ABSTRACT
The upregulation of autophagosome formation in response to nutrient deprivation requires significant intracellular membrane rearrangements that are poorly understood. Recent findings have implicated COPII-coated vesicles, well known as ER-Golgi cargo transport carriers, as key players in macroautophagy. The role of COPII vesicles in macroautophagy and how they interact with autophagy-related (Atg) proteins was unknown. In our recent report, we show that during nutrient deprivation, phosphorylation of the membrane-distal surface of the COPII coat subunit Sec24 facilitates the interaction of Sec24 with the Atg machinery (specifically, Atg9) to regulate the abundance of autophagosomes during starvation. Phosphorylation of Sec24 is specifically required for macroautophagy, but not ER-Golgi transport. These findings begin to unravel the unique function of COPII vesicles during starvation-induced macroautophagy.
KEYWORDS: autophagosome formation, autophagy and secretory pathway crosstalk, cell homeostasis, COPII vesicles, phosphorylation
Macroautophagy (hereafter referred to as autophagy) is a highly conserved catabolic process that uses phagophores (precursors to autophagosomes that are double-membrane bounded structures), which capture cytoplasmic proteins and organelles and deliver them to the lysosome or vacuole for degradation. Approximately 40 autophagy-related (ATG) genes, whose products are required for autophagy, have been identified in the yeast Saccharomyces cerevisiae. Much is known about the Atg proteins and how they assemble at a specific site called the phagophore assembly site (PAS), to make autophagosomes. Recent studies, however, have revealed that the Atg machinery, and the membranes that traffic this machinery to the PAS, are not sufficient for autophagosome formation. How other membranes and machinery are directed to the autophagy pathway remains a major unanswered question in the field.
COPII-coated vesicles, which bud from specialized regions of the ER called ER exit sites (ERES), are required for ER-Golgi traffic and autophagy. Under nutrient-rich conditions, COPII vesicles mediate anterograde ER-Golgi traffic. However, when cells are starved for nutrients, a mounting body of evidence suggests these vesicles contribute to autophagosome formation (Fig. 1). The mechanism by which COPII vesicles are redirected to the autophagy pathway, and the role these vesicles play in autophagy, have been unclear. COPII vesicle formation is initiated with the recruitment of the Sec23-Sec24 complex. Sec24 is one of 3 cargo adaptors (Sec24, Sfb2/Iss1 and Sfb3/Lst1) that sort cargo into the nascent vesicle. The Sec23-Sec24 complex then recruits the coat outer shell, Sec13-Sec31, which leads to coat polymerization and vesicle budding. In addition to its role in vesicle budding, the COPII coat also confers directionality to maintain the anterograde direction of ER-Golgi traffic.
Figure 1.
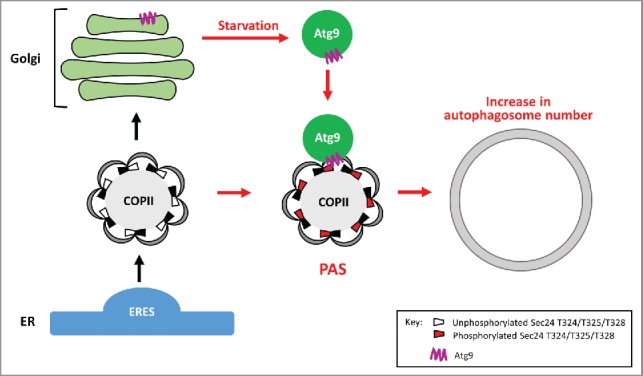
Phosphorylation of Sec24 T324, T325 and T328 regulates autophagosome abundance in nutrient-deprived cells. Under nutrient-rich conditions, COPII vesicles bud from the ERES and transport secretory cargo to the Golgi (black arrows). During starvation (red arrows), autophagosome formation is initiated and Atg9 vesicles traffic from the late Golgi to the PAS. The membrane distal surface of Sec24 becomes phosphorylated, facilitating the interaction of Sec24 with the C terminus of Atg9. These events increase autophagosome number to rapidly make more nutrients for the starving cell.
