermeer, van IJzendoorn, & Linting, 2012; Ouellet-Morin et al., 2010), but this may be because in these studies they sampled earlier in the afternoon right after children finished napping. We have noted that cortisol levels decline when children nap and then rebound after the nap both at home (Larson, Gunnar, & Hertsgaard, 1991) and child care (Watamura, Sebanc, & Gunnar, 2002). Thus, the story appears to be that for infants, toddlers and young preschoolers, being away from home and with other children all day produces elevations in cortisol by late afternoon and the presence of the child care provider does not seem to buffer this stress response. It is noteworthy that there is evidence that cortisol levels drop when parents pick the child up and are not different during the evening on child care and non-child care days (Sumner, Bernard, & Dozier, 2010). Together these data fail to provide any support for the idea that the child care provider is substituting for the parents as a stress buffer. However, before we finally draw this conclusion, it will be important to examine the child accompanied by a care provider versus a parent in laboratory stress-buffering paradigms that allow for greater experimental control. It would also be useful to study whether cortisol elevates over the day in a child care setting in which the target child's mother is providing care to other children while at the same time caring for them.
Parental Stress Buffering and Pubertal Change
Until my students and I began to explore how long into childhood the parent remained a powerful buffer, there was very little on parents as stress buffers after the preschool years. There was one fascinating study by Seltzer and colleagues (Seltzer, Ziegler, & Pollak, 2010) in which they had 7- to 12-year-old girls perform the Trier Social Stress Test for Children (TSST-C). The TSST-C involves a 5-minute speech preparation period, then a 5-minute speech follow by 5 minutes of mental arithmetic. All of this is done in front of a camera and a set of judges. The child is told their performance is being evaluated and the videos will be shown to children their age who will also rate them. The adult version of this task is a most effective cortisol-elevating laboratory task and is viewed as a social evaluative performance stressor (Dickerson, Gruenewald, & Kemeny, 2004). Following the task, Seltzer and colleagues evaluated the stress-reducing effects of three conditions: recover on own, call your mother, or be with your mother. Compared with being alone, calling one's mother helped, but the fastest return to baseline was obtained when the mother was present during recovery. In this study the researchers also collected urinary oxytocin and found that both talking to and being with their mother elevated oxytocin to levels greater than when the girls were alone with the researchers. Thus, parents remain powerful stress buffers throughout childhood, at least for girls.
Would the effectiveness of parental stress buffering survive the transition to adolescence? There were good reasons to expect that the answer could go either way. Certainly, adolescents remain emotionally attached to their parents (Doyle, Lawford, & Markiewicz, 2009). However, with puberty the HPA axis increases in basal activity (Gunnar & Herrera, 2013) as does sensitivity to social evaluative threat, particularly in anticipation of the stressor (Stroud et al., 2009; van den Bos, van Duijvenvoorde, & Westenberg, 2016). Thus, with greater physiological activity and greater psychological reactivity to social threat the parent's presence might not be enough.
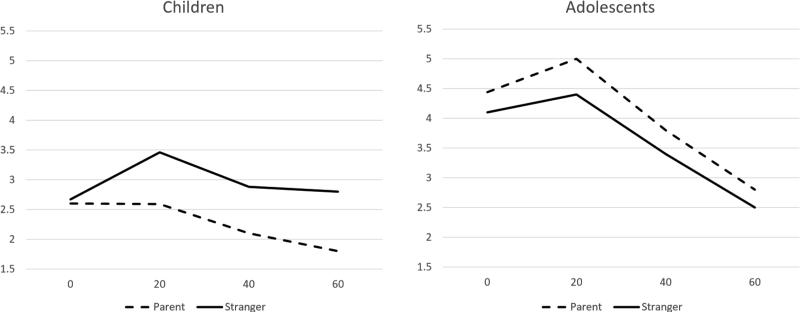
We (Hostinar, Johnson, & Gunnar, 2015c) studied children aged 9 and 10 years and adolescents aged 15 and 16 years using the TSST-C. To assess parental social buffering, we borrowed from a paradigm used with adults (Kirschbaum, Klauer, Filipp, & Hellhammer, 1995). Accordingly, we had either the parent or the researcher support the participant during the 5-minute speech preparation period. Once the preparation period was over, the supportive person left and was not present during the public performance part of the task. Thus, the social support was during preparation for the speech, which is believed to be the period that is critical in stimulating the axis. The results showed that there was no response to the task among children if the parent was the speech preparation supporter, but for adolescents, large and equivalent cortisol responses were seen in both conditions [(Hostinar, Johnson, & Gunnar, 2015b) figure 3)]. Adolescents and children described their relationships with parents as equally close and rated the TSST-C as equally stressful.
Figure 3.
Cortisol Response to TSST-C estimated from HLM analysis in Children (left panel) and Adolescents (right panel) with Social Support from Parent versus Stranger. Note that these levels are higher in Adolescents than Children, reflecting the increase in basal cortisol activity with pubertal development. (Reprinted with permission from Hostinar et al., 2015b).
Why were parents losing their potency? To help us get closer to the mechanism, we asked whether the shift was more closely correlated with age or pubertal stage (Doom, Hostinar, VanZomeren-Dohn, & Gunnar, 2015). To tease apart age and pubertal stage we focused on children 11 to 14 years when same-aged youth can be at very different pubertal stages and we preselected our participants to completely cross puberty with age. We then conducted the same paradigm as in Hostinar et al., 2015. The results supported pubertal stage and not age as being the place to look for the mechanism(s) underlying parents losing their stress buffering potency.
Overall, these studies indicate that parents remain powerful stress buffers throughout childhood. With puberty their potency begins to wane. Of course, teens still rely on their parents and report that they perceive high levels of support from them (Bokhorst, Sumter, & Westenberg, 2010). Attachment to parents also persist through adolescence (Freeman & Brown, 2001). What our data suggests, however, is that adolescents do not get as deep a physiological pay-off from the presence of their parents as they did prior to puberty. I should note, however, that the question of parents becoming less potent social buffers has only been studied using the TSST-C. We do not know whether they lose their potency when the threat is physical harm or social rejection. Furthermore, the reduction in parental stress buffering potency could be particular to the HPA axis. We do not know whether it extends to buffering of the cardiovascular or other stress-sensitive systems. But even if the lost buffering potency is only an HPA axis phenomenon, what does this imply for stress vulnerability and resilience in adolescence?
Beyond Parents as Social Stress Buffers
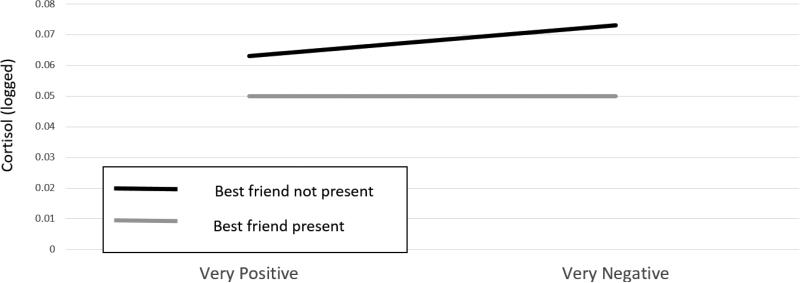
As already noted, there is little evidence that non-attachment figures can be potent stress buffering agents early in life. However, what about as children develop and their relationships increasingly extend beyond the family? We have very few studies to go on to answer this question and those we have focus on peers. A relevant study was conducted by Adams and colleagues with 5th and 6th graders (Adams, Santo, & Bukowski, 2011). They collected cortisol, measures of self-esteem, and self-reports of experiences rated from positive to negative repeatedly across four school days. The students also recorded whether their best friend was with them or not during the experience. For both boys and girls, the presence of the best friend seemed to block cortisol increases 20 minutes after negative experiences (figure 4). We do not know what those experiences were or whether the friend was caught up in the experience (e.g., both were on the losing team, both being teased), nor do we know what the friend did in relation to the experience (emotional support, instrumental support). Nonetheless, this study suggests that for negative school experiences, the presence of a best friend may reduce children's stress responding. This conclusion supports the friendship protection hypothesis (Kendrick, Jutengren, & Stattin, 2012) that argues that having friends protects children from the negative effects of being victims of bullies. However, while there is ample evidence that being victimized is stressful, increases the risk for psychopathology, and has impacts on the HPA axis (Ouellet-Morin et al., 2011), there is no clear evidence that the HPA axis is buffered from the effects of peer victimization when children have friends available. This information would be very valuable in helping to explain how friends help to reduce the long term negative impacts of peer victimization.
