Synopsis
Gastroesophageal cancer (GEC) remains a major cause of cancer-related mortality worldwide. Although the incidence of distal gastric adenocarcinoma (GC) is declining in the United States, proximal esophagogastric junction adenocarcinoma (EGJ) incidence is rising. GC and EGJ, together, are treated uniformly in the metastatic setting as GEC. Overall survival in the metastatic setting remains poor, with few molecular targeted approaches having been successfully incorporated into routine care to date – only first line anti-HER2 therapy for ERBB2 amplification and second line anti-VEGFR2 therapy. Here we review aberrations in EGFR, MET, and ERBB2, their therapeutic implications, and future directions in targeting these pathways.
Keywords: Gastroesophageal, gastric, MET, EGFR, HER2, ERBB2, treatment, targeted
Background
Distal gastric adenocarcinoma (GC) incidence remains the fifth most common cancer globally, and the third highest for cancer-related mortality.1–3 Approximately twenty-five thousand new GC cases and eleven thousand deaths were predicted in the United States in 2015.4 Further, esophagogastric junction adenocarcinoma (EGJ) incidence is increasing. When assessing GC and EGJ cancers, together known as gastroesophageal cancer (GEC), the majority of patients present with metastatic or locally advanced disease with a high risk of recurrence despite aggressive perioperative therapy. In the metastatic/recurrent setting, median overall survival remains approximately 11 months with optimal palliative chemotherapy in ERRB2 non-amplified patients. Over the past decade, molecular subtyping of GEC has highlighted the inter-patient heterogeneity of GEC and uncovered potentially actionable molecular pathways.5 Routine next generation sequencing identified that at least 37% of GC patients harbor genetic alterations, namely amplifications, in receptor tyrosine kinases (RTKs), including ERBB2, MET, EGFR, KRAS, and FGFR2.6–8 Clinical trials of agents targeting these pathways have had mixed results. However, interpretation of these results requires understanding both the agents used as well as the study population. These genomic events, as well as recently derived key subsets of the disease, namely microsatellite instability-high (MSI-high), EBV-associated (EBV), chromosomal instability (CIN), and genomically stable (GS), provide for more molecularly targeted therapeutic possibilities.9
ERBB2
ERBB2, or HER2, is a transmembrane RTK within the epidermal growth factor receptor (EGFR) family, encoded at chromosome17q21. HER2 regulates proliferation, adhesion, differentiation, and migration via activation of the RAS-MAPK and PI3K-AKT pathways (Figure 1). HER2 lacks an exogenous ligand and is transactivated via heterodimerization with other HER family members leading to downstream kinase activation. Significant and therapeutically relevant protein over-expression results predominantly from gene amplification; less commonly, other genomic events may include activating mutation. HER2 IHC expression localizes to the cell membrane in well-differentiated adenocarcinoma and to the cytoplasm in poorly-differentiated adenocarcinomas, which may affect treatment response.10 HER2 amplified tumors are more common with EGJ (15–32%) compared with the distal GC (10–15%), and the prognostic impact of HER2 amplification remains controversial (Table 1).11–18
Figure 1.
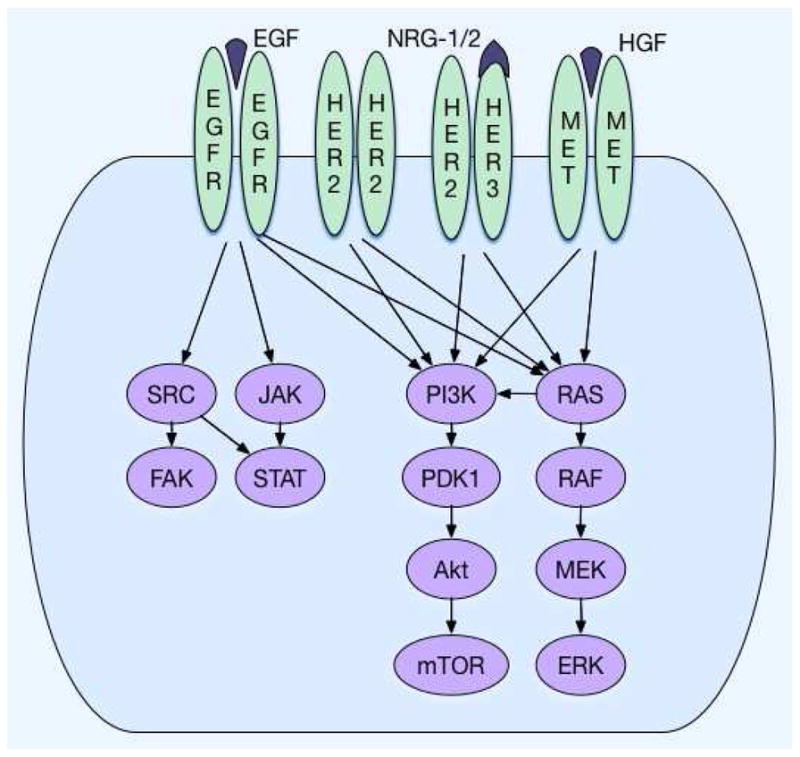
EGFR, HER2, and c-MET kinase cascade.
Table 1.
Rates of key receptor tyrosine kinase overexpression and gene amplification in gastroesophageal cancer
| ERBB2 | EGFR | MET | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Study | Amp (%) | Exp (%) | Amp (%) | Exp (%) | Amp (%) | Exp (%) | Population | Site |
| Nagatsuma46 | 9 | 11.8 | 2.4 | 24.9 | 1.3 | 24.9 | 950 | Gastric, resected |
| Nakajima66 | 11.7 | 16.4 | 10.2 | 46.1 | 128 | Gastric, resected | ||
| Lennerz47 | 8.9 | 4.7 | 2 | 489 | Gastroesophageal, all comers | |||
| Terashima14 | 13.3 | 13.6 | 9 | 829 | Gastric, resected | |||
| Van Cutsem87 | 4.1 | 21.4/32.2 (G/EGJ) | 3280 | Gastric/EGJ,advanced | ||||
| Kim44 | 2.3 | 27.4 | 511 | Gastric, resected | ||||
| Luber59 | 3 | 39 | Gastric/EGJ, advanced | |||||
| Petty60 | 6.1 | 450 | Esophageal/EGJ, advanced | |||||
| Catenacci63 | 5.2 | 35.2 | 394 | Gastroesophageal,resected | ||||
| Graziano68 | 10 | 216 | Gastric, resected | |||||
| Lee67 | 3.4 | 62.3 | 439 | Gastric, resected | ||||
| Jardim75 | 6 | 77 | Gastroesophageal, all comers | |||||
Amp: Amplification, Exp: Over-expression
Effective targeting of HER2 in GEC was initially demonstrated using trastuzumab, a humanized monoclonal anti-HER2 antibody against the HER2 ectodomain (Table 2). The phase III ToGA trial evaluated a first line fluoropyrimidine/cisplatin chemotherapy doublet with or without trastuzumab in patients with HER-2 over-expressing (any IHC 3+ or FISH HER2:CEP17 ratio ≥2) unresectable or metastatic GEC.18 Patients receiving trastuzumab survived a median of 13.8 months versus 11.1 months with chemotherapy alone (HR 0.74, p=0.0046), and response rates were 47% and 35%, respectively, in the intention-to-treat (ITT) population. In a subset analysis, median survival was 16 vs 11.8 months in the combined IHC2+/FISH+ and IHC3+ groups, accounting for 77% of the patients enrolled, whereas IHC0-1+/FISH+ patients appeared to derive no benefit. This trial therefore led to the approval of trastuzumab in HER2 over-expressing gastric cancer for the IHC2+/FISH+ and IHC3+ subsets of the trial.18,19
Table 2.