COPII coat subunits are phosphorylated by the highly conserved CSNK1/CK1 kinase, Hrr25, which is required for both ER-Golgi traffic and autophagy. As COPII vesicles have been proposed to be a membrane source for autophagosome formation and Hrr25 is required for autophagy, we asked if coat phosphorylation plays a role in redirecting COPII vesicles from the ER-Golgi pathway to autophagy. To address this question, we focused on Sec24 as it is the major cargo adaptor. Sec24 was purified from yeast cells induced for autophagy, and 27 high confidence phosphorylation sites were identified. Phosphorylation of only 3 amino acids, T324, T325 and T328, on a conserved helix on the Sec24 membrane distal surface is required for autophagy. Single and double mutant combinations of T324A, T325A and T328A show a range of defects in autophagy, with the triple alanine mutant showing the most dramatic defect (hereafter referred to as Sec24-3A). None of the viable phosphomimetic mutants show a defect in autophagy. Multiple autophagy assays, including vacuolar phosphatase (Pho8Δ60), translocation of GFP-Atg8 to the vacuole, and cleavage of GFP from GFP-Atg8 were used to confirm that phosphorylation of the Sec24 membrane distal surface is required for autophagy. Phosphorylation of Sec24 T324, T325 and T328 is not involved in ER-Golgi traffic, as the processing of Prc1/carboxypeptidase Y in Sec24-3A cells is indistinguishable from wild type. Thus, phosphorylation of the Sec24 membrane distal surface is specifically required for autophagy, but not ER-Golgi traffic.
Importantly, phosphorylation of Sec24 T324, T325 and T328 regulates autophagosome number in starved cells, but not in nutrient-rich medium. When autophagy is induced, the transmembrane protein Atg9 traffics in vesicles from the late Golgi to the PAS (Fig. 1). As with Sec24, Atg9 regulates autophagosome number, and proteomic studies have linked Atg9 to the ERES machinery. We showed that Sec24 co-immunoprecipitates with Atg9 and the Sec24-Atg9 interaction is enhanced by both starvation and phosphorylation of the Sec24 membrane distal surface. Furthermore, Hrr25 kinase activity is required for the Sec24-Atg9 interaction and the Sec24-Atg9 interaction is needed for autophagy. Sec24 binds directly to the C terminus of Atg9 in vitro and this interaction is enhanced by sec24T325E or sec24T324,328E mutations. Whereas phosphorylation of the Sec24 membrane distal surface is needed for the Sec24-Atg9 interaction, it is dispensable for Atg9 trafficking and Atg complex assembly at the PAS, indicating that Sec24 phosphorylation is not indirectly disrupting autophagy by perturbing Atg protein function.
In summary, we have shown that during starvation, phosphorylation of the Sec24 membrane-distal surface drives the interaction of Sec24 with Atg9, committing COPII vesicles to the autophagy pathway (Fig. 1). These events lead to an increase in autophagosome number and the rapid degradation of cytoplasmic components to make nutrients for the starving cell. Our findings tease apart the distinct roles of COPII vesicles in autophagy and the secretory pathway, and conclusively demonstrate a direct role for COPII vesicles in autophagy. These results also ascribe a new role for Sec24 in maintaining homeostasis during cell stress, and provide a possible explanation for why the coat is retained on COPII vesicles until they are primed for fusion. The mechanism we describe for yeast cells is likely to be conserved in higher eukaryotes as the Sec24 phosphosites and the domain of Atg9 that binds to Sec24, are conserved in vertebrates. The function of Hrr25 (CSNK1D/CK1δ in mammals) is also highly conserved.
Our findings raise several important questions that need to be addressed in the future. First, as neither Hrr25 expression, nor its activity is altered upon nitrogen starvation, other kinases are likely to be involved in phosphorylating the membrane-distal surface of Sec24. Second, a major role of COPII vesicles is to transport cargo in the secretory pathway. Additional studies will be needed to address if COPII vesicles bring needed cargo to the autophagy pathway. Finally, while our findings apply to starvation-induced autophagy, protein modification of vesicle coat proteins may serve as a global mechanism for rearranging membranes in response to different environmental cues.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
JW is supported by the Beijing Overseas Talent Pooling Program. S F-N, SD and MZ received support from NIGMS under award numbers R01GM114111 and R01GM115422 to S F-N; EAM received support from R01GM085089 and MC_UP_1201/10.