Figure 4.
Associations between the negativity of the experience and changes in cortisol levels when a best friend was present and when the best friend was not present. (Reprinted with permission from Adams, Santo and Bukowski, 2011).
While friends may provide stress buffering in childhood, what happens in adolescence? It seems logical to think that they will become even more important, perhaps taking over the job of stress buffers that parents once served. Adolescents spend more time affiliating with friends and they show more positive affect when they are with friends (Scholte & van Aken, 2006). They also experience more distress than do children when a negative event is peer-related, especially if they are girls (Conley & Rudolph, 2009). To address whether peers became potent stress buffers with adolescence, we used the same paradigm as in the original Hostinar et al (2014) study. Children aged 9 and 10 and adolescents aged 15 and 16 prepared for the TSST-C with either their parent or their same sexed, best friend (Doom, Doyle, & Gunnar, 2016). So that the only difference between conditions was the 5-minute speech preparation period, we had all participants come to the laboratory with both their parent and best friend. Just before speech preparation we told them with whom they would preparing their speeches. The results were quite clear and unequivocal. Preparing with your best friend versus your parent increased the reactivity of the HPA axis for adolescents but not for children. Indeed, it produced some of the nicest cortisol stress responses we had seen in the lab. In retrospect, the TSST-C is a social evaluative stressor and we were asking the best friend to help our participants to prepare a speech where they would talk about what was good and not so good about themselves! What we do not know is whether best friends might have provided a stress buffer to a different type of stressor or even a different type of speech.
It is notable, however, that the Doom et al. (2016) paper is not our only evidence of friends producing higher levels of cortisol. In one study (Calhoun et al., 2014) researchers had adolescents debrief from a social speech stressor with a best friend for four minutes. Being with a close friend from a more negative quality friendship prolonged cortisol levels long after the youth were done with the stressor task. Likewise, there is evidence that even in emotionally supportive friendships, debriefing with your friend might backfire if you and your friend end up ruminating on the negative aspects of your experiences. Indeed, in youth at risk for depression, rumination has been shown to impair cortisol recovery (Stewart, Mazurka, Bond, Wynne-Edwards, & Harkness, 2013). Thus, the situation for friends as social stress buffers in adolescence appears to be complex and nuanced. This may be part of why adolescence is a time when we are both near the peak of our capacities and at our most vulnerable (Dahl & Gunnar, 2009).
Sex Differences in Social Buffering
We do not see any sex differences in the potency of the attachment figure to buffer stress in early childhood, but what about after infancy and early childhood? In our own work, we have not found significant interactions between social buffering partner conditions (parent vs researcher; parent vs best friend) and sex-of-participant (Doom et al., 2016; Doom et al., 2015; Hostinar et al., 2015b). However, in all honestly we have not really had numbers in these sex by condition groups, particularly if we also include age or pubertal stage, to really be confident that gendered differences are not emerging with development. Certainly, if we examine the adult literature on social support, it looks as if we should be observing sex differences as children get older. It also helps clarify how complex the issue of sex differences can become.
First, there is much stronger evidence that social support buffers the activity of the HPA axis for men than for women. This is despite the argument that women cope with threat by tending and befriending one another, while men cope with fighting or fleeing (Taylor et al., 2000). Thus, in one early study (Kirschbaum et al., 1995), the researchers asked a third of participants to bring their significant other to the stress session and then had them prepare for the speech with that person. They had another third go through the task with a supportive stranger of the opposite sex and a third were tested without a supportive partner (i.e., alone). As in our work, the supportive person (partner or stranger) was not present when they delivered their speech or performed the arithmetic part of the task. The results were unambiguous. Men were buffered by the presence of their significant other, while women showed larger cortisol increases if their significant other was there to support them. Men judged their significant other to be more effective in supporting them than the stranger. Women judged the supportiveness of the stranger and their partner to be about equally effective.
In a later study (Ditzen et al., 2007), these researchers examined whether there was anything that men could do to buffer the HPA system in their female partners. They contrasted verbal support as in the Kirschbaum et al. (1995) study with massaging the woman's neck while not talking to her. The neck massage worked to both reduce cortisol and heart rate responses to the stressor. Despite the fact that describing the results of these two studies to female audiences always gets a good laugh and knowing nods, I really do not think we know what is happening that produces this gendered difference. Is it the nature of the support provider? Would women gain more stress relief it they brought their best girlfriend with them? Is it how men provide verbal support that is not working for their female partners? Is this the product of social experiences that lead a woman who is being helped by a man to feel less competent and thus more anxious about social evaluation in a public speaking task? Or does the difference lie in the neurobiology of how social support impacts the stress response systems of men and women?
This same research group conducted an additional study that helps address at least one aspect of this problem. Specifically, is it the sex of the support provider that matters? In this study of social buffering the participant was asked to come with their best friend or come alone (Heinrichs et al., 2003). The participants were all male, and the sex of the best friend was not specified by the researchers, thus some participants brought male and some female best friends. Over half of the time the friend was male (Heinrichs, personal communication, August, 2016). Despite the support provider often being male, the presence of a best friend did reduce the HPA response for these male participants. It did so even better if the participant was administered a dose of nasal oxytocin. Thus at least for men, support from a best guy friend seems to be effective. Of course, the findings for men does not answer the question of whether a best same-sex friend would buffer the HPA axis for women.
In all three of these studies, the friend helped the participant prepare for the stress task. There is another way to conduct this type of study. Specifically, the friend or partner can be in the waiting room, but then the participant is taken away for the study. The participant then comes back to the waiting room once the stressor is over and waits a bit more with their friend or partner. This type of study mimics what often happens in medical settings. When the study is conducted in this manner, there is some evidence that women (and men) in relationships with partners who engage in supportive interactions before and after the stressor task show a faster recovery in cortisol than do those with friends or partners who engage in less supportive or more negative support behaviors (Lepore, Allen, & Evans, 1993). These findings raise the possibility that for women, what interferes with the effectiveness of social buffering by male relationship partners is having the man “help” the woman prepare for her speech. Uchino (2009) has shown that received support, which would involve helping the person plan their speech, can backfire and increase stress precisely when it reduces the recipient's sense of being able to master the situation, placing them in a more helpless position which lowers self-esteem.
One other possibility should be considered. It might be that for women it is not the HPA axis that is buffered by the presence of a companion. Perhaps for women the cardiovascular system is more affected. The weight of the evidence suggests this might be true. College women but not men wearing ambulatory blood pressure devices had lower systolic blood pressure if they reported more support during the recording period (Linden, Chambers, Maurice, & Lenz, 1993). Similar results were reported in two other studies (Phillips, Carroll, Hunt, & Der, 2006; Phillips, Gallagher, & Carroll, 2009); notably in these studies of social evaluative threat women supported by their male partners showed cardiovascular buffering, while men supported by their female partners did not. This is the reverse of the studies examining the HPA axis. Some studies of social buffering of the cardiovascular system did not involve both sexes but instead used women as the subjects. Many of these reported significant reductions in blood pressure or other indices of cardiovascular reactivity in the presence of a friend or otherwise supportive person, typically same sex (e.g., (Gerin, Pieper, Levy, & Pickering, 1992; Kamarck, Manuck, & Jennings, 1991). Importantly, however, the effectiveness of social support for the cardiovascular system was shown to be disrupted in women if the friend could observe their performance and thus became part of the evaluative audience (Kors, Linden, & Gerin, 1997).