HER2-directed phase III clinical trials for gastroesophageal cancer
| Line | Trial | N | Treatment | Primary Endpoint (Met?) | mOS (mo) | HR | mPFS (mo) | HR | RR |
|---|---|---|---|---|---|---|---|---|---|
| 1L |
Bang et al18 TOGA |
584 | Cis/FP Cis/FP + Trastuzumab |
OS (Yes) | 11.8* 16* |
0.74 0.65* P<0.05* |
5.5 6.7 |
0.71 P<0.001 |
35% 47% |
| 1L |
Hecht et al22 LOGiC |
545 (487) | Cis/FP Cis/FP + Lapatinib |
OS (No) | 10.5 12.2 |
0.91 p=N.S. |
5.4 6 |
0.82 P=0.03 |
39% 53% |
| 2L |
Satoh et al26 TYTAN |
261 | Paclitaxel Paclitaxel+Lapatinib |
OS (No) | 8.9 11 |
0.84 p=N.S. |
4.4 5.5 |
0.84 p<0.001 |
9% 27% |
| 2L |
Kang et al27 GATSBY |
345 (1:2) | Pac38% / Doc 62% T-DM1 |
OS (No) | 8.6 7.9 |
1.15 p=N.S. |
2.9 2.7 |
1.13 p=N.S |
20% 21% |
Cis/FP: Cisplatin+Fluoropyrimidine; Pac: Paclitaxel; Doc: Docetaxel
Finally, whereas trastuzumab binds domain IV of HER2, pertuzumab binds domain II, and thereby prevents dimerization with other RTKS, namely HER3. The CLEOPATRA trial in breast cancer revealed progression-free and overall survival benefits with the addition of pertuzumab to trastuzumab and chemotherapy as first line therapy,20 and initial results from the counter-part JACOB trial for GEC evaluating pertuzumab in combination with trastuzumab and chemotherapy are pending.21 A large trial with appropriate HER2 selection (ie not allowing IHC0-1+/FISH+ patients), and without the concern of later line evolution (ie not second or later line of therapy) nor lack of ADCC (ie like the lapatinib trials) will likely allow JACOB to adequately test the hypothesis whether there is benefit of adding pertuzumab to standard cytotoxic plus trastuzumab therapy for these patients. However, intratumoral and spatial HER2 heterogeneity (at higher rates than compared to breast cancer) may still have implications on the overall trial results. The results of this trial remain eagerly awaited. Lapatinib, a selective intracellular tyrosine kinase inhibitor (TKI) of ERBB1 and ERBB2 was also studied in first and second line GEC (Table 2). The phase III TRIO-013/LOGiC trial randomized 545 untreated first line HER2 positive (HER2:CEP17 ratio ≥2 by FISH or IHC 3+ if FISH not available) GEC patients to receive capecitabine and oxaliplatin in addition to either lapatinib or placebo.
Lapatinib increased objective response from 39% to 53%, and modestly increased median PFS from 5.4 to 6 months, but failed to confer an overall survival benefit in the ITT population.22 Younger and Asian patients appeared to derive the most benefit in subset analyses. The absolute level of amplification positively correlated with outcome, as previously described,23,24 signifying heterogeneity of benefit within the current ‘HER2 positive’ classification. HER2 amplification varies depending on the report, ranging from 4 to 20% of GEC patients (Table 1). Recently, the degree of amplification has been shown to correlate closely with both absolute protein expression level and clinical benefit.25 The inter-trial variations in absolute amplification/expression and lapatinib’s lack of antibody-dependent cell-mediated cytotoxicity (ADCC) as compared to trastuzumab, serve as two of many potential explanations when contrasting outcomes of ToGA and LOGiC.
In the second line, the phase III Asian TyTAN trial enrolled patients regardless of HER2 expression (FISH ratio ≥2 were eligible), where 31% of patients enrolled were IHC 0-1+/FISH+.26 Patients received paclitaxel alone or in combination with lapatinib. Despite response rates of 27% versus 9%, no statistically significant PFS or OS benefit was demonstrated in the ITT population. Of note, when limiting the evaluation to only those patients with 3+ HER2 expression by IHC, median survival improved to 14 months from 7.6 months in this subgroup (p =0.0176), with progression-free survival of 5.6 versus 4.2 months, respectively (p=0.0101).
Trastuzumab emtansine (T-DM1), an antibody-drug conjugate that is approved in HER2 positive metastatic breast cancer, was also studied in the second line GATSBY trial for ‘HER2+’ GEC (Table 2), but this failed to support a response or survival benefit versus paclitaxel monotherapy.27 Possible explanations for this negative trial include intra-patient HER2 tumor heterogeneity, which is more frequent in GEC than observed in breast cancer.28 With clonal heterogeneity, it has been hypothesized that HER2-negative (or low expressing) clones are not controlled by HER2-targeted cytotoxic therapy. Furthermore, HER2 expression/amplification has been demonstrated to ‘convert’ after first line therapy. Archived specimen testing, as used in both second line trials (GATSBY and TyTAN), may therefore lead to inadequate HER2+ patient selection in subsequent line trials.29–32 Specifically, a recent phase II randomized study of paclitaxel plus trastuzumab versus paclitaxel, trastuzumab plus MM-111 (a bivalent antibody towards HER2 and HER3) was conducted in the second line setting after failure of first line cytotoxic therapy for HER2+ cancers. Eligibility for trial enrollment was determined by archived original diagnostic samples, and correlative studies demonstrated this HER2 amplification molecular evolution concern. In this trial, the median overall survival of 44 patients receiving trastuzumab and paclitaxel was 14 months versus 8 months in the 44 patients receiving MM-111, trastuzumab and paclitaxel (HR 2.12, p = 0.045, 95% CI 1.0–4.5). Interestingly, central HER2 testing of the 66% of available original archived samples revealed that 30% of cases were actually considered IHC0/1 and 8% IHC2+/FISH-, demonstrating an overall 38% of cases that would never have been considered HER2 positive. This could be due to a combination of factors including intra-tumoral HER2 heterogeneity testing different regions spatially within a tumor, or technique/assay variability and subjective scoring. Importantly, of ~40 patients having matched archival and fresh biopsy prior to initiating second line therapy, ~15% of patients initially considered HER2+ (ratio ≥2) were later found to be HER2- prior to second line therapy initiation. Regardless, approximately 50% of patients enrolled into this ‘HER2+’ selection trial were considered HER2 negative in retrospect. Future trials evaluating the role of anti-HER2 therapy for GEC patients after failure of prior anti-HER2 therapy should therefore mandate fresh biopsy (and/or possibly cfDNA assessment) to confirm the presence of HER2 positivity at the time of enrollment and thereby ensure proper treatment arm stratification by HER2 status.
