Abstract
Cognitive dysfunction in schizophrenia (SCZ) and Alzheimer’s disease (AD) is a major driver of functional disability but is largely unresponsive to current therapeutics. Animal models of cognitive dysfunction relevant to both disorders suggest the α7 nicotinic acetylcholine receptor (nAChR) may be a promising drug development target, with multiple clinical trials subsequently testing this hypothesis in individuals with SCZ and AD. However, the translational value of rodent cognitive tasks for predicting the overall efficacy of this therapeutic target in clinical trials is unknown. To compare effect sizes between rodent and human studies, we searched PubMed and the Cochrane Library for all randomized, placebo-controlled trials of compounds with pharmacological activity at the α7 nAChR for treatment of cognitive dysfunction in SCZ and AD and identified 18 studies comprising 2670 subjects treated with eight different compounds acting as full or partial agonists. Cognitive outcomes were standardized, and random-effects meta-analyses revealed no statistically significant effects of α7 nAChR agonists on overall cognition or any of eight cognitive subdomains when all doses were included (Range of all cognitive outcomes: Cohen’s d = −0.077 to 0.12, negative favoring drug). In contrast, analysis of 29 rodent studies testing the same α7 agonists revealed large effect sizes in multiple commonly used preclinical behavioral tests of cognition (Range: d = −1.18 to −0.73). Our results suggest that targeting the α7 nAChR with agonists is not a robust treatment for cognitive dysfunction in SCZ or AD and necessitate a better understanding of the translational gap for therapeutics targeting the α7 nAChR.
Keywords: Alpha7 nicotinic receptor, Cognition, Schizophrenia, Alzheimer’s disease, Meta-analysis, Clinical trial
1. Introduction
Multiple lines of evidence suggest that alterations of the α7 nicotinic acetylcholine receptor (nAChR) may play a role in the pathophysiology of several neuropsychiatric disorders that manifest with cognitive impairment, including schizophrenia (SCZ) and Alzheimer’s disease (AD). Several brain regions important for cognition, including the hippocampus (Freedman et al., 1995) and cortex (Marutle et al., 2001), show reduced availability of α7 nAChR binding sites in patients with SCZ compared to controls. Many patients with SCZ demonstrate impaired sensory gating to paired stimuli, a deficit thought to contribute to attentional dysfunction, which is normalized by nicotine (Adler et al., 1993) and by α7-selective agonists (Olincy et al., 2006; Stevens et al., 1998). Substantive genetic evidence further links the α7 nAChR to P50 gating-deficits in individuals with SCZ and their relatives (Freedman et al., 2003). Similarly, individuals with AD demonstrate altered levels of α7 nAChR protein in peripheral blood leukocytes (Chu et al., 2005) and in the brain (Burghaus et al., 2000).
Given these electrophysiological, neurochemical, and genetic findings, treatments directed toward normalization or augmentation of signaling through the α7 nAChR have been proposed for SCZ and AD, most specifically to address cognitive deficits (Freedman, 2014). Cognitive decline is the hallmark of AD, while impaired cognition in SCZ plays a major role in driving functional deficits (Bowie and Harvey, 2005). Just as cognitive domains are diverse in their fundamental neurophysiology, the mechanism by which targeting α7 nAChRs is hypothesized to enhance cognition is multifactorial. The normalization of the P50 deficit in SCZ by nicotine and α7 nAChR agonists is arguably the most studied of these effects and believed to be due to α7 nAChR stimulation of inter-neuron populations that inhibit excitatory pyramidal cells in the hippocampus (Freedman, 2014). Additional pro-cognitive effects of α7 nAChRs are hypothesized to result from regulation of neurotransmitter release through presynaptic mechanisms (Wonnacott et al., 2006), enhancement of synaptic plasticity in circuits important for cognition (Gu and Yakel, 2011), and initiation of signal transduction cascades through calcium influx (Bitner et al., 2007). Thus, α7 nAChR targeting in individuals with disorders such as SCZ or AD may correct an established α7-signaling deficit, or independently enhance cognition through complementary mechanisms.