Taken together, it seems possible that it may be easier to socially buffer the HPA axis response to threat in men and the cardiovascular response in women. The sex of the supporter may play a role, but it might also be a red herring. Why we observe sex differences in social buffering is not clear. These differences could be the product of gendered culture and thus be mediated by gendered psychological interpretations of support. Alternatively, these differences may reflect sex differences in the neural and endocrine pathways mediating the impact of social partners on stress reactivity and regulation. In either case, these differences seem to emerge between childhood and adulthood and this needs explication as the emergence of these differences is likely important for understanding sex differences in physical and mental health.
Relationship Quality, Social Buffering and Early Experiences
Because relationships can be both stress protective and stress inducing, the quality of the relationship matters in stress buffering throughout life. In early development we have clearly shown that the security of the attachment relationship influences how potent a stress buffer the attachment figure provides (e.g., (Nachmias et al., 1996). In work by Calhoun and colleagues (2014), negative aspects of friendships hindered stress recovery. In research on adult relationships, social buffering is sometimes referred to as dyadic coping (Bodenmann, Meuwly, & Kayser, 2011). Here the focus is on what partner A is doing to respond supportively to partner B's communication about the stress they are experiencing. The goal of successful dyadic coping is to reduce the stressed partner's perception of stress. These studies rely on self-report measures of success (Cutrona, Russell, & Gardner, 2005), which we know do not always match the recipient's physiological stress patterns. Nonetheless, a focus on relationship and/or friendship quality is likely critical for understanding how and whether the presence and availability of a partner will help or hinder physiological stress buffering. But while it is clear that relationship quality matters, it is not clear which aspects of quality matter. For example, in the Calhoun et al. (2014) work, they measured positive and negative dimensions of friendships and only the negative aspects affected cortisol recovery.
Of particular importance to developmentalists is understanding how early experiences of support from attachment figures influences how easily adolescents and adults can use social partners to buffer stress. This is the big question that I described early in this essay. Does the security of one's attachment relationships undergird one's capacity to gain stress relief from social partners throughout life? If so, how does this work? Is the effect direct, in that early experiences organize neurobiological and/or psychological mechanisms that permit the individual to gain stress-relieving benefit from social partners? Is it indirect, in that early experiences in a supportive, caring family shapes the psychological capacities, such as self-esteem and social skills, that are involved in establishing the type of social relationships that buffer stress? Are both direct and indirect mechanisms responsible?
To my knowledge, no studies have prospectively examined attachment security in infancy or early childhood and then used laboratory-based paradigms to examine social buffering in adulthood. There is one study, described in more detail below, that found that higher quality relationships between mothers and their 16-year-old adolescents predicted how well those individuals could use holding a friend's hand to reduce neural responses to the threat of electric shocks at 24 years (Coan, Beckes, & Allen, 2013). There is also good evidence that adults with different attachment working models when made frightened or anxious will engage in different types of social support seeking (Simpson, Rholes, & Nelligan, 1992). Notably, those with avoidant orientations seek less comfort from others than those with other attachment orientations. There are plenty of studies that have examined adult's perceptions of social support and related that to their reports of feeling loved and supported in their families as children (Uchino, 2009). Likewise, adults who report better relationships in childhood have lower levels of inflammation and cortisol (Fagundes, Bennett, Derry, & Kiecolt-Glaser, 2011). However, in these cases the use of retrospective reports of childhood experiences makes the results suspect. There are fewer, but very important, prospective studies of attachment security in infancy and adult outcomes. These tend to corroborate the studies using retrospective report. Thus, attachment security measured in infancy and early childhood predicts perceived health in adulthood, e.g., (Puig, Englund, Simpson, & Collins, 2013), and good adult relationships with romantic partners (Simpson, Collins, & Salvatore, 2011). It seems likely that early attachment relationships provide the foundation of our ability to benefit from social buffering.
The type of early relationship quality differences discussed above are in the normative, non-abusive range. Outside that range we do have more evidence that early experiences can impair later responsiveness to social buffering stimuli. Specifically, the absence of attachment relationships, as studied in nursery-reared monkeys, has been shown to result in an inability to use a familiar cage-mate to buffer the HPA axis to a novel-environment stressor (Winslow, Nobel, Lyons, Sterk, & Insel, 2003). The nursery- versus mother-rearing also resulted in lower cerebral spinal fluid levels of oxytocin, a potential mediator of social buffering impacts on the HPA axis. In human children, researchers in Wisconsin found that children adopted from orphanages did not increase oxytocin and reduce cortisol when in contact with their mothers versus a stranger, while children reared normally in families did (Wismer Fries, Shirtcliff, & Pollak, 2008). Likewise, we (Hostinar, Johnson, & Gunnar, 2015a) found that 9- and 10-year-olds adopted in early childhood from orphanages did not show any buffering of the HPA axis when they prepared for the TSST-C with their mothers versus a researcher. In contrast, as previously noted, youth reared from birth in their families of origin did. This latter finding held even though the adopted and non-adopted youth rated their relationships to parents as equally supportive and close. These findings from nursery-reared monkeys and children who spent their infancy in orphanages raise the possibility that there may be a sensitive period for organizing neural systems so that they are response to the stress-buffering potential of social partners. This is an important issue as it affects our understanding of ways in which early experiences enhance or impair our ability to gain protection from the negative consequences of stressor exposure via our close relationships throughout life.
Psychological and Neurobiological Mechanisms of Social Buffering
Obviously, the neurobiological mechanisms of social buffering must connect with the neurobiology and neuroendocrinology of stress that was outlined earlier in this essay. There are numerous avenues through which this could happen across different levels of the nervous and endocrine system. As Hennessy and colleagues (2009) argue, it is likely that social buffering occurs at all of these levels depending on the nature of the stressor, the type of contact with the partner, the nature of the relationship with the partner and the stage of development. Nonetheless, most theories of the mechanisms of social buffering argue that these operate through psychological pathways, particularly reductions in perceived threat and reductions in or inhibition of fear and anxiety. There is remarkably little evidence for such models in the social support literature or more narrowly in the social buffering literature (Uchino et al., 2012). Indeed, in the social buffering literature there are many instances in which autonomic and/or neuroendocrine activity is buffered by the presence of a social partner, but this does not affect reports of the aversiveness of the stressor or, in children the level of crying and behavioral distress observed. Nonetheless, neurobiological models of social buffering in adults have emphasized the role of the social partners as safety signals that increase the activity of regulatory regions in the prefrontal cortex (PFC) and therefore reduce activity in fear and pain circuits.
Animal and human studies of adults strongly support a focus on circuits in the PFC. In rodent studies, for example, lesions in prelimbic regions have been shown to block social buffering (Herman, Ostrander, Mueller, & Figueiredo, 2005). In humans, Eisenberger and her colleagues (2011) exposed female participants to pain stimuli while looking at an image of their romantic partner on some trials and a stranger on others Eisenberger et al., 2011). When participants looked at their romantic partner, pain ratings were lower and activity in the ventromedial prefrontal cortex (vmPFC) was greater and inversely related to activity in pain-reactive regions, including the dorsal anterior cingulate (dACC) and anterior insula (Hornstein, Fanselow & Eisenberger, 2016).
We have very few developmental studies to draw on in considering how these adult patterns emerge; however, one study is suggestive. In this study (Gee et al., 2014), children aged 4 through 10 and adolescents aged 11 through 17 years were imaged while looking at either their mother's face or a stranger's (other child's mother) face. Faces are known to evoke strong responses of the amygdala. The authors examined both amygdala and amygdala-PFC connectivity. Results showed that among adolescents, amygdala-PFC connectivity was strong regardless of the image they were observing and there were no condition differences in amygdala reactivity. Among children, however, connectivity was stronger and the amygdalar response was less when observing the mother's versus the stranger's face. Thus when looking at a picture of one's mother, neural emotion-regulatory circuitry was functionally more mature.
Unfortunately, in all of these studies measures of cardiovascular activity and cortisol were not obtained, thus we do not know whether the effects of images of the romantic partner or of the mother were actually effective in buffering the activity of autonomic and neuroendocrine components of the stress system. Currently, we have a body of literature on social buffering of pain and fear, and a body of literature on social buffering of stress responses of the SAM and HPA system, but no studies that actually link the two.