As alluded to above, though HER-2 over-expression/ERBB2 amplification predict benefit from the anti-HER2 antibody trastuzumab in the first line setting,18,33 the definitions of positivity and trial inclusion criteria have evolved over time. Current clinical diagnostic testing requires evaluation by a combination of IHC (membranous reactivity in ≥10% of cancer cells in a surgical specimen or a cluster of at least 5 cells in a biopsy specimen), and fluorescence in-situ hybridization (FISH with HER2:CEP17 ratio ≥2). IHC 0/1 is now considered negative, and IHC3+ is considered positive, while IHC2+ requires reflex to FISH assessment. Higher throughput assays, including mass spectrometry and next-generation sequencing (NGS), have emerged with the potential to refine diagnostic accuracy and allow multiplexing capability to assess for other relevant aberrations with limited tissue samples.5,25,32 Similarly, circulating cell-free DNA (cfDNA) is emerging as a potential non-invasive method, particularly for serial ERBB2 amplification,34 which may provide further insight into tumor genetic evolution.29–32
Nevertheless, to date, no standard anti-HER2 directed approaches are recognized in trastuzumab refractory HER2+ GEC using any available diagnostic testing. Standard chemotherapy with irinotecan or taxane based regimens are recommended. Notably, while second line ramucirumab trials included HER2 positive and trastuzumab-treated patients, this accounted for only ~6% of patients enrolled in the RAINBOW trial and <1% of patients enrolled to the REGARD trial (Medical Letter).35,36 Other strategies under evaluation in the second and later lines include novel TKIs like apatinib,37 trastuzumab beyond progression (and ensuring persisting HER2 positivity),29,30 novel HER2 antibodies,38 and combination therapy with immune checkpoint inhibitors (NCT02689284).
Neoadjuvant HER2-directed therapy has been integrated into routine breast cancer therapy based upon the phase III NeOAdjuvant Herceptin (NOAH) trial, which identified an improved 5 year event-free survival and overall survival with the addition of trastuzumab to neoadjuvant chemotherapy.39 Similarly, the GeparQuattro trial demonstrated increased pathologic complete remission with neoadjuvant trastuzumab in HER2-positive breast cancer patients,40 which was further increased by combining trastuzumab and lapatinib with chemotherapy in the NeoALTTO trial.41 Based on these results and the ToGA trial,18 RTOG1010 explores neoadjuvant chemoradiation in EGJ with carboplatin and paclitaxel with or without trastuzumab. Accrual has completed, and results are awaited (NCT01196390). Similarly, the neoadjuvant INNOVATION trial is evaluating the addition of trastuzumab and pertuzumab to cisplatin and fluoropyrimidine doublet therapy in GEC.42 The phase II HER-FLOT trial identified an R0 resection rate of 93.3% and pathologic complete remission in 22.2% of patients when trastuzumab was added to peri-operative 5-FU, oxaliplatin, leucovorin, and docetaxel (FLOT).43 A similar phase II study combining FOLFIRINOX and trastuzumab in the peri-operative setting remains underway (NCT02581462). These findings will be further explored in the phase III PETRARCA study, which randomizes patients to receive peri-operative FLOT with or without trastuzumab and pertuzumab (NCT02581462). However, until these results from these trials are available, HER2-targeted therapy is not considered standard-of-care for GEC in the neoadjuvant setting.
EGFR
Epidermal growth factor receptor (EGFR) or ERBB1 is a transmembrane receptor and a well-recognized mediator of oncogenic phenotype that is expressed in approximately 30% of GEC.44,45 EGFR-overexpressing tumors are associated with higher stage, more poorly differentiated histology, increased vascular invasion, and potentially shorter survival.14,46 EGFR amplification and consequent overexpression is found in only 2–6% of GEC patients, and mutations in less than 2%, though the functional and therapeutic implications of these aberrations are yet to be clearly defined (Table 1).9,47,48
EGFR-directed therapies include monoclonal antibodies such as cetuximab and panitumumab, which antagonize the extracellular binding domain. Pre-clinical data also suggests that cetuximab, a recombinant human-murine chimeric monoclonal antibody of a murine Fv region and a human IgG1 heavy and k light chain Fc region, also induces ADCC similar to trastuzumab.49 Small molecule TKIs, such as gefitinib, erlotinib, lapatinib, and afatinib competitively bind intracellular to the tyrosine kinase domain at varying potencies and specificities. Early phase II trials combining cetuximab, panitumumab, or erlotinib with cytotoxic chemotherapy in unselected GEC patients identified first line therapy response rates from 41–65%.50–53 Second line phase II evaluations of gefitinib or erlotinib monotherapy led to more modest responses of ~9-11%, and responses appeared higher in proximal EGJ cancers rather than distal GC.54,55
Subsequent phase III GEC trials targeting EGFR included EXPAND (cetuximab plus Capecitabin/Cisplatin, first line), REAL-3 (panitumumab plus Epirubicin/Oxaliplatin/Capecitabine, first line), and COG (gefitinib monotherapy, second line) (Table 3).15,56,57 Disappointingly, each trial was resoundingly negative, and panitumumab actually resulted in worse survival compared to the control. Notably, each of these trials enrolled all-comers without biomarker selection of any kind.
Table 3.
EGFR-directed phase III clinical trials for gastroesophageal cancer
| Line | Trial | N | Treatment | 1° Endpt (Met?) | mos (mo) | HR | mPFS(mo) | HR | RR |
|---|---|---|---|---|---|---|---|---|---|
| 1L |
Lordick et al
15 EXPAND |
904 | Cis/Cape/Placebo Cis/Cape/Cetuximab |
PFS (No) | 10.7 9.4 |
1.00 | 5.6 4.4 |
1.09 p=0.32 |
29% 30% |
| 1L |
Waddell et al16 REAL-3 |
553 | Epi/Oxali/Cape-Pl Epi/Oxali/Cape-P |
OS (No) | 11.3 8.8 |
1.37 p=0.013 |
7.4 6.0 |
1.22 | 42% 46% |
| 2L |
Dutton et al57 COG |
450 | Placebo Gefitinib |
OS (No) | 3.67 3.73 |
0.9 | 1.17 1.57 |
0.8 | ~1% ~4% |
Cis/Cape: Cisplatin/Capecitabine; Epi/Oxali/Cape:
Epirubicin/Oxaliplatin/Capecitabine; Pl: Placebo; P: Panitumumab
Preclinically, 20% of patient-derived xenografts responded to cetuximab, and of these responders, half were later found to harbor EGFR amplification 58 In the phase II study combining FOLFOX with cetuximab, 22% of patients had greater than 4 EGFR copies, which correlated with increased overall survival.59 Similarly, in TRANS-COG, the preplanned translational correlative study of COG, 15.6% of patients had increased gene copy number (GCN) including true EGFR amplification (ratio EGFR/CEP7 ≥2) (~5%); this latter small subset of EGFR amplified patients derived a statistically significant survival benefit with the addition of gefitinib (HR 0.19, p=0.007).60 The EXPAND trial also demonstrated survival benefit in the small subset with extremely high EGFR expression by IHC H-Score (likely representing EGFR amplified tumors, but yet to be confirmed).61 Thus, with these recent promising subset analyses of EGFR amplification and consequent overexpression, future studies assessing the benefits of anti-EGFR therapy in these patients are being pursued.62 A Phase III trial of second line nimotuzumab with irinotecan (NCT01813253) is also currently recruiting patients deemed to harbor EGFR overexpressing (IHC 2/3+) tumors.