The majority of evidence supporting the therapeutic use of α7 nAChR-selective drugs for cognitive impairment derives from rodent preclinical tasks in genetic, environmental, or pharmacological models of cognitive impairment (Levin, 2012) – however, pharmaceutical development of compounds targeting neuronal nAChRs has been challenging. For instance, the α4β2* nAChR-selective agonist TC-1734/AZD3480 (ispronicline) showed efficacy in early clinical trials for mild, age-associated memory loss (Dunbar et al., 2011) but failed to show clear efficacy in follow-up studies for cognitive impairment in AD (Frolich et al., 2011) or SCZ (Velligan et al., 2012). The ultimate failure of drugs such as ispronicline in large clinical trials despite encouraging preclinical and early clinical data suggests that the translational pathway for drugs targeting neuronal nAChRs may benefit from a reappraisal to identify specific barriers. With this goal in mind, the past ten years have witnessed the assessment of multiple drugs active at α7 nAChRs in randomized, placebo-controlled clinical trials for cognitive impairment in SCZ and AD, most of which have extensive preclinical assessment. We thus sought to apply the technique of meta-analysis to this collection of rodent and human data to evaluate the strategy of α7 nAChR targeting for cognitive symptoms in SCZ and AD. Specifically, we compare evidence of efficacy for this strategy in humans with that from the preclinical rodent models which supported initial drug development of these compounds. Taken together, our overall objective is to quantitatively evaluate preclinical and clinical data seeking to alleviate a major unanswered clinical burden and facilitate the successful development of therapeutics targeting nAChRs.
2. Methods and materials
2.1. Identification of studies
2.1.1. Search strategy
PubMed was searched on April 1, 2016 for relevant studies using the search term: (alpha7 OR alpha-7) AND (nicotinic receptor OR nicotinic acetylcholine receptor) AND (agonist OR positive allosteric modulator) AND placebo. References from appropriate papers and clinicaltrials.gov were searched for additional relevant publications or unpublished studies that might contribute to the meta-analysis. The Cochrane Library was also searched using the same search terms and no new additional references were identified. Upon identification of α7 nAChR-targeting compounds used in clinical trials, PubMed was again searched to identify rodent studies of these compounds using the search term: ((choline AND galantamine) OR (ABT-126) OR (DMXB-A OR GTS-21) OR (encenicline OR EVP-6124) OR RG3487 OR TC-5619 OR tropisetron OR varenicline) AND (rat OR mouse) AND (cognition OR cognitive). References were reviewed for additional relevant published and unpublished research.
2.1.2. Selection of studies
Two reviewers examined the titles, abstracts, and in some cases texts of studies obtained by the above search strategies to determine inclusion in the meta-analyses. Discrepancies were resolved by a final reviewer. Eligibility for inclusion in the human meta-analysis was based upon analysis of the full articles for the following criteria: randomized, double-blind, placebo-controlled trials of single drug or drug combination with a potential mechanism of action targeting the α7 nAChR, compared with placebo; and subject diagnosis of SCZ or AD. Studies were excluded if no cognitive outcomes were measured or reported. We excluded studies solely of galantamine (without an additional α7-selective agonist) on the basis of its relative non-specificity as a positive allosteric modulator (PAM) at α7 (Samochocki et al., 2003). Study eligibility for inclusion in the rodent meta-analysis was based upon analysis of the full articles for the following criteria: study tested α7 compound or combination used in clinical trials; reported outcome of cognitive behavioral task(s); adequate statistical description of sample size and outcomes to calculate effect size (ES). Studies were excluded if only juvenile animals were examined, rats or mice were not used, or the paper was solely a review article.