Failure to acquire physiological indices of stress in these imaging studies was due, in part, to how difficult it is to activate the HPA axis in imaging environments. Earlier, Eisenberger and colleagues (Eisenberger, Taylor, Gable, Hilmert, & Lieberman, 2007) used two different laboratory paradigms to examine the neural systems linking social support to reductions in reactivity of the HPA axis. They had participants keep diaries of their daily experiences of social support for ten days as they went about their everyday lives. Then they brought these participants to the laboratory twice, once for the TSST during which they collected cortisol, and once to be imaged while they played a social rejection task called Cyberball. Individuals who reported more supportive relationships in their daily diaries had lower cortisol responses to the TSST. These individuals also showed diminished activity in the dACC during rejection in Cyberball. Increased vmPFC activity was not found in this study. Thus in this study reporting a good deal of social support on a regular basis seemed to reduce the participant's experience of social pain when rejected during the Cyberball computer game and may also have decreased their sense of threat during the social evaluative TSST task. It did not, however, increase the activation of emotion-inhibiting circuits in the PFC.
Other studies have also found more direct effects of social partners on pain and emotion circuit activity. What is notable about these studies is that the partner is actually and not just symbolically present. Thus, in a study in which adult women were threatened with electric shock, neural activity was recorded during blocks when she held her husband's hand, the hand of an anonymous male experimenter, or no hand (Coan, Schaefer, & Davidson, 2006). Compared to no contact blocks, holding the husband's hand resulted in significantly less activation in systems supporting emotional and behavioral threat responses, including the right anterior insula, superior frontal gyrus, and hypothalamus. The biggest effects were obtained for women who reported the highest marital quality. No increase in activity in the vmPFC or other regions with inhibitory input to limbic regions was noted.
Importantly, from a developmental perspective, similar results were reported by this group when they collected observational measures of the quality of mother-adolescent interactions at 16 years and then assessed these same individuals at 24 years while they were imaged facing threat. While being imaged, individuals held the hand of an opposite-sex platonic friend, an anonymous opposite-sex experimenter, or did not hold anyone's hand (Coan et al., 2013). Again the threat was an electric shock and here the results showed less threat-related activation in the bilateral orbitofrontal cortex, inferior frontal gyrus and left insula during friend handholding for individuals who had more positive observed relationships with their mothers at age 16. The quality of the mother-adolescent relationship did not predict neural reactivity in the stranger and no handholding blocks of trials. These findings provide more support for the attachment relationship being the foundation of our capacity to use social partners to buffer stress.
Taken together the studies discussed in this section raise questions about the role of inhibitory circuits in the PFC in mediating the effects of social buffering. Do partners increase the ability to inhibit pain and fear reactions that are stimulated by the stressful situation? Or are we simply less reactive to painful and threatening stimuli when we are with partners, especially those we trust and feel close to? It is hard not to notice one of the major differences in the paradigms that implicate the PFC emotion-regulating circuits from those that seem to have a more direct impact on pain- and emotion-activating systems. Specifically, in the former case the individual is alone in the scanner and the presence of the partner is only symbolic, while in the latter case there is actual physical contact with the partner. Perhaps the pathways to regulation of stress differs under symbolic versus actual contact conditions, involving more frontal inhibitory activity when the partner is only symbolically present. Alternatively, Beckes and Coan (2011) argue that the effects reported above are not due to the presence of a social partner, but rather to their absence. They argue that being with others is our natural condition and when social partners are not available this activates regions of the brain that are involved in processing threat. The presence of relationship partners directly impacts these systems and allows us to deal with threat without using as many costly metabolic resources. If they are correct, then we many only need to bring on PFC inhibitory circuits when we confront threat on our own. When that is the case, thinking about our loved ones helps us recruit that inhibitory circuitry and contain our tendency to experience pain and fear. Of course, while the threat of a mild shock may be mildly fear-evoking, threat of significant damage to our relationships, social standing and/or our body can be extremely frightening. It seems possible that whether we observe direct effects of the partner or effects that appear to be mediated by activation of PFC regulatory circuitry might depend on both the intensity of the threat we are facing and the degree of access we have to our social partner. The circuitry that is recruited also likely depends on the developmental status of the brain.
So far we have noted one study of the brain activity during social buffering in children and adolescents. What about earlier in development? It seems highly unlikely that social buffering in infancy and very early childhood is operating through the pathways thus far described. These regions and circuits are immature in infancy, yet as we have noted, attachment figures become powerful buffering agents by the end of the first year. There are two animal studies, one of infant guinea pigs and one of rat pups that suggest that early in development the mother's presence may rather directly control regions in the hypothalamus and brain stem that regulate stress and arousal (Harvey, Moore, Lucot, & Hennessy, 1994; Shionoya, Moriceau, Bradstock, & Sullivan, 2007). Specifically, in these studies norepinephrine in the hypothalamus was blocked by the mother's presence. Maternal stimuli also increased oxytocin levels in the infant, and oxytocin can operate directly at the level of the pituitary to suppress adrenocorticotropic hormone (Gibbs, 1986) and at the PVN to suppress corticotropin-releasing hormone (Gimpl & Fahrenholz, 2001); although most often oxytocin's effects are indirect through receptors in forebrain regions that regulate autonomic and neuroendocrine stress responses (Windle et al., 2004). Clearly, we need to know much more than we do about the neurobiology of social buffering early in life. We also need to know how experiences early in development are shaping the neurobiological pathways regulating the stress buffering impact of social partners as neural system becomes mature.
Conclusions and Future Directions
There is little doubt that social buffering serves as a critical process that protects us from over-exposure to stress. There is growing evidence that social buffering by attachment figures early in life is not an immature form of adult social buffering, but is the foundation out of which our capacity to gain stress-relieving benefits from the presence and availability of friends and family emerges. There are some hints in the available data that there may be critical or sensitive periods in early development when different neurobiological systems are open to being organized around social relationships in ways that may influence the capacity of social partners to access these systems to foster stress buffering later in development. This hypothesis has the most support with regards to the oxytocin system, but it may also extend to the HPA axis. If there is a sensitive or critical period, we do not know whether there are later periods when these systems become open to reorganization, perhaps during puberty or, for women, during pregnancy. Understanding the role of early experiences in organizing stress-moderating and stress-mediating systems around social buffers is a major direction for future research.
Addressing the sensitive/critical period question, however, involves vastly increasing our understanding of the neurobiology of social buffering in adults and across development. We also need to connect our understanding of the neurobiology of social buffering of pain and fear circuits with our understanding of the neurobiology of social buffering of the HPA and SAM systems. The former is advancing in very interesting ways. Connecting our understanding of the neurobiology of the social buffering of fear with the neurobiology of the social buffering of the HPA and SAM systems has challenges that make it quite difficult to accomplish. However, this direction of work is truly needed. This direction will also require integration of the animal and human research if we are to go young, as below a certain age it is very difficult to obtain imaging data on awake and active human children. A group of us, including Regina Sullivan, Mar Sanchez, Nim Tottenham, and Camelia Hostinar have been working to integrate the animal and human work on social buffering, fear learning, and stress regulation with the help of a three-year conference grant from NSF. The challenges, we have learned, are not trivial, but the opportunities are also exciting (Gunnar, Hostinar, Sanchez, Tottenham, & Sullivan, 2015; Gunnar & Sullivan, 2016).
Social relationships cut both ways. They can be our greatest source of stress relief and our greatest source of stress and sorrow. Throughout this essay I have focused on the presence of social partners in reducing stress; however, even in the studies described, there was evidence that in some cases, the social partner was amplifying stress reactions. Thus in Calhoun and colleagues (2014) study, debriefing by a friend with whom the participant had a negative relationship produced prolonged elevations in cortisol, despite a quick return to baseline when the debrief was with a friend from a low negative relationship. The presence of the parent in an insecure attachment relationship does not provide a particularly effective stress buffer, but when we examined the behavior of those parents we found ample evidence that they might be interfering with their children's attempts to regulate distress (Nachmias et al., 1996). In other work, we found that preparing for a speech with a friend versus one's parent increased cortisol responses to the TSST-C (Doom et al., 2016). When does the presence of a partner help and when does it hinder our attempts to regulate stress? This is a critical question that needs more work, particularly from a developmental perspective.