MET
The MET protooncogene encodes the c-MET receptor tyrosine kinase, which is involved in cell proliferation, angiogenesis, and migration. MET over-expressing and MET amplified tumors are each associated with worse survival.46,47,63–71 Canonical MET activation occurs via binding of its ligand, hepatocyte growth factor (HGF), but MET activation can also occur in an HGF-independent manner through RTK cross-talk (Figure 1).72,73 MET amplification leads to constitutive receptor activation independent of ligand, and is reported in ~4-10% of GEC cases,47,74–76 but over-expression ranges from 25–70% by IHC in GEC (Table 1).66–68,77–79
Early phase reports and trials suggested that MET-expression may serve as a predictive biomarker for MET-directed therapeutic response in GEC patients.71,77 However, a subsequent phase II,80 and two Phase III MET-directed trials in GEC have all reported overall negative results (Table 4).80,81 The first line MetGastric phase III study evaluated onartuzumab, a humanized IgG1 antibody against the extracellular domain of c-MET, in combination with mFOLFOX6, in patients with c-MET-expressing tumors (≥1+, ≥50% cells).82 However, METGastric was terminated prematurely (70% of planned accrual) due to negative results (in any predefined MET expression subgroup) reported from the prior/parallel YO28252 phase II biomarker evaluation trial of onartuzumab enrolling unselected GEC patients.80 With this in mind, no benefit was seen in the METGastric ITT, nor in the MET IHC 2/3+ pre-planned subgroup analysis (which accounted for ~38% of enrolled patients, HR 0.64, p=0.06); this subgroup notably now possessed less power to identify a true benefit due to early termination of the trial.82 Similarly, RILOMET-1, which evaluated first line epirubicin, cisplatin, and capecitabine (ECX) with or without the addition of rilotumomab, a fully human IgG2 antibody against HGF ligand, for ‘MET expressing’ GEC was terminated due to an increased risk of death from the study drug.81
Table 4.
MET-directed phase II/III clinical trials for gastroesophageal cancer
| Line | Trial | N | Treatment | 1° Endpoint (Met?) | mOS (mo) | HR | mPFS (mo) | HR | RR (%) |
|---|---|---|---|---|---|---|---|---|---|
| 1L |
Cunningham et al
88 RILOMET-1 |
609 | Epi/Cis/5FU/Placebo Epi/Cis/5FU/Rilotumumab |
OS (No) | 11.5 9.6 |
1.37 p=0.016 |
5.7 5.7 |
1.3 | 39 30 |
| 1L |
Shah et at
82 MET-GASTRIC |
562 | FOLFOX/Placebo FOLFOX-Onartuzumab |
OS (No) | 11.3 11 |
0.82 p=0.24 |
6.8 6.7 |
0.9 | 41 46 |
| 1L |
Shah et al
80 Y028252 |
123 | FOLFOX6/Placebo FOLFOX6-Onartuzumab |
PFS (No) | 11.27 10.61 |
1.06 p=0.83 |
6.97 6.77 |
1.08 0.71 |
57.1 60.5 |
Epi/Cis/5FU: Epirubicin/Cisplatin/5-fluorouracil
One pitfall of these phase III trials was their loose definition of MET expression. In RILOMET-1, patient selection was defined as ≥1+ MET expression by IHC in ≥25% of tumor cells to be eligible, accounting for 81% of patients screened, and of all patients enrolled only 21% of tumors had high expression (≥2+, ≥50% cells). Similarly, only 38% of METGastric were IHC 2/3+ in ≥50% of cells, yet as above, these patients demonstrated a near-significant benefit, in an under-accrued trial. Thus, even with the large phase III MET inhibitor trials, one could argue that the selection for ‘MET-dependent’ cancers was too lenient and inadequate, and the highest expressing tumors clearly under-represented, particularly in RILOMET-1.47,74–76
However, more promising results have been reported in smaller earlier phase trials of MET inhibitors in MET amplified patients (4–5% of GEC),47,67,74,76 with consequent over-expression.74 AMG-337, a relatively highly selective MET TKI, demonstrated clinical responses in MET-amplified advanced GEC patients (ORR 50%), but the phase II expansion phase of the trial has been on hold (results not publically available).83 Similarly, half of MET-amplified patients treated with crizotinib in a phase I expansion cohort experienced response,47 and 75% of MET amplified patients receiving ABT-700 monoclonal antibody monotherapy demonstrated an objective response.84 The challenge of molecular heterogeneity,29,85 particularly in the CIN subset of GEC,9 may account for lack of response and/or rapid development of resistance to MET-directed monotherapy of MET amplified GEC. Any future therapeutic attempts of the MET pathway will likely be directed towards the small MET amplified subset of patients,86 in conjunction with cytotoxic agents,55 other targeted therapies, and/or immune checkpoint inhibitors either in combination or in sequential fashion to achieve optimal benefit.
Summary
Development of molecularly targeted therapies in GEC has been hindered by inadequate predictive biomarkers. With respect to HER2, despite the breast cancer and ToGA experiences, patients with HER2 FISH+ but IHC negative disease were included in the Tytan (36% of patients) and LOGiC (17% of patients) trials,26 Moreover, 10.4% of patients in the GATSBY trial harbored IHC3+/FISH- tumors compared to the ToGA trial’s 2.3%, raising the question as to whether this molecular subset IHC3+/FISH- should be considered similar or dissimilar to genomic driver subset IHC3+/FISH+. Furthermore, nearly one third of cases in the second line trastuzumab with/without MM-111 were later reclassified as HER2 negative and another 15% were no longer HER2+ upon repeat biopsy after first line progression, suggesting that updated HER2 testing prior to each line might be necessary for optimal patient selection.30 For MET, although early trials suggested MET expression as a predictive biomarker for anti-MET therapies, the RILOMET-1 trial MET expression requirements may have been too loose, and the power for METGastric to identify a more likely HR of ~0.7–0.8 was low given that the trial was under-accrued due to early termination.81 Finally, in the phase III EGFR trials, no biomarkers of selection were utilized. All of the evidence suggests that targeted therapies may have a role, but in more targeted select patient populations. Finally, using alternative diagnostic platforms, including DNA amplification and mass spectroscopy, it may be feasible to better select the appropriate patient population in future studies.62
Although approximately one-third to half of patients with GEC harbor potentially actionable alterations, patient population selection with varying scoring, heterogeneity, and/or infrequent incidence has stifled clinical trial success. As of 2016, only trastuzumab has been approved for first line GEC patients in a select HER2 positive population. Subset analyses have identified patients with MET and EGFR amplifications that are more likely to benefit from respective targeted therapies, albeit for a finite period of time before various developed resistance mechanisms emerge - molecular evolution over time. Intra-patient molecular heterogeneity is also emerging as a considerable hurdle for targeted therapies.29 However, designing traditional trials for such infrequent genomic aberrations remains difficult. One solution may be further development of novel trial designs such as the PANGEA type II expansion platform trial (particularly with serial assessment and ‘re-targeting’ over time) that may better identify and treat these uncommon actionable aberrations by testing an overall treatment strategy composed of various biomarker/drug pairings, and compare this personalized treatment strategy outcome to a treatment control arm.62
Key Points.