2.2. Meta-analytic procedures
Data were extracted by the first author and corroborated independently by a second reviewer. The primary outcome measure for clinical trials was performance on cognitive tasks. To standardize the cognitive measures across trials for ES calculation, we created nine cognitive subdomains relevant to assessment of cognition in SCZ and AD (Keefe et al., 1999): overall cognitive index, attention, working memory, executive function, speed of processing, verbal learning, visual learning, social cognition, and language (Supplemental Table 1). Reviewers also gathered data on dose, trial design, smoking status, number of participants, duration of active treatment, and other relevant attributes of the studies. When additional information was required for analysis, a request for the data was made to the corresponding author of the study by email. For the rodent studies, primary outcome measure was performance on behavioral tasks relevant to human cognition. For water maze (WM) tasks, results from the probe trial were preferentially used to calculate ES. If these data were not available, group differences in learning were used. Data was gathered on behavioral task, dose, duration of treatment, method of drug delivery (oral, intraperitoneal, subcutaneous, intraventricular), sample size, and other relevant attributes.
Meta-analysis was conducted using Open Meta-Analyst (Wallace et al., 2012). Effect sizes of drug treatment were calculated as Cohen’s d using Open Meta-Analyst, freely available web tools for ES calculation (Lipsey and Wilson, 2001; Wilson, 2010), and Microsoft Excel. Egger’s test and Duval and Tweedie’s trim and fill correction were performed using Comprehensive Meta-Analysis (Biostat, Englewood, NJ). The convention throughout the study is negative ES favor drug treatment, whereas positive ES favor placebo/vehicle. Effect sizes from clinical trials were calculated using one of the following: 1) Group mean change from baseline to trial endpoint, sample size, and SD; 2) trial endpoint group means, sample size, and SD; 3) p-value between groups and sample size, or; 4) ES for outcome and duration of interest reported directly from the study text. For all ES, 95% confidence intervals (CIs) were calculated. In the case of multiple doses of α7 agonist, ES of each comparison to placebo were weighted by participant number and averaged to create a single ES with the exception of subgroup analysis examining effect sizes of the highest doses used or the doses corresponding to the largest ES. When studies reported the results of multiple cognitive tasks that fell within the same cognitive subdomain without a subdomain index, we utilized a task hierarchy ordered by the most commonly used tasks across trials.
For meta-analysis of the rodent studies, ES were calculated using 1) t-statistic and sample size; 2) F-test and sample size; 3) p-value between groups and sample size. When a range of p-values (i.e. “p < 0.05”) was reported, the least significant p-value was used to calculate the ES for statistically significant results, and a p-value of 0.50 was used when the p-value was simply reported as “non-significant” without further specification (Moran et al., 2016). The effect direction was concluded by visual inspection of graphical results. As in the analysis of human studies, a weighted average was performed across doses to obtain a single ES. No additional correction for multiple comparisons beyond what was employed in the source data was performed. For meta-analysis within a single rodent cognitive task (i.e., novel object recognition (NOR) task or WM task, a single ES was calculated for each independent cognitive deficit model and entered into the meta-analysis. For both human and rodent studies, a random-effects model for meta-analysis was used. Publication bias was assessed by visual inspection of funnel plots and Egger’s test. We assessed the association between sample size and ES using meta-regression. For secondary analyses of human trials we performed subgroup analysis based on the diagnosis of patient group (SCZ vs. AD). p < 0.05 were considered statistically significant.