We also need to integrate research on social buffering of the autonomic and cardiovascular system with work on the HPA axis. As noted in the section on sex differences, stress buffering of the two arms of the mammalian stress system may not proceed of a piece. These two arms tell us different things about the state of the body and have different impacts on health and disease. Studying both arms together, however, is not as simple as measuring both autonomic and neuroendocrine systems. Sadly, they seem to require different paradigms to be assessed well. Situations that are potent enough to activate the HPA axis, seem maximal for measures of heart rate and vagal tone, for example. While stressors that allow nuanced evaluation of autonomic measures are often submaximal for activation of the HPA axis. Nonetheless, we need to examine social buffering using both autonomic and neuroendocrine end points if we are to obtain a full picture of the development of social buffering, including the emergence of sex differences with development.
Finally, as the evidence mounts that experiences in our first relationships shape our ability to use social partners to relieve and buffer us from stress, we need to increase our focus on when and how to intervene to rescue individuals whose experiences have impaired their capacity to form and/or use close relationships in stress buffering. We also need to develop a two generation perspective on social buffering. Early in my career I was part of a study that examined whether adult rhesus monkeys who were rehoused with infants versus alone would show a more rapid return to baseline cortisol levels (Gonzales, Gunnar, & Levine, 1981). The mothers caged with their infants did return to baseline faster than non-maternal females who were caged alone, but there was one mother who had a very disturbed relationship with her infant who maintained high cortisol levels for a prolonged period despite the baby being with her. Subsequently, this mother's infant actually had lower cortisol levels when he was separated from her than he had 24 hours after reunion (Gunnar et al., 1981). We do not know the early life history of this mother, but it is possible that it was traumatic and/or devoid of appropriate attachments. Mothers do gain stress buffering benefits from holding and nursing their babies (Heinrichs et al., 2001), and it is possible that failure to experience these benefits may add to problems mothers who experienced maltreatment early in life often have relating to and forming secure relationships with their own children.
As can be seen, the study of social buffering of stress is an active and generative research area in adult research in health and social psychology and in development science. There are a number of avenues of research that are ripe for pursuit. The answers promise to fill in gaps in our understanding of how supportive relationships protect our health and psychological well-being, how our capacity to form and use supportive relationships develops, as well as ways to intervene and the best timing of interventions to foster recovery of the capacity to gain stress-relief from social partners.
Acknowledgments
Work on this review was supported by NSF grant on the Neurodevelopment of stress, social buffering and fear learning: Integration and crosstalk, BCS-1439258 and NICHD grant, Pubertal Stress Recalibration Hypothesis, R01 HD075349 to Megan R. Gunnar and JPOB Foundation: Research Network on Toxic Stress and Health to Jack Shonkoff with a subcontract to Megan R. Gunnar. I am also grateful to my colleagues on the NSF grant, Regina Sullivan, Mar Sanchez, Nim Tottenham, and Cam Hostinar for the many discussions we have had that have furthered my thinking about social buffering and development. My thanks also to my many colleagues, students and staff without whom the work described here could not have been completed.
References
- Adams RE, Santo JB, Bukowski WM. The presence of a best friend buffers the effects of negative experiences. Developmental Psychology. 2011;47:1786–1791. doi: 10.1037/a0025401. [DOI] [PubMed] [Google Scholar]
- Ahnert L, Gunnar MR, Lamb ME, Barthel M. Transition to child care: Associations with infant-mother attachment, infant negative emotion, and cortisol elevations. Child Development. 2004;75(3):639–650. doi: 10.1111/j.1467-8624.2004.00698.x. [DOI] [PubMed] [Google Scholar]
- Albers EM, Riksen-Walraven JM, Sweep FC, de Weerth C. Maternal behavior predicts infant cortisol recovery from a mild everyday stressor. Journal of Child Psychology & Psychiatry. 2008;49:97–103. doi: 10.1111/j.1469-7610.2007.01818.x. [DOI] [PubMed] [Google Scholar]
- Beckes L, Coan JA. Social baseline theory: The role of social proximity in emotion and economy of action. Social and Personality Psychology Compass. 2011;5(12):976–988. [Google Scholar]
- Bernard K, Peloso E, Laurenceau JP, Zhang Z, Dozier M. Examining change in cortisol patterns during the 10-week transition to a new child-care setting. Child Development. 2015;86:456–471. doi: 10.1111/cdev.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blass E,M, Ciaramitaro V. Oral determinants of state, affect and action in newborn humans. Monographs of the Society for Research in Child. 1994;59:1–96. [PubMed] [Google Scholar]
- Bodenmann G, Meuwly N, Kayser K. Two conceptualizations of dyadic coping and their potential for predicting relationship quality and individual well-being: A comparison. European Psychologist. 2011;16:255–266. [Google Scholar]
- Bokhorst CL, Sumter SR, Westenberg PM. Social support from parents, friends, classmates, and teachers in children and adolescents aged 9 to 18 years: Who is perceived as most supportive? Social Development. 2010;19:417–426. [Google Scholar]
- Bowlby J. Attachment and Loss: Attachment. Vol. 1. Basic Books; New York: 1969. [Google Scholar]
- Bowlby J. Attachment and Loss: Separation. Vol. 2. Basic Books; New York: 1973. [Google Scholar]
- Calhoun CD, Helms SW, Heilbron N, Rudolph KD, Hastings PD, Prinstein MJ. Relational victimization, friendship, and adolescents' hypothalamic-pituitaryadrenal axis responses to an in vivo social stressor. Development and Psychopathology. 2014;26:605–618. doi: 10.1017/S0954579414000261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coan JA, Beckes L, Allen JP. Childhood maternal support and social capital moderate the regulatory impact of social relationships in adulthood. International Journal of Psychophysiology. 2013;88:224–231. doi: 10.1016/j.ijpsycho.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coan JA, Schaefer HS, Davidson RJ. Lending a hand: social regulation of the neural response to threat. Psychological Science. 2006;17:1032–1039. doi: 10.1111/j.1467-9280.2006.01832.x. [DOI] [PubMed] [Google Scholar]
- Coe CL, Mendoza SP, Smotherman WP, Levine S. Mother-infant attachment in the squirrel monkey: Adrenal response to separation. Behavioral Biology. 1978;22:256–263. doi: 10.1016/s0091-6773(78)92305-2. [DOI] [PubMed] [Google Scholar]
- Conley C,S, Rudolph KD. The emerging sex difference in adolescent depression: interacting contributions of puberty and peer stress. Development and Psychopathology. 2009;21:593–620. doi: 10.1017/S0954579409000327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowell RA, Cicchetti D, Rogosch FA, Toth SL. Childhood maltreatment and its effect on neurocognitive functioning: Timing and chronicity matter. Developmental Psychopathology. 2015;27:521–533. doi: 10.1017/S0954579415000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutrona CE, Russell DW, Gardner KA. The relationship enhancement model of social support. In: Revenson T, Kayser K, Bodenmann G. p.-W., editors. Couples coping with stress: Emerging perspectives on dyadic coping. American Psychological Association; Washington, DC: 2005. pp. 73–95. DC: American Psychological Association (Eds.) [Google Scholar]