Trastuzumab is a treatment standard for HER2 amplified/overexpressed gastroesophageal adenocarcinoma, yet benefit has not been demonstrated in second and later lines of therapy, or beyond progression in first line therapy.
Anti-EGFR therapy warrants further investigation for gene amplification/over-expression despite lack of benefit demonstrated in unselected gastroesophageal patients to date.
Anti-MET therapy has not demonstrated benefit in ‘over-expressing’ gastroesophageal patients in any line of therapy, but evidence supports further investigation in patients with gene amplification/overexpression.
Footnotes
Disclosure statement
DVTC has received research funding from Genentech/Roche, Amgen, OncoplexDx/Nantomics and honoraria from Genentech/Roche, Amgen, Eli Lilly, Five Prime, OncoplexDx/Nantomics, Guardant Health, Foundation Medicine.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Steven B. Maron, Fellow, University of Chicago Comprehensive Cancer Center, Section of Hematology/Oncology, Chicago, IL.
Daniel V.T. Catenacci, Associate Director, Gastrointestinal Oncology Program, Assistant Professor of Medicine, University of Chicago Comprehensive Cancer Center, Section of Hematology/Oncology, Chicago, IL.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Sehdev A, Catenacci DV. Gastroesophageal cancer: focus on epidemiology, classification, and staging. Discov Med. 2013;16(87):103–111. [PubMed] [Google Scholar]
- 3.Sehdev A, Catenacci DV. Perioperative therapy for locally advanced gastroesophageal cancer: current controversies and consensus of care. J Hematol Oncol. 2013;6:66. doi: 10.1186/1756-8722-6-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 5.Ali SM, Sanford EM, Klempner SJ, et al. Prospective comprehensive genomic profiling of advanced gastric carcinoma cases reveals frequent clinically relevant genomic alterations and new routes for targeted therapies. Oncologist. 2015;20(5):499–507. doi: 10.1634/theoncologist.2014-0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deng N, Goh LK, Wang H, et al. A comprehensive survey of genomic alterations in gastric cancer reveals systematic patterns of molecular exclusivity and co-occurrence among distinct therapeutic targets. Gut. 2012;61(5):673–684. doi: 10.1136/gutjnl-2011-301839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stachler MD, Taylor-Weiner A, Peng S, et al. Paired exome analysis of Barrett's esophagus and adenocarcinoma. Nat Genet. 2015;47(9):1047–1055. doi: 10.1038/ng.3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zang ZJ, Ong CK, Cutcutache I, et al. Genetic and structural variation in the gastric cancer kinome revealed through targeted deep sequencing. Cancer Res. 2011;71(1):29–39. doi: 10.1158/0008-5472.CAN-10-1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cancer Genome Atlas Research N. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513(7517):202–209. doi: 10.1038/nature13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kameda T, Yasui W, Yoshida K, et al. Expression of ERBB2 in human gastric carcinomas: relationship between p185ERBB2 expression and the gene amplification. Cancer Res. 1990;50(24):8002–8009. [PubMed] [Google Scholar]
- 11.Okines AF, Thompson LC, Cunningham D, et al. Effect of HER2 on prognosis and benefit from peri-operative chemotherapy in early oesophago-gastric adenocarcinoma in the MAGIC trial. Ann Oncol. 2013;24(5):1253–1261. doi: 10.1093/annonc/mds622. [DOI] [PubMed] [Google Scholar]
- 12.Van Cutsem E, de Haas S, Kang YK, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a biomarker evaluation from the AVAGAST randomized phase III trial. J Clin Oncol. 2012;30(17):2119–2127. doi: 10.1200/JCO.2011.39.9824. [DOI] [PubMed] [Google Scholar]
- 13.Gordon MA, Gundacker HM, Benedetti J, et al. Assessment of HER2 gene amplification in adenocarcinomas of the stomach or gastroesophageal junction in the INT-0116/SWOG9008 clinical trial. Ann Oncol. 2013;24(7):1754–1761. doi: 10.1093/annonc/mdt106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Terashima M, Kitada K, Ochiai A, et al. Impact of expression of human epidermal growth factor receptors EGFR and ERBB2 on survival in stage II/III gastric cancer. Clin Cancer Res. 2012;18(21):5992–6000. doi: 10.1158/1078-0432.CCR-12-1318. [DOI] [PubMed] [Google Scholar]
- 15.Lordick F, Kang YK, Chung HC, et al. Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): a randomised, open-label phase 3 trial. Lancet Oncol. 2013;14(6):490–499. doi: 10.1016/S1470-2045(13)70102-5. [DOI] [PubMed] [Google Scholar]
- 16.Waddell T, Chau I, Cunningham D, et al. Epirubicin, oxaliplatin, and capecitabine with or without panitumumab for patients with previously untreated advanced oesophagogastric cancer (REAL3): a randomised, openlabel phase 3 trial. Lancet Oncol. 2013;14(6):481–489. doi: 10.1016/S1470-2045(13)70096-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kurokawa Y, Matsuura N, Kimura Y, et al. Multicenter large-scale study of prognostic impact of HER2 expression in patients with resectable gastric cancer. Gastric Cancer. 2015;18(4):691–697. doi: 10.1007/s10120-014-0430-7. [DOI] [PubMed] [Google Scholar]
- 18.Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2- positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376(9742):687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 19.Hofmann M, Stoss O, Shi D, et al. Assessment of a HER2 scoring system for gastric cancer: results from a validation study. Histopathology. 2008;52(7):797–805. doi: 10.1111/j.1365-2559.2008.03028.x. [DOI] [PubMed] [Google Scholar]
- 20.Swain SM, Kim SB, Cortes J, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2013;14(6):461–471. doi: 10.1016/S1470-2045(13)70130-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tabernero J, Hoff PM, Shen L, et al. Pertuzumab (P) with trastuzumab (T) and chemotherapy (CTX) in patients (pts) with HER2-positive metastatic gastric or gastroesophageal junction (GEJ) cancer: An international phase III study (JACOB). Paper presented at: ASCO Annual Meeting Proceedings; 2013. [Google Scholar]