3. Results
3.1. Included studies
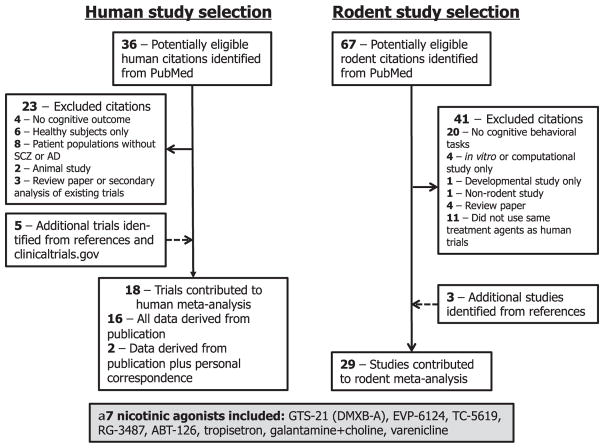
Fig. 1 depicts the selection strategy of studies for inclusion in the human and rodent meta-analyses. Thirty-six potential references were identified in PubMed of placebo-controlled clinical trials of α7 nAChR agonists or PAMs for cognitive impairment in SCZ and AD. After trial exclusion and addition of eligible trials from review of references and clinicaltrials.gov, 18 trials were eligible for inclusion. Of these, 16 trials provided data sets within the publication, and we were able to ascertain additional cognitive subdomain data from two trials via email request. Altogether, we included 18 studies involving 2670 participants testing eight drugs or drug combinations, all of which have agonist activity at the α7 nAChR (Table 1, Supplemental Table 2). No new references were identified from a Cochrane Library search using the same terms. We then searched PubMed for rodent studies using cognitive tasks following treatment with these eight drugs or drug combinations. We identified 67 potential rodent studies, and after study exclusion and addition of studies identified from the study references, included 29 studies in our rodent meta-analysis (Supplemental Table 3). We were unable to identify any published rodent studies of ABT-126.
Fig. 1.
Study selection for clinical trial and rodent preclinical meta-analytic calculations.
Table 1.
Included trials in the meta-analysis of α7 nicotinic agonists for cognitive dysfunction in schizophrenia and Alzheimer’s disease.
| First author | Year | Agonist | Dose(s) (total mg/day) | Design | Duration | N | Subject diagnosis | Smoking status | Cognitive outcomes in meta-analysis | References |
|---|---|---|---|---|---|---|---|---|---|---|
| Haig | 2016 | ABT-126 | 10, 25 | Parallel | 12 weeks | 207 | SCZ | Mix | OCI, P, VeL, ViL, WM, EF, A, SC | (Haig et al., 2016) |
| Florian | 2016 | ABT-126 | 25, 75 | Parallel | 24 weeks | 434 | AD | Mix | OCI | (Florian et al., 2016) |
| Gault | 2015 | ABT-126 | 5, 25 | Parallel | 12 weeks | 274 | AD | Mix | OCI | (Gault et al., 2015) |
| Keefe | 2015 | EVP-6124 | 0.27, 0.90 | Parallel | 12 weeks | 319 | SCZ | Mix | OCI, P, A, WM, VeL, ViL, EF, SC | (Keefe et al., 2015) |
| Preskorn | 2014 | EVP-6124 | 0.30, 1.0 | Parallel | 3 weeks | 21 | SCZ | Mix | OCI, WM, EF, SC, ViL | (Preskorn et al., 2014) |
| Deutsch | 2013 | Galantamine + choline | Gal: 24 Chol: 2000 |
Parallel | 16 weeks | 43 | SCZ | Mix | P, A, WM, VeL, ViL | (Deutsch et al., 2013) |
| Freedman | 2008 | GTS-21 | 150, 300 | Crossover | 4 weeks | 31 | SCZ | NS | P, A, WM, VeL, ViL, EF | (Freedman et al., 2008) |
| Olincy | 2006 | GTS-21 | 112.5, 225 | Crossover | 1 day | 12 | SCZ | NS | OCI, A, WM, VeL, ViL, P, L | (Olincy et al., 2006) |
| Umbricht | 2014 | RG3487 | 5, 15, 50 | Parallel | 8 weeks | 215 | SCZ | Mix | OCI, P, A, VeL, ViL, EF, SC | (Umbricht et al., 2014) |
| Lieberman | 2013 | TC-5619 | 25 | Parallel | 12 weeks | 185 | SCZ | Mix | OCI, EF, P, A, VeL, ViL, WM, SC | (Lieberman et al., 2013) |