- Dahl RE, Gunnar MR. Heightened stress responsiveness and emotional reactivity during pubertal maturation: Implications for psychopathology. Development & Psychopathology. 2009;21:1–6. doi: 10.1017/S0954579409000017. [DOI] [PubMed] [Google Scholar]
- Dallman MF, Akana SF, Pecoraro NC, Warne JP, la Fleur SE, Foster MT. Glucocorticoids, the etiology of obesity and the metabolic syndrome. Current Alzheimers Research. 2007;4:199–204. doi: 10.2174/156720507780362236. [DOI] [PubMed] [Google Scholar]
- Dettling AC, Gunnar MR, Donzella B. Cortisol levels of young children in fullday childcare centers: Relations with age and temperament. Psychoneuroendocrinology. 1999;24(5):505–518. doi: 10.1016/s0306-4530(99)00009-8. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Gruenewald TL, Kemeny ME. When the social self is threatened: shame, physiology, and health. Journal of Personality. 2004;72:1191–1216. doi: 10.1111/j.1467-6494.2004.00295.x. [DOI] [PubMed] [Google Scholar]
- Ditzen B, Neumann ID, Bodenmann G, von Dawans B, Turner RA, Ehlert U, Heinrichs M. Effects of different kinds of couple interaction on cortisol and heart rate responses to stress in women. Psychoneuroendocrinology. 2007;32:565–574. doi: 10.1016/j.psyneuen.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Doom JR, Doyle C, Gunnar MR. Social stress buffering by friends in childhood and adolescence: Effects on HPA and xxytocin activity. Social Neuroscience. 2016;25:1–14. doi: 10.1080/17470919.2016.1149095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doom JR, Hostinar CE, VanZomeren-Dohn, Gunnar MR. The roles of puberty and age in explaining the diminished effectiveness of parental buffering of HPA reactivity and recovery in adolescence. 2015. Under review. [DOI] [PMC free article] [PubMed]
- Doyle AB, Lawford H, Markiewicz D. Attachment style with mother, father, best friend, and romantic partner during adolescence. Journal of Research on Adolescence. 2009;19:690–714. [Google Scholar]
- Eisenberger NI, Mastera SL, Inagakia TK, Taylor SE, Shirinyan D, Lieberman MD, Naliboff BD. Attachment figures activate a safety signal-related neural region and reduce pain experience. Proceedings of the National Academy of Sciences. 2011;108:11721–11726. doi: 10.1073/pnas.1108239108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Taylor SE, Gable SL, Hilmert CJ, Lieberman MD. Neural pathways link social support to attenuated neuroendocrine stress responses. Neuroimage. 2007;35:1601–1612. doi: 10.1016/j.neuroimage.2007.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagundes CP, Bennett JM, Derry HM, Kiecolt-Glaser JK. Relationships and inflammation across the lifespan: Social developmental pathways to disease. Social and Personality Psychology Compass. 2011;5:891–903. doi: 10.1111/j.1751-9004.2011.00392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman F, Brown BB. Primary attachment to parents and peers during adolescence: Differences in attachment style. Journal of Youth and Adolescence. 2001;6:653–674. [Google Scholar]
- Gee DG, Gabard-Durnam L, Telzer EH, Humphreys KL, Goff B, Shapiro M, Tottenham N. Maternal buffering of human amygdala-prefrontal circuitry during childhood but not during adolescence. Psychological Science. 2014;25:2067–2078. doi: 10.1177/0956797614550878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geoffroy M, Cote SM, Parent S, Seguin JR. Daycare attendance, stress, and mental health. Canadian Journal of Psychiatry. 2006;51:607–615. doi: 10.1177/070674370605100909. [DOI] [PubMed] [Google Scholar]
- Gerin W, Pieper C, Levy R, Pickering TG. Social support in social interaction: A moderator of cardiovascular reactivity. Psychosomatic Medicine. 1992;52:324–336. doi: 10.1097/00006842-199205000-00008. [DOI] [PubMed] [Google Scholar]
- Gibbs DM. Stress-specific modulation of ACTH secretion by oxytocin. Neuroendocrinology. 1986;42:456–458. doi: 10.1159/000124487. [DOI] [PubMed] [Google Scholar]
- Gimpl G, Fahrenholz F. The oxytocin receptor system: Structure, function, and regulation. Physiological Reviews. 2001;81:629–668. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- Gonzales C, Gunnar MR, Levine S. Behavioral and hormonal responses to social disruption and infant stimuli in female rhesus monkeys. Psychoneuroendocrinology. 1981;6(1):55–65. doi: 10.1016/0306-4530(81)90048-2. [DOI] [PubMed] [Google Scholar]
- Groeneveld MG, Vermeer HJ, van IJzendoorn MH, Linting M. Stress, cortisol and well-being of caregivers and children in home-based child care: a case for differential susceptibility. Child: Care Health and Development. 2012;38:251–260. doi: 10.1111/j.1365-2214.2010.01194.x. [DOI] [PubMed] [Google Scholar]
- Gunnar MR. Reactivity of the hypothalamic-pituitary-adrenocortical system to stressors in normal infants and children. Pediatrics. 1992;90(3):491–497. [PubMed] [Google Scholar]
- Gunnar MR, Brodersen L, Krueger K, Rigatuso J. Dampening of adrenocortical responses during infancy: Normative changes and individual differences. Child Development. 1996;67(3):877–889. [PubMed] [Google Scholar]
- Gunnar MR, Brodersen L, Nachmias M, Buss KA, Rigatuso J. Stress reactivity and attachment security. Developmental Psychobiology. 1996;29(3):191–204. doi: 10.1002/(SICI)1098-2302(199604)29:3<191::AID-DEV1>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Davis EP. The developmental psychobiology of stress and emotion in early childhood. In: Weiner IB, Lerner RM, Easterbrooks MA, Mistry J, editors. Comprehensive Handbook of Psychology: Vol. 6. Developmental Psychology. Wiley; New York: 2003. pp. 113–143. [Google Scholar]
- Gunnar MR, Gonzales C, Goodlin C, Levine S. Behavioral and pituitary-adrenal responses during a prolonged separation period in infant Rhesus macaques. Psychoneuroendocrinology. 1981;6(1):65–75. doi: 10.1016/0306-4530(81)90049-4. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Gonzalez C, Levine S. The role of peers in modifying behavioral distress and pituitary-adrenal response to a novel environment in year-old Rhesus monkeys. Physiology and Behavior. 1980;25:795–798. doi: 10.1016/0031-9384(80)90387-x. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Herrera A. The development of stress reactivity: A neurobiological perspective. In: Zelazo PD, editor. Oxford Handbook of Developmental Psychology: Self and Other. Vol. 2. Oxford University Press; New York: 2013. pp. 45–80. [Google Scholar]
- Gunnar MR, Hostinar CE, Sanchez MM, Tottenham N, Sullivan RM. Parental buffering of fear and stress neurobiology: Reviewing parallels across rodent, monkey, and human models. Social Neuroscience. 2015;10(5):474–478. doi: 10.1080/17470919.2015.1070198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Kryzer E, vanRyzin MJ, Phillips DA. The rise in cortisol in family day care: Associations with aspects of care quality, child behavior, and child sex. Child Development. 2010;81:851–869. doi: 10.1111/j.1467-8624.2010.01438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Mangelsdorf S, Kestenbaum R, Lang S, Larson M, Andres D. Stress and coping in early development. In: Cicchetti D, editor. The emergence of a discipline. The Rochester symposium on developmental psychopathology. Vol. 1. Cambridge University Press; New York: 1989. pp. 119–138. [Google Scholar]
- Gunnar MR, Sullivan RM. The neurodevelopment of social buffering and fear learning: Integration and crosstalk. Social Neuroscience. 2016 doi: 10.1080/17470919.2016.1151824. e-pub a head of print. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Talge NM, Herrera A. Stressor paradigms in developmental studies: What does and does not work to produce mean increase in salivary cortisol. Invited paper Psychoneuroendocrinology. 2009;34:953–567. doi: 10.1016/j.psyneuen.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Vazquez D. Stress neurobiology and developmental psychopathology. In: Cicchetti D, Cohen D, editors. Developmental Psychopathology:Developmental Neuroscience. 2nd ed. Vol. 2. Wiley; New York: 2006. pp. 533–577. [Google Scholar]
