ABSTRACT
Macroautophagy/autophagy is vital for cellular homeostasis and helps cells respond to various stress situations. Macropinocytosis enables cells to nonselectively engulf and take up large volumes of fluid and is known to supply amino acids to cells. The stem cell-enriched limbal epithelium has the machinery necessary to carry out both autophagy and macropinocytosis; however, both processes are relatively understudied in this tissue. We have demonstrated that these processes are linked via MIR103-MIR107, a microRNA family that is limbal epithelial-preferred. Loss of MIR103-MIR107 causes the accumulation of large vacuoles that originate, in part, from a dysregulation in macropinocytosis via activation of SRC-RAS signaling. We found that these vacuoles were autophagic in nature and retained in cells due to inappropriate regulation of end-stage autophagy. Specifically, MIR103-MIR107 regulates diacylglycerol-PRKC/protein kinase C and CDK5 (cyclin dependent kinase 5) signaling, which enables DNM1 (dynamin 1) to function in vacuole clearance.
KEYWORDS: dynamin, lysosomal reformation, microRNA, miR-103/107, stem cell
The corneal epithelium is a self-renewing stratified squamous epithelium that protects the underlying delicate structures of the eye, supports a tear film and maintains transparency so light can be transmitted to the interior of the eye. A unique feature of the corneal epithelium is that its stem cells reside in the basal layer of the adjacent limbal epithelium. Consequentially, the corneal epithelial basal layer is enriched in transit amplifying cells, which represent the stem cell progeny. The stem cell-enriched limbal epithelium is essential for assuring corneal epithelial homeostasis and the ability of the epithelial cells to rapidly re-epithelialize the surface following perturbations. This separation of stem cells and their progeny into 2 morphologically and biochemically distinct epithelia provides a unique opportunity to investigate the impact of autophagy in these 2 proliferative populations.
microRNA (miRNA) expression profiling in limbal versus corneal epithelial basal cells revealed that MIR103-MIR107 are preferentially expressed in the limbal epithelium. MIR103-MIR107 belong to a miRNA family that have an identical seed sequence with only a difference of 1 nucleotide in 5’ region and thus target common genes. We reported that MIR103-MIR107: (1) contribute to quiescence by targeting RPS6KA2/p90RSK2; (2) enhance proliferative capacity through negatively regulating WNT3A and MAP3K7; (3) regulate degradation of CDH1/E-cadherin via targeting NEDD9; and (4) function in repression of GJA1/Cx43 by inhibiting PTPRM. Poor GJA1/Cx43 expression is a characteristic of stem cell-enriched epithelia. Taken together, these findings suggest that MIR103-MIR107 regulate key aspects associated with stem cell behavior.
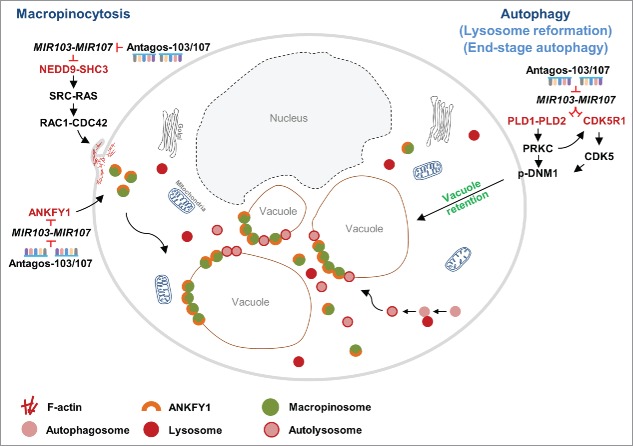
To determine the global effects of MIR103-MIR107 in human limbal epithelial keratinocytes (HLEKs) in an unbiased manner, we conducted an mRNA profiling study using HLEKs treated with an irrelevant antagomir vs antagomirs to MIR103-MIR107 (antagos-103/107). Bioinformatic analysis predicted that apoptosis, metabolic processes and response to stress are major biological events that are affected by depletion of this miRNA family. Interestingly, 6 h after depletion of MIR103-MIR107 in HLEKs, there is an accumulation of large vacuoles. We demonstrated that such vacuoles are developed, in part, due to dysregulated macropinocytosis. Depletion of MIR103-MIR107 in HLEKs upregulates: (1) NEDD9 and SHC3, which activates SRC and RAS signaling, respectively, and consequently increases macropinocytosis; and (2) ANKFY1, a key protein on macropinosomes, resulting in the escalation of macropinosome biogenesis (Fig. 1). Macropinocytosis is a process of uptake and degradation of extracellular proteins, which is critical for supplying amino acids. In MIR103-MIR107-depleted HLEKs, macropinocytotic uptake is functional but proteolytic cleavage in these macropinocytotic-derived vacuoles is deficient. This observation led to the idea that this microRNA family plays a critical role in ensuring proper macropinocytosis and correct metabolic processes. Dysregulation of macropinocytosis in other cell types has been associated with cell death. Therefore, we postulate that in the stem cell-enriched limbal epithelium, MIR103-MIR107 function to prevent such cell death induced by dysregulation of macropinocytosis and thus help maintain the homeostasis of limbal epithelial stem cells.