| Walling | 2016 | TC-5619 | 5, 50 | Parallel | 24 weeks | 477 | SCZ | Mix | OCI | (Walling et al., 2016) |
| Shiina | 2010 | Tropisetron | 10 | Parallel | 8 weeks | 40 | SCZ | Mix | WM, EF, A | (Shiina et al., 2010) |
| Zhang | 2012 | Tropisetron | 5, 10, 20 | Parallel | 10 days | 40 | SCZ | NS | OCI, WM, P, L, A, VeL, ViL | (Zhang et al., 2012) |
| Shim | 2012 | Varenicline | 2 | Parallel | 8 weeks | 120 | SCZ | Mix | A, P, WM, EF | (Shim et al., 2012) |
| Kim | 2014 | Varenicline | 2 | Crossover | 6 weeks | 66 | AD | NS | OCI, ViL, P, A, WM | (Kim et al., 2014) |
| Smith | 2016 | Varenicline | 2 | Parallel | 8 weeks | 87 | SCZ | S | OCI, P, A, WM, VeL, ViL, EF | (Smith et al., 2016) |
| Roh | 2014 | Varenicline | 1 | Crossover | 1 day | 30 | SCZ | NS | A, P, WM | (Roh et al., 2014) |
| Hong | 2011 | Varenicline | 1 | Parallel | 8 weeks | 69 | SCZ | Mix | OCI, A, P | (Hong et al., 2011) |
Abbreviations: OCI, overall cognitive index. P, speed of processing, VeL, verbal learning; ViL, visual learning; WM, working memory; EF, executive function; A, attention; SC, social cognition; L, language; NS, non-smoking; S, all smokers; AD, Alzheimer’s disease; SCZ, schizophrenia.
3.2. α7 agonist efficacy in two common rodent tasks of cognition
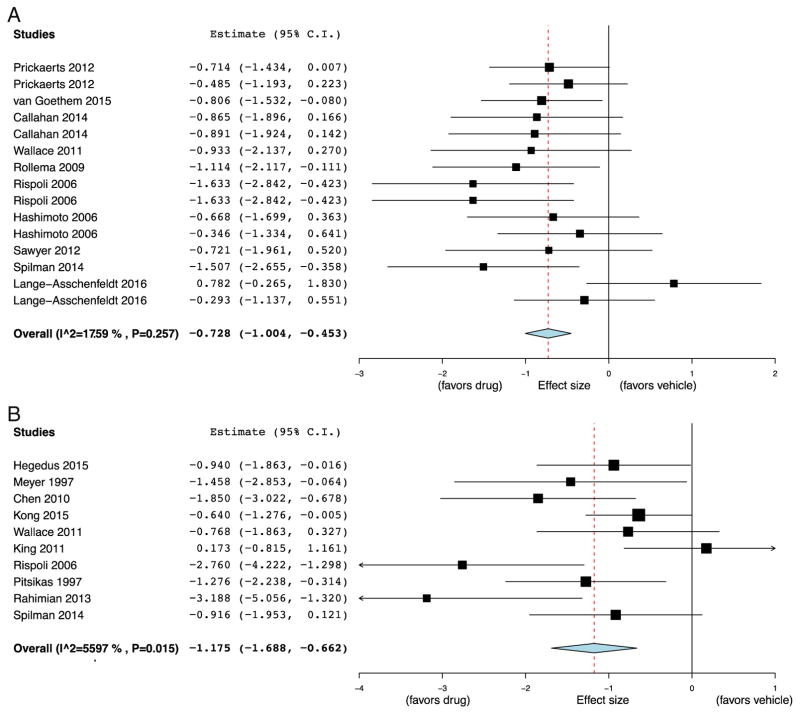
The two most commonly used preclinical cognitive models were the NOR task, a test of rodent non-spatial memory, and the Morris WM or water labyrinth, tests of rodent spatial memory, used in 10/29 (34%) and 11/29 studies (38%), respectively (Supplemental Table 3). The remainder included tests of social interaction, fear conditioning, passive and active avoidance, additional tests of spatial memory, impulsivity, and attention. Models of cognitive impairment were diverse (listed in Supplemental Table 3), and included pharmacological, genetic, neuro-degenerative, and acquired injury models. Cognitive enhancement in unmanipulated animals was also commonly tested. To determine the ES of α7 nAChR agonists in the most common paradigms, NOR and WM, random-effects meta-analysis was performed. Large ES were found for both NOR (ES = −0.73, 95% CI = −1.00 to −0.45, p < 0.001, Fig. 2A) and WM (ES = −1.18, 95% CI = −1.69 to −0.66, p < 0.001, Fig. 2B). There was minimal heterogeneity between studies for the NOR (I2 = 18%, p = 0.26), though heterogeneity was significant in studies using WM (I2 = 56%, p = 0.015). Visual inspection of funnel plots revealed asymmetry for both paradigms (Supplemental Fig. 1), and Egger’s test provided evidence of publication bias for WM studies (p = 0.0092) but not for NOR studies (p = 0.24). Correction for publication bias in the WM using Duval and Tweedie’s trim and fill yielded an effect size similar to that derived from the NOR studies (corrected ES = −0.84, 95% CI = −1.39 to −0.51). Taken together, we found that rodent preclinical testing of α7 nAChR drugs focused on tests of spatial and non-spatial memory across a wide variety of cognitive impairment models, with published results demonstrating large ES of these agents.