- 22.Hecht JR, Bang YJ, Qin SK, et al. Lapatinib in Combination With Capecitabine Plus Oxaliplatin in Human Epidermal Growth Factor Receptor 2-Positive Advanced or Metastatic Gastric, Esophageal, or Gastroesophageal Adenocarcinoma: TRIO-013/LOGiC--A Randomized Phase III Trial. J Clin Oncol. 2016;34(5):443–451. doi: 10.1200/JCO.2015.62.6598. [DOI] [PubMed] [Google Scholar]
- 23.Gomez-Martin C, Plaza JC, Pazo-Cid R, et al. Level of HER2 gene amplification predicts response and overall survival in HER2-positive advanced gastric cancer treated with trastuzumab. J Clin Oncol. 2013;31(35):4445–4452. doi: 10.1200/JCO.2013.48.9070. [DOI] [PubMed] [Google Scholar]
- 24.Ock CY, Lee KW, Kim JW, et al. Optimal Patient Selection for Trastuzumab Treatment in HER2-Positive Advanced Gastric Cancer. Clin Cancer Res. 2015;21(11):2520–2529. doi: 10.1158/1078-0432.CCR-14-2659. [DOI] [PubMed] [Google Scholar]
- 25.Catenacci DV, Liao WL, Zhao L, et al. Mass-spectrometry-based quantitation of Her2 in gastroesophageal tumor tissue: comparison to IHC and FISH. Gastric Cancer. 2015 doi: 10.1007/s10120-015-0566-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Satoh T, Xu RH, Chung HC, et al. Lapatinib plus paclitaxel versus paclitaxel alone in the second-line treatment of HER2-amplified advanced gastric cancer in Asian populations: TyTAN--a randomized, phase III study. J Clin Oncol. 2014;32(19):2039–2049. doi: 10.1200/JCO.2013.53.6136. [DOI] [PubMed] [Google Scholar]
- 27.Kang Y-K, Shah MA, Ohtsu A, et al. A randomized, open-label, multicenter, adaptive phase 2/3 study of trastuzumab emtansine (T-DM1) versus a taxane (TAX) in patients (pts) with previously treated HER2-positive locally advanced or metastatic gastric/gastroesophageal junction adenocarcinoma (LA/MGC/GEJC). Paper presented at: ASCO Annual Meeting Proceedings; 2016. [Google Scholar]
- 28.Yang J, Luo H, Li Y, et al. Intratumoral heterogeneity determines discordant results of diagnostic tests for human epidermal growth factor receptor (HER) 2 in gastric cancer specimens. Cell Biochem Biophys. 2012;62(1):221–228. doi: 10.1007/s12013-011-9286-1. [DOI] [PubMed] [Google Scholar]
- 29.Catenacci DV. Next-generation clinical trials: Novel strategies to address the challenge of tumor molecular heterogeneity. Mol Oncol. 2015;9(5):967–996. doi: 10.1016/j.molonc.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Denlinger CS, Alsina Maqueda M, Watkins DJ, et al. Randomized phase 2 study of paclitaxel (PTX), trastuzumab (T) with or without MM-111 in HER2 expressing gastroesophageal cancers (GEC). Paper presented at: ASCO Annual Meeting Proceedings; 2016. [Google Scholar]
- 31.Janjigian YY, Riches JC, Ku GY, et al. Loss of human epidermal growth factor receptor 2 (HER2) expression in HER2-overexpressing esophagogastric (EG) tumors treated with trastuzumab. Paper presented at: ASCO Annual Meeting Proceedings; 2015. [Google Scholar]
- 32.Sellappan S, Blackler A, Liao WL, et al. Therapeutically Induced Changes in HER2, HER3, and EGFR Protein Expression for Treatment Guidance. J Natl Compr Canc Netw. 2016;14(5):503–507. doi: 10.6004/jnccn.2016.0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hofmann M, Stoss O, Gaiser T, et al. Central HER2 IHC and FISH analysis in a trastuzumab (Herceptin) phase II monotherapy study: assessment of test sensitivity and impact of chromosome 17 polysomy. J Clin Pathol. 2008;61(1):89–94. doi: 10.1136/jcp.2006.043562. [DOI] [PubMed] [Google Scholar]
- 34.Shoda K, Masuda K, Ichikawa D, et al. HER2 amplification detected in the circulating DNA of patients with gastric cancer: a retrospective pilot study. Gastric Cancer. 2015;18(4):698–710. doi: 10.1007/s10120-014-0432-5. [DOI] [PubMed] [Google Scholar]
- 35.Wilke H, Muro K, Van Cutsem E, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15(11):1224–1235. doi: 10.1016/S1470-2045(14)70420-6. [DOI] [PubMed] [Google Scholar]
- 36.Fuchs CS, Tomasek J, Yong CJ, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014;383(9911):31–39. doi: 10.1016/S0140-6736(13)61719-5. [DOI] [PubMed] [Google Scholar]
- 37.Janjigian YY, Capanu M, Imtiaz T, et al. A phase II study of afatinib in patients (pts) with metastatic human epidermal growth factor receptor (HER2)- positive trastuzumab-refractory esophagogastric (EG) cancer. Paper presented at: ASCO Annual Meeting Proceedings; 2014. [Google Scholar]
- 38.Rugo HS, Pegram MD, Gradishar WJ, et al. SOPHIA: A phase 3, randomized study of margetuximab (M) plus chemotherapy (CTX) vs trastuzumab (T) plus CTX in the treatment of patients with HER2+ metastatic breast cancer (MBC) ASCO Meeting Abstracts. 2016;34(15_suppl):TPS630. [Google Scholar]
- 39.Gianni L, Eiermann W, Semiglazov V, et al. Neoadjuvant and adjuvant trastuzumab in patients with HER2-positive locally advanced breast cancer (NOAH): follow-up of a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet Oncol. 2014;15(6):640–647. doi: 10.1016/S1470-2045(14)70080-4. [DOI] [PubMed] [Google Scholar]
- 40.Untch M, Rezai M, Loibl S, et al. Neoadjuvant treatment with trastuzumab in HER2-positive breast cancer: results from the GeparQuattro study. J Clin Oncol. 2010;28(12):2024–2031. doi: 10.1200/JCO.2009.23.8451. [DOI] [PubMed] [Google Scholar]
- 41.Baselga J, Bradbury I, Eidtmann H, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet. 2012;379(9816):633–640. doi: 10.1016/S0140-6736(11)61847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wagner AD, Kang Y-K, van Dieren J, et al. EORTC-1203: Integration of trastuzumab (T), with or without pertuzumab (P), into perioperative chemotherapy (CT) of HER-2 positive stomach cancer--INNOVATION trial. ASCO Meeting Abstracts. 2016;34(15_suppl):TPS4133. [Google Scholar]
- 43.Hofheinz R, Hegewisch-Becker S, Thuss-Patience PC, et al. HER-FLOT: Trastuzumab in combination with FLOT as perioperative treatment for patients with HER2-positive locally advanced esophagogastric adenocarcinoma: A phase II trial of the AIO Gastric Cancer Study Group. Paper presented at: ASCO Annual Meeting Proceedings; 2014. [Google Scholar]
- 44.Kim MA, Lee HS, Lee HE, Jeon YK, Yang HK, Kim WH. EGFR in gastric carcinomas: prognostic significance of protein overexpression and high gene copy number. Histopathology. 2008;52(6):738–746. doi: 10.1111/j.1365-2559.2008.03021.x. [DOI] [PubMed] [Google Scholar]