Figure 1.
A schematic representation of how MIR103-MIR107 coordinately regulate aspects of both macropinocytosis and autophagy. Loss of MIR103-MIR107 upregulates macropinocytosis via: (1) activation of NEDD9 and SHC3, which collectively activates SRC-RAS and consequently RAC1-CDC42; and (2) upregulation of ANKFY1, which is critical for macropinosome formation. This yields numerous vacuoles. Depletion of MIR103-MIR107 inhibits end-stage autophagy through activating PLD1, PLD2 and PRKC as well as CDK5R1-CDK5 pathways, which inactivate DNM1 causing vacuole retention. Red, inhibition; black, activation.
The question arose as to why the large vacuoles were retained in antagos-103/107-treated HLEKs. Since the morphology of the vacuoles was similar to autophagic vesicles, we investigated the possibility that their retention was due to a defect in autophagy. Consistent with this idea, LC3 and RAB11, markers commonly associated with autophagosomes, colocalize with lysosomal markers (e.g., LysoTracker) on the large vacuoles. Furthermore, autophagy flux is decreased in antagos-103/107-treated HLEKs. Pharmacological and genetic inhibition of autophagy at early stages prevents accumulation of large vacuoles in antagos-103/107-treated HLEKs. Collectively, these observations strongly suggest that loss of MIR103-MIR107 impairs end-stage autophagy resulting in large vacuole retention. This finding provided a novel insight into the mechanisms underlying end-stage autophagy. It has been reported that DNM1 plays an essential role in lysosome reformation and clearance during the end stage of autophagy. Phosphorylation of DNM1 blocks its binding to phospholipid resulting in inactivation. We demonstrated that loss of MIR103-MIR107 increases phosphorylation (inhibition) of DNM1. MIR103-MIR107 ensure DNM1 activity via: (i) targeting PLD1 and PLD2 and consequently downregulating phosphatidic acid and diacylglycerol synthesis, which diminishes the activity of PRKC (a kinase of DNM1); and (ii) targeting CDK5R1 (an activator of CDK5), leading to inhibition of CDK5, a kinase for DNM1 (Fig. 1). Repression of PRKC and CDK5 results in decreased phosphorylation of DNM1 (activation), which ensures lysosome recycling and clearance. It is well known that autophagy plays essential roles in stem cells, which are quiescent and require active elimination of unnecessary proteins and organelles. However, the biological significance autophagy in the stem cell-enriched limbal epithelium is understudied. Using a GFP-LC3 transgenic mouse, we observed a dramatically higher activity of autophagy in the basal layer of the limbal epithelium compared with the corneal epithelium. In HLEK cultures, inhibition of autophagy results in a decrease of holoclone colonies, considered to be derived from stem cells and a measure of proliferative capacity. These data suggest that autophagy functions, in part, to preserve the proliferative capacity of limbal epithelial basal cells.
Collectively, our findings show that MIR103-MIR107 coordinately repress macropinocytosis and preserve end-stage autophagy, which is the first demonstration that these 2 processes are linked. The regulation of both processes by MIR103-MIR107 is critical for limbal epithelial homeostasis. However, major questions remain unanswered. For example: (i) what are the molecular mechanism(s) underlying the autophagy-related increase in limbal epithelial proliferation; (ii) does autophagy directly affect limbal epithelial stem cell activation in vivo; (iii) is nucleophagy, a form of autophagy, essential for terminal differentiation in the limbal and corneal epithelia; and (iv) what role(s) does macropinocytosis play in corneal epithelial physiology?
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work is supported by National Institutes of Health grants EY06769, EY017539, and EY019463 (to R.M. L.), Dermatology Foundation research grant and Career Development Award (to H. P.), and a MidWest Eye Bank research grant (to H. P.).