- Harlow HF. The primate socialization motives. Transactions and studies of the College of Physicians of Philadelphia. 1966;33:224–237. [PubMed] [Google Scholar]
- Harvey AT, Moore H, Lucot JB, Hennessy MB. Monoamine activity in the anterior hypothalamus of guinea pig pups seprated from their mothers. Behavioral Neuroscience. 1994;108:171–176. doi: 10.1037//0735-7044.108.1.171. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biological Psychiatry. 2003;54:1389–1398. doi: 10.1016/s0006-3223(03)00465-7. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Meinlschmidt G, Neumann I, Wagner S, Kirschbaum C, Ehlert U, Hellhammer DH. Effects of suckling on hypothalamic-pituitary-adrenal axis responses to psychosocial stress in postpartum lactating women. Journal of Clinical Endocrinology and Metabolism. 2001;86:4798–4804. doi: 10.1210/jcem.86.10.7919. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Kaiser S, Sachser N. Social buffering of the stress response: diversity, mechanisms, and functions. Frontiers in Neuroendocrinology. 2009;30(4):470–482. doi: 10.1016/j.yfrne.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE. Neurocircuitry of stress: Central control of the hypothalamo-pituitary-adrenocortical axis. Trends in Neurosciences. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- Herman JP, McKlveen JM, Solomon MB, Carvalho-Netto E, Myers B. Neural regulation of the stress response: glucocorticoid feedback mechanisms. Brazilian Journal of Medical Biological Research. 2012;45:292–298. doi: 10.1590/S0100-879X2012007500041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Ostrander MM, Mueller NK, Figueiredo H. Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Progress in Neuropsychopharmacology and Biological Psychiatry. 2005;29:1201–1213. doi: 10.1016/j.pnpbp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Hermans EJ, Henckens MJ, Joëls M, Fernández G. Dynamic adaptation of large-scale brain networks in response to acute stressors. Trends in Neuroscience. 2014;37:304–314. doi: 10.1016/j.tins.2014.03.006. [DOI] [PubMed] [Google Scholar]
- Hornstein EA, Fanselow MS, Eisenberger NI. A safe haven: Investigating social-support figures as prepared safety stimuli. Psychological Science. 2016;27:1051–1060. doi: 10.1177/0956797616646580. [DOI] [PubMed] [Google Scholar]
- Hostinar CE, Johnson AE, Gunnar MR. Early social deprivation and the social buffering of cortisol stress responses in late childhood: An experimental study. Developmental Psychology. 2015a;51:1597–1698. doi: 10.1037/dev0000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostinar CE, Johnson AE, Gunnar MR. Parent support is less effective in buffering cortisol stress reactivity for adolescents compared to children. Developmental Science. 2015b;18:281–297. doi: 10.1111/desc.12195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostinar CE, Johnson AE, Gunnar MR. Parent support is less effective in buffering cortisol stress reactivity for adolescents compared to children. Developmental Science. 2015c;18(2):281–297. doi: 10.1111/desc.12195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HosDtinar CE, Sullivan RM, Gunnar MR. Psychobiological mechanisms underlying the social buffering of the hypothalamic-pituitary-adrenocortical axis: A review of animal models and human studies across development. Psychological Bulletin. 2014;140(1):256–282. doi: 10.1037/a0032671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson SW, Bihun JT, Chiodo LM, Berube RL. Differences in cortisol levels in infants exposed prenatally to alcohol and cocaine. Infant Behavior and Development. 1994;17:722. [Google Scholar]
- Joëls M, Baram TZ. The neuro-symphony of stress. Nat Rev Neurosci. 2009;10:459–466. doi: 10.1038/nrn2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AE, Bruce J, Tarullo AR, Gunnar MR. Growth delay as an index of allostatic load in young children: Predictions to disinhibited social approach and diurnal cortisol activity. Development and Psychopathology. 2011;23:859–871. doi: 10.1017/S0954579411000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamarck T, Manuck SB, Jennings JR. Social support reduces cardiovascular reactivity to psychological challenge: A laboratory model. Psychosomatic Medicine. 1991;52:42–58. doi: 10.1097/00006842-199001000-00004. [DOI] [PubMed] [Google Scholar]
- Kaplan BH, Cassal JC, Gore S. Social support and health. Medical Care. 1977;15:47–58. doi: 10.1097/00005650-197705001-00006. [DOI] [PubMed] [Google Scholar]
- Kaufman IC, Rosenblum LA. Effects of separation from mothers on the emotional behavior of infant monkeys. Annals of the New York Academy of Sciences. 1969;159 doi: 10.1111/j.1749-6632.1969.tb12971.x. [DOI] [PubMed] [Google Scholar]
- Kendrick K, Jutengren G, Stattin H. The protective role of supportive friends against bullying perpetration and victimization. journal of Adolescence. 2012;36:1069–1080. doi: 10.1016/j.adolescence.2012.02.014. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Klauer T, Filipp SH, Hellhammer DH. Sex-specific effects of social support on cortisol and subjective responses to acute psychological stress. Psychosomatic Medicine. 1995;57:23–31. doi: 10.1097/00006842-199501000-00004. [DOI] [PubMed] [Google Scholar]
- Kors DJ, Linden W, Gerin W. Evaluation interferes with social support: Effects on cardiovascular stress reactivity in women. Journal of Social and Clinical Psychology. 1997;16:1–23. [Google Scholar]
- Larson MC, Gunnar MR, Hertsgaard L. The effects of morning naps, car trips, and maternal separation on adrenocortical activity in human infants. Child Development. 1991;62(2):362–372. [PubMed] [Google Scholar]
- Lepore SJ, Allen KA, Evans GW. Social support lowers cardiovascular reactivity to an acute stressor. Psychosomatic Medicine. 1993;55:518–524. doi: 10.1097/00006842-199311000-00007. [DOI] [PubMed] [Google Scholar]
- Levine S. Infantile experience and resistance to physiological stress. Science. 1957;126:405–406. doi: 10.1126/science.126.3270.405. [DOI] [PubMed] [Google Scholar]
- Levine S, Chevalier JA, Korchin SJ. The effects of early handling and shock on later avoidance behavior. Journal of Personality. 1956;24:475–493. doi: 10.1111/j.1467-6494.1956.tb01283.x. [DOI] [PubMed] [Google Scholar]
- Lewis M, Ramsay DS. Stability and change in cortisol and behavioral responses to stress during the first 18 months of life. Developmental Psychobiology. 1995;28(8):419–428. doi: 10.1002/dev.420280804. [DOI] [PubMed] [Google Scholar]
- Linden W, Chambers L, Maurice J, Lenz JW. Sex differences in social support, self-deception, hostility, and ambulatory cardiovascular activity. Health Psychology. 1993;12(5):376–380. doi: 10.1037//0278-6133.12.5.376. [DOI] [PubMed] [Google Scholar]
- Maccoby EE, Masters JC. Attachment and dependency Carmicael's Maual of Child Psychology. 1970;2:73–157. [Google Scholar]
- McKlveen JM, Myers B, Herman JP. The medial prefrontal cortex: coordinator of autonomic, neuroendocrine and behavioural responses to stress. Journal of Neuroendocrinology. 2015;27:446–456. doi: 10.1111/jne.12272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza SP, Smotherman WP, Miner MT, Kaplan J, Levine S. Pituitaryadrenal response to separation in mother and infant squirrel monkeys. Developmental Psychobiology. 1978;11(2):169–175. doi: 10.1002/dev.420110209. [DOI] [PubMed] [Google Scholar]
- Myers B, Scheimann JR, Franco-Villanueva A, Herman JP. Ascending mechanisms of stress integration: Implications for brainstem regulation of neuroendocrine and behavioral stress responses. Neuroscience and Biobehaval Reviews. 2016;S0149-7634(16):30092–30096. doi: 10.1016/j.neubiorev.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachmias M, Gunnar MR, Mangelsdorf S, Parritz R, Buss KA. Behavioral inhibition and stress reactivity: Moderating role of attachment security. Child Development. 1996;67(2):508–522. [PubMed] [Google Scholar]