- 45.Wang KL, Wu TT, Choi IS, et al. Expression of epidermal growth factor receptor in esophageal and esophagogastric junction adenocarcinomas: association with poor outcome. Cancer. 2007;109(4):658–667. doi: 10.1002/cncr.22445. [DOI] [PubMed] [Google Scholar]
- 46.Nagatsuma AK, Aizawa M, Kuwata T, et al. Expression profiles of HER2, EGFR, MET and FGFR2 in a large cohort of patients with gastric adenocarcinoma. Gastric Cancer. 2015;18(2):227–238. doi: 10.1007/s10120-014-0360-4. [DOI] [PubMed] [Google Scholar]
- 47.Lennerz JK, Kwak EL, Ackerman A, et al. MET amplification identifies a small and aggressive subgroup of esophagogastric adenocarcinoma with evidence of responsiveness to crizotinib. J Clin Oncol. 2011;29(36):4803–4810. doi: 10.1200/JCO.2011.35.4928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dulak AM, Stojanov P, Peng S, et al. Exome and whole-genome sequencing of esophageal adenocarcinoma identifies recurrent driver events and mutational complexity. Nat Genet. 2013;45(5):478–486. doi: 10.1038/ng.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kimura H, Sakai K, Arao T, Shimoyama T, Tamura T, Nishio K. Antibody-dependent cellular cytotoxicity of cetuximab against tumor cells with wild-type or mutant epidermal growth factor receptor. Cancer Sci. 2007;98(8):1275–1280. doi: 10.1111/j.1349-7006.2007.00510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Enzinger PC, Burtness BA, Niedzwiecki D, et al. CALGB 80403 (Alliance)/E1206: A Randomized Phase II Study of Three Chemotherapy Regimens Plus Cetuximab in Metastatic Esophageal and Gastroesophageal Junction Cancers. J Clin Oncol. 2016 doi: 10.1200/JCO.2015.65.5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wainberg ZA, Lin LS, DiCarlo B, et al. Phase II trial of modified FOLFOX6 and erlotinib in patients with metastatic or advanced adenocarcinoma of the oesophagus and gastro-oesophageal junction. Br J Cancer. 2011;105(6):760–765. doi: 10.1038/bjc.2011.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lordick F, Luber B, Lorenzen S, et al. Cetuximab plus oxaliplatin/leucovorin/5-fluorouracil in first-line metastatic gastric cancer: a phase II study of the Arbeitsgemeinschaft Internistische Onkologie (AIO) Br J Cancer. 2010;102(3):500–505. doi: 10.1038/sj.bjc.6605521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tebbutt NC, Price TJ, Ferraro DA, et al. Panitumumab added to docetaxel, cisplatin and fluoropyrimidine in oesophagogastric cancer: ATTAX3 phase II trial. Br J Cancer. 2016;114(5):505–509. doi: 10.1038/bjc.2015.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ferry DR, Anderson M, Beddard K, et al. A phase II study of gefitinib monotherapy in advanced esophageal adenocarcinoma: evidence of gene expression, cellular, and clinical response. Clin Cancer Res. 2007;13(19):5869–5875. doi: 10.1158/1078-0432.CCR-06-1970. [DOI] [PubMed] [Google Scholar]
- 55.Dragovich T, McCoy S, Fenoglio-Preiser CM, et al. Phase II trial of erlotinib in gastroesophageal junction and gastric adenocarcinomas: SWOG 0127. J Clin Oncol. 2006;24(30):4922–4927. doi: 10.1200/JCO.2006.07.1316. [DOI] [PubMed] [Google Scholar]
- 56.Okines AF, Ashley SE, Cunningham D, et al. Epirubicin, oxaliplatin, and capecitabine with or without panitumumab for advanced esophagogastric cancer: dose-finding study for the prospective multicenter, randomized, phase II/III REAL-3 trial. J Clin Oncol. 2010;28(25):3945–3950. doi: 10.1200/JCO.2010.29.2847. [DOI] [PubMed] [Google Scholar]
- 57.Dutton SJ, Ferry DR, Blazeby JM, et al. Gefitinib for oesophageal cancer progressing after chemotherapy (COG): a phase 3, multicentre, double-blind, placebo-controlled randomised trial. Lancet Oncol. 2014;15(8):894–904. doi: 10.1016/S1470-2045(14)70024-5. [DOI] [PubMed] [Google Scholar]
- 58.Zhang L, Yang J, Cai J, et al. A subset of gastric cancers with EGFR amplification and overexpression respond to cetuximab therapy. Sci Rep. 2013;3:2992. doi: 10.1038/srep02992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Luber B, Deplazes J, Keller G, et al. Biomarker analysis of cetuximab plus oxaliplatin/leucovorin/5-fluorouracil in first-line metastatic gastric and oesophago-gastric junction cancer: results from a phase II trial of the Arbeitsgemeinschaft Internistische Onkologie (AIO) BMC Cancer. 2011;11:509. doi: 10.1186/1471-2407-11-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Petty RD, Dahle-Smith A, Miedzybrodzka Z, et al. Epidermal growth factor receptor copy number gain (EGFR CNG) and response to gefitinib in esophageal cancer (EC): Results of a biomarker analysis of a phase III trial of gefitinib versus placebo (TRANS-COG) J Clin Oncol (Meeting Abstracts) 2014;32(15_suppl):4016. [Google Scholar]
- 61.Lordick F, Kang Y-K, Salman P, et al. Clinical outcome according to tumor HER2 status and EGFR expression in advanced gastric cancer patients from the EXPAND study. Paper presented at: ASCO Annual Meeting Proceedings; 2013. [Google Scholar]
- 62.Catenacci DVT, Polite BN, Henderson L, et al. Toward personalized treatment for gastroesophageal adenocarcinoma (GEC): Strategies to address tumor heterogeneity--PANGEA. Paper presented at: ASCO Annual Meeting Proceedings; 2014. [Google Scholar]
- 63.Catenacci DVT, Tang R, Oliner KS, et al. MET as a prognostic biomarker of survival in a large cohort of patients with gastroesophageal cancer (GEC). Paper presented at: ASCO Annual Meeting Proceedings; 2015. [Google Scholar]
- 64.Hack SP, Bruey JM, Koeppen H. HGF/MET-directed therapeutics in gastroesophageal cancer: a review of clinical and biomarker development. Oncotarget. 2014;5(10):2866–2880. doi: 10.18632/oncotarget.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Metzger ML, Behrens HM, Boger C, Haag J, Kruger S, Rocken C. MET in gastric cancer--discarding a 10% cutoff rule. Histopathology. 2016;68(2):241–253. doi: 10.1111/his.12745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nakajima M, Sawada H, Yamada Y, et al. The prognostic significance of amplification and overexpression of c-met and c-erb B-2 in human gastric carcinomas. Cancer. 1999;85(9):1894–1902. doi: 10.1002/(sici)1097-0142(19990501)85:9<1894::aid-cncr3>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 67.Lee HE, Kim MA, Lee HS, et al. MET in gastric carcinomas: comparison between protein expression and gene copy number and impact on clinical outcome. Br J Cancer. 2012;107(2):325–333. doi: 10.1038/bjc.2012.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Graziano F, Galluccio N, Lorenzini P, et al. Genetic activation of the MET pathway and prognosis of patients with high-risk, radically resected gastric cancer. J Clin Oncol. 2011;29(36):4789–4795. doi: 10.1200/JCO.2011.36.7706. [DOI] [PubMed] [Google Scholar]