Fig. 2.
Forest plot of effect sizes of α7 nicotinic agonists on the novel object recognition task and water maze tasks in rodent models of cognitive impairment. Meta-analysis demonstrated a significant effect in the novel object recognition task (A) compared to vehicle (Effect size (ES) = −0.73, 95% CI = −1.00 to −0.45, p < 0.001), and a significant effect in the water maze task (B) compared to vehicle (ES = − 1.18, 95% CI = −1.69 to −0.66, p < 0.001). Note: negative effect size favors drug treatment.
3.3. α7 agonists for cognitive impairment in clinical trials of SCZ and AD
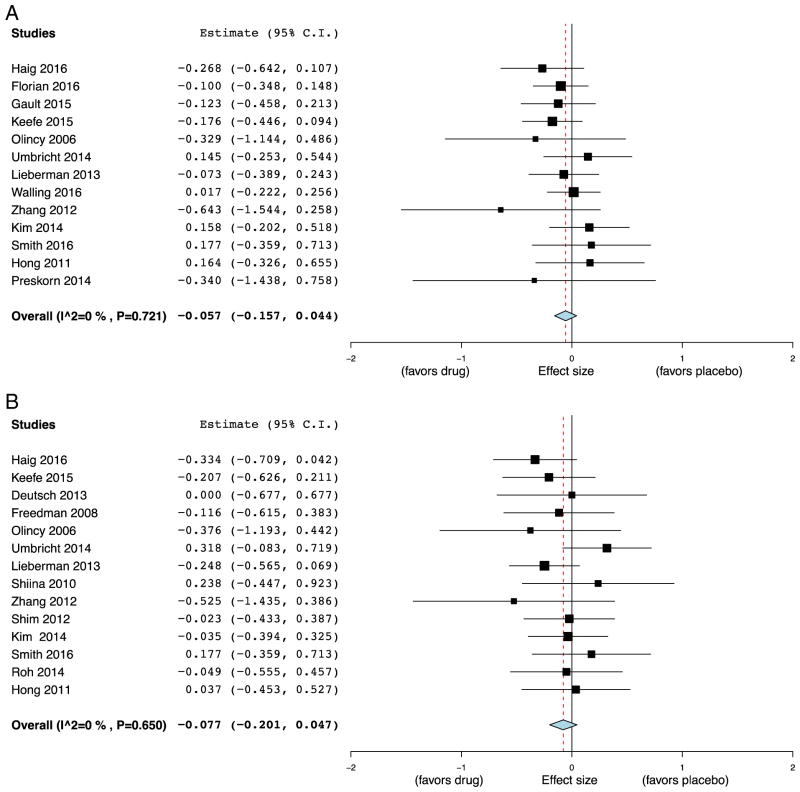
Cognitive outcomes in the clinical trials were grouped into nine cognitive subdomains (Supplemental Table 1). Meta-analysis of studies reporting an overall cognitive index (13/18, 72%) demonstrated no significant benefit of α7 nAChR agonists over placebo (ES = −0.057, 95% CI = −0.16 to 0.044, p = 0.27; Fig. 3A). No significant heterogeneity was found (I2 = 0, p = 0.72). Visual inspection of the funnel plot suggested minimal publication bias (Supplemental Fig. 2A). Varenicline’s affinity at α7 nAChR is many orders of magnitude lower than at α4β2* nAChRs and at low doses may exert insignificant agonism at α7 (Coe et al., 2005; Hong et al., 2011). We thus performed a subgroup analysis of studies leaving out varenicline. Random-effect meta-analysis again revealed a small, non-significant ES of remaining α7 agonists on overall cognition (ES = −0.097, 95% CI = −0.21 to 0.012, p = 0.081). Subgroup analysis by patient diagnosis found no significant difference in effect of α7 agonists on overall cognitive index in patients with SCZ (ES = − 0.062, 95% CI = −0.19 to 0.061, p = 0.32) and AD (ES = −0.046, 95% CI = −0.22 to 1.29, p = 0.61). Meta-regression demonstrated no significant association between trial size and ES on overall cognitive index (β = −0.000, 95% CI = −0.001 to 0.001, p = 0.75). The results of the individual rodent studies demonstrate cognitive effects of α7 nAChR agonists are highly dose-dependent. We thus repeated the meta-analysis using two additional subgroups: 1) the highest dose employed in each study, and 2) the highest ES in each study. Meta-analysis of the highest dose in each study did not meaningfully change the overall ES (ES = − 0.055, 95% CI = −0.16 to 0.046, p = 0.28). However, when only the most effective doses were included in the meta-analysis, we detected a small but significant effect of α7 agonists on overall cognitive index (ES = −0.12, 95% CI = −0.22 to −0.02, p = 0.020).