- Ouellet-Morin I, Odgers CL, Danese A, Bowes L, Shakoor S, Papadopoulos AS, Arseneault L. Blunted cortisol responses to stress signal social and behavioral problems among maltreated/bullied 12-year-old children. Biological Psychiatry. 2011;70:1016–1023. doi: 10.1016/j.biopsych.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellet-Morin I, Tremblay RE, Boivin M, Meaney M, Kramer M, Côté SM. Diurnal cortisol secretion at home and in child care: a prospective study of 2-year-old toddlers. Journal of Child Psychology & Psychiatry. 2010;51:295–303. doi: 10.1111/j.1469-7610.2009.02167.x. [DOI] [PubMed] [Google Scholar]
- Patterson FG, Bonvillian JD, Reynolds PC, Maccoby EE. Mother and peer attachment under conditions of fear in Rhesus monkeys (Macaca mulatta). Primates. 1975;16:75–81. [Google Scholar]
- Phillips AC, Carroll D, Hunt K, Der G. The effects of the spontaneous presence of a spouse/partner and others on cardiovascular reactions to an acute psychological challenge. Psychophysiology. 2006;43:633–640. doi: 10.1111/j.1469-8986.2006.00462.x. [DOI] [PubMed] [Google Scholar]
- Phillips AC, Gallagher S, Carroll D. Social support, social intimacy, and cardiovascular reactions to acute psychological stress. Annals of Behavioral Medicine. 2009;37:38–45. doi: 10.1007/s12160-008-9077-0. [DOI] [PubMed] [Google Scholar]
- Porges SW, Furman SA. The early development of the autonomic nervous system provides a neural platform for social behavior: A polyvagal perspective. Infant and Child Development. 2011;20:106–118. doi: 10.1002/icd.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puig J, Englund MM, Simpson JA, Collins WA. Predicting adult physical illness from infant attachment: a prospective longitudinal study. Health Psychology. 2013;32:409–417. doi: 10.1037/a0028889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachter S. The Psychology of Affiliation. Stanford University Press; Stanford: 1959. [Google Scholar]
- Scholte RHJ, van Aken M. Peer relations in adolescence. In: Jackson S, Goossens L, editors. Handbook of adolescent development. Psychology Press; New York, New York: 2006. pp. 175–199. [Google Scholar]
- Seltzer LJ, Ziegler TE, Pollak SD. Social vocalizations can release oxytocin in humans. Proceedings of the Royal Society of Biological Sciences. 2010;277:2661–2666. doi: 10.1098/rspb.2010.0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shionoya K, Moriceau S, Bradstock P, Sullivan RM. Maternal attenuation of hypothalamic paraventricular nucleus norephinphrine switches avoidance learning to preference learning in preweanling rat pups. Hormones and Behavior. 2007;52:391–400. doi: 10.1016/j.yhbeh.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson JA, Collins WA, Salvatore JE. The impact of early interpersonal experience on adult romantic relationship functioning: Recent findings from the Minnesota Longitudinal Study of Risk and Adaptation. Current Directions in Psychological Science. 2011;20:355–359. doi: 10.1177/0963721411418468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson JA, Rholes WS, Nelligan JS. Support seeking and support giving within couples in an anxiety-provoking situation: The role of attachment styles. Journal of Personality and Social Psychology. 1992;62:434–446. [Google Scholar]
- Smotherman WP, Brown CP, Levine S. Maternal responsiveness following differential pup treatment and mother-pup interactions. Hormones and Behavior. 1977;8:242–253. doi: 10.1016/0018-506x(77)90041-1. [DOI] [PubMed] [Google Scholar]
- Smotherman WP, Hunt LE, McGinnis LM, Levine S. Mother-infant separation in group-living rhesus macaques: A hormonal analysis. Developmental Psychobiology. 1979;12(3):211–217. doi: 10.1002/dev.420120304. [DOI] [PubMed] [Google Scholar]
- Spangler G, Schieche M, Ilg U, Maier U, Ackermann C. Maternal sensitivity as an external organizer for biobehavioral regulation in infancy. Developmental Psychobiology. 1994;27(7):425–437. doi: 10.1002/dev.420270702. [DOI] [PubMed] [Google Scholar]
- Sroufe LA, Waters E. Heart rate as a convergent measure in clinical and developmental research. Merrill-Palmer Quarterly. 1979;23(1):3–27. [Google Scholar]
- Stang HJ, Snellman LW, Condon LM, Conroy MM, Liebo R, Brodersen L, Gunnar MR. Beyond DPNB: A more humane circumcision. Pediatrics. 1997;100(2) doi: 10.1542/peds.100.2.e3. [DOI] [PubMed] [Google Scholar]
- Stewart JG, Mazurka R, Bond L, Wynne-Edwards KE, Harkness KL. Rumination and impaired cortisol recovery following a social stressor in adolescent depression. Journal of Abnormal Child Psychology. 2013;41:1015–1026. doi: 10.1007/s10802-013-9740-1. [DOI] [PubMed] [Google Scholar]
- Stroud L, Foster E, Handwerger K, Papandonatos GD, Granger D, Kivlighan KT, Niaura R. Stress response and the adolescent transition: performance versus peer rejection stress. Development & Psychopathology. 2009;21:47–68. doi: 10.1017/S0954579409000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchecki D, Rosenfeld P, Levine S. Maternal regulation of the hypothalamicpituitary-adrenal axis in the rat: The roles of feeding and stroking. Developmental Brain Research. 1993;75(2):185–192. doi: 10.1016/0165-3806(93)90022-3. [DOI] [PubMed] [Google Scholar]
- Sumner MM, Bernard K, Dozier M. Young children's full-day patterns of cortisol production on child care days. Archives of Pediatric and Adolescent Medicine. 2010;164:567–571. doi: 10.1001/archpediatrics.2010.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE, Klein LC, Lewis BP, Gruenewald TL, Gurung RAR, Updegraff JA. Biobehavioral responses to stress in females: Tend-and-befriend, not fight-orflight. Psychological Review. 2000;107(3):411–429. doi: 10.1037/0033-295x.107.3.411. [DOI] [PubMed] [Google Scholar]
- Uchino BN. Understanding the links between social support and physical health: A life-span perespective with emphasis on the separability of perceived and received support. Perspectives in Psychological Science. 2009;4:236–255. doi: 10.1111/j.1745-6924.2009.01122.x. [DOI] [PubMed] [Google Scholar]
- van den Bos E, van Duijvenvoorde AC, Westenberg PM. Effects of adolescent sociocognitive development on the cortisol response to social evaluation. Developmental Psychology. 2016;52:1151–1163. doi: 10.1037/dev0000133. [DOI] [PubMed] [Google Scholar]
- Vermeer HJ, Van IJzendoorn MH. Children's elevated cortisol levels at childcare: a review and meta-analysis. Early Childhood Research Quarterly. 2006;21:390–401. [Google Scholar]
- Watamura SE, Sebanc AM, Gunnar MR. Rising cortisol at childcare: Relations with nap, rest, and temperament. Developmental Psychobiology. 2002;40:33–42. doi: 10.1002/dev.10011. [DOI] [PubMed] [Google Scholar]
- Wiik KL, Loman MM, Van Ryzin MJ, Armstrong JM, Essex MJ, Pollak SD, Gunnar MR. Behavioral and emotional symptoms of post-institutionalized children in middle childhood. Journal of Child Psychology & Psychiatry. 2011;52(1):56–63. doi: 10.1111/j.1469-7610.2010.02294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windle RJ, Kershaw YM, Shanks N, Wood SA, Lightman SL, ngram CD. Oxytocin attenuates stress-inducedc-fos mRNA expression in specific forebrain regions associated with modulation of hypothalamo-pituitary-adrenal activity. Journal of Neuroscience. 2004;24:2974–2982. doi: 10.1523/JNEUROSCI.3432-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow JT, Nobel PL, Lyons CK, Sterk SM, Insel TR. Rearing effects on cerebrospinal fluid oxytocin concentrations and social buffering in rhesus monkeys. Nepsychopharmacology. 2003;28:910–918. doi: 10.1038/sj.npp.1300128. [DOI] [PubMed] [Google Scholar]
- Wismer Fries AB, Shirtcliff EA, Pollak SD. Neuroendocrine dysregulation following early social deprivation in children. Developmental Psychobiology. 2008;50:588–599. doi: 10.1002/dev.20319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang TY, Labonté B, Wen XL, Turecki G, Meaney MJ. Epigenetic mechanisms for the early environmental regulation of hippocampal glucocorticoid receptor gene expression in rodents and humans. Neuropsychopharmacology. 2013;38:111–123. doi: 10.1038/npp.2012.149. [DOI] [PMC free article] [PubMed] [Google Scholar]