- 69.Catenacci DV, Cervantes G, Yala S, et al. RON (MST1R) is a novel prognostic marker and therapeutic target for gastroesophageal adenocarcinoma. Cancer Biol Ther. 2011;12(1):9–46. doi: 10.4161/cbt.12.1.15747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yu S, Yu Y, Zhao N, Cui J, Li W, Liu T. C-Met as a prognostic marker in gastric cancer: a systematic review and meta-analysis. PLoS One. 2013;8(11):e79137. doi: 10.1371/journal.pone.0079137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Iveson T, Donehower RC, Davidenko I, et al. Rilotumumab in combination with epirubicin, cisplatin, and capecitabine as first-line treatment for gastric or oesophagogastric junction adenocarcinoma: an open-label, dose de-escalation phase 1b study and a double-blind, randomised phase 2 study. Lancet Oncol. 2014;15(9):1007–1018. doi: 10.1016/S1470-2045(14)70023-3. [DOI] [PubMed] [Google Scholar]
- 72.Jo M, Stolz DB, Esplen JE, Dorko K, Michalopoulos GK, Strom SC. Cross-talk between epidermal growth factor receptor and c-Met signal pathways in transformed cells. J Biol Chem. 2000;275(12):8806–8811. doi: 10.1074/jbc.275.12.8806. [DOI] [PubMed] [Google Scholar]
- 73.Yamaguchi H, Chang SS, Hsu JL, Hung MC. Signaling cross-talk in the resistance to HER family receptor targeted therapy. Oncogene. 2014;33(9):1073–1081. doi: 10.1038/onc.2013.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Catenacci DV, Liao WL, Thyparambil S, et al. Absolute quantitation of Met using mass spectrometry for clinical application: assay precision, stability, and correlation with MET gene amplification in FFPE tumor tissue. PLoS One. 2014;9(7):e100586. doi: 10.1371/journal.pone.0100586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jardim DL, Tang C, de Gagliato DM, et al. Analysis of 1,115 patients tested for MET amplification and therapy response in the MD Anderson Phase I Clinic. Clin Cancer Res. 2014;20(24):6336–6345. doi: 10.1158/1078-0432.CCR-14-1293. [DOI] [PubMed] [Google Scholar]
- 76.Smolen GA, Sordella R, Muir B, et al. Amplification of MET may identify a subset of cancers with extreme sensitivity to the selective tyrosine kinase inhibitor PHA-665752. Proc Natl Acad Sci U S A. 2006;103(7):2316–2321. doi: 10.1073/pnas.0508776103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Catenacci DV, Henderson L, Xiao SY, et al. Durable complete response of metastatic gastric cancer with anti-Met therapy followed by resistance at recurrence. Cancer Discov. 2011;1(7):573–579. doi: 10.1158/2159-8290.CD-11-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Toiyama Y, Yasuda H, Saigusa S, et al. Co-expression of hepatocyte growth factor and c-Met predicts peritoneal dissemination established by autocrine hepatocyte growth factor/c-Met signaling in gastric cancer. Int J Cancer. 2012;130(12):2912–2921. doi: 10.1002/ijc.26330. [DOI] [PubMed] [Google Scholar]
- 79.Zhao J, Zhang X, Xin Y. Up-regulated expression of Ezrin and c-Met proteins are related to the metastasis and prognosis of gastric carcinomas. Histol Histopathol. 2011;26(9):1111–1120. doi: 10.14670/HH-26.1111. [DOI] [PubMed] [Google Scholar]
- 80.Shah MA, Cho JY, Tan IB, et al. A Randomized Phase II Study of FOLFOX With or Without the MET Inhibitor Onartuzumab in Advanced Adenocarcinoma of the Stomach and Gastroesophageal Junction. Oncologist. 2016 doi: 10.1634/theoncologist.2016-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cunningham D, Tebbutt NC, Davidenko I, et al. Phase III, randomized, double-blind, multicenter, placebo (P)-controlled trial of rilotumumab (R) plus epirubicin, cisplatin and capecitabine (ECX) as first-line therapy in patients (pts) with advanced MET-positive (pos) gastric or gastroesophageal junction (G/GEJ) cancer: RILOMET-1 study. Paper presented at: ASCO Annual Meeting Proceedings; 2015. [Google Scholar]
- 82.Shah MA, Bang Y-J, Lordick F, et al. METGastric: A phase III study of onartuzumab plus mFOLFOX6 in patients with metastatic HER2-negative (HER2-) and MET-positive (MET+) adenocarcinoma of the stomach or gastroesophageal junction (GEC). Paper presented at: ASCO Annual Meeting Proceedings; 2015. [Google Scholar]
- 83.Kwak EL, LoRusso P, Hamid O, et al. Clinical activity of AMG 337, an oral MET kinase inhibitor, in adult patients (pts) with MET-amplified gastroesophageal junction (GEJ), gastric (G), or esophageal (E) cancer. Paper presented at: ASCO Annual Meeting Proceedings; 2015. [Google Scholar]
- 84.Kang Y-K, LoRusso P, Salgia R, et al. Phase I study of ABT-700, an anti-c-Met antibody, in patients (pts) with advanced gastric or esophageal cancer (GEC). Paper presented at: ASCO Annual Meeting Proceedings; 2015. [Google Scholar]
- 85.Kwak EL, Ahronian LG, Siravegna G, et al. Molecular Heterogeneity and Receptor Coamplification Drive Resistance to Targeted Therapy in MET-Amplified Esophagogastric Cancer. Cancer Discov. 2015;5(12):1271–1281. doi: 10.1158/2159-8290.CD-15-0748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mullard A. NCI-MATCH trial pushes cancer umbrella trial paradigm. Nat Rev Drug Discov. 2015;14(8):513–515. doi: 10.1038/nrd4694. [DOI] [PubMed] [Google Scholar]
- 87.Van Cutsem E, Bang YJ, Feng-Yi F, et al. HER2 screening data from ToGA: targeting HER2 in gastric and gastroesophageal junction cancer. Gastric Cancer. 2015;18(3):476–484. doi: 10.1007/s10120-014-0402-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cunningham Dea. Phase III, randomized, double-blind, multicenter, placebo (P)-controlled trial of rilotumumab (R) plus epirubicin, cisplatin and capecitabine (ECX) as first-line therapy in patients (pts) with advanced MET-positive (pos) gastric or gastroesophageal junction (G/GEJ) cancer: RILOMET-1 study. J Clin Oncol. 2015;33(Suppl) [Google Scholar]