Fig. 3.
Forest plot of effect sizes of α7 nicotinic agonists on overall cognitive index and attention for subjects with schizophrenia and Alzheimer’s disease. Meta-analysis demonstrated no significant effect on overall cognition (A) compared to placebo (Effect size (ES) = −0.057, 95% CI = −0.16 to 0.044, p = 0.27), and no significant effect on attention (B) compared to placebo (ES = −0.077, 95% CI = −0.20 to 0.047, p = 0.22). Note: negative effect size favors drug treatment.
Random effects meta-analysis on the most commonly reported cognitive outcome, attention (14/18 studies, 78%), again found that α7 agonists did not significantly differ from placebo (ES = −0.077, 95% CI = −0.20 to 0.047, p = 0.22; Fig. 3B). No significant heterogeneity was found (I2 = 0, p = 0.65) or evidence of publication bias by inspection of funnel plot (Supplemental Fig. 2B). Subgroup analysis of non-varenicline studies was of small, non-significant ES (ES = −0.13, 95% CI = −0.30 to 0.044, p = 0.15). Only one study reported attentional measures in subjects with AD and thus subgroup analysis by disorder was not performed. Meta-regression demonstrated no significant association between trial size and ES on attentional measures (β = −0.000, 95% CI = −0.002 to 0.001, p = 0.55). Similar to overall cognitive index, subgroup analysis taking only the highest dose of α7 nAChR agonist did not substantially alter the result of the meta-analysis for attention (ES = −0.033, 95% CI = −0.17 to 0.11, p = 0.65), while meta-analysis incorporating only the most effective doses found a small but significant effect of α7 agonists on attention (ES = −0.13, 95% CI = −0.25 to −0.00, p = 0.049). Meta-analysis incorporating all doses of the remaining seven cognitive subdomains failed to demonstrate significant effects of α7 nAChR drugs (Table 2), with some subdomains including only a small number of studies.
Table 2.
Summary of results from random-effects meta-analysis for all cognitive subdomains across all drug doses studied.
| Cognitive subdomain | Effect size (95% CI) | I2 (p-value) | Number of studies included |
|---|---|---|---|
| Overall cognitive index | −0.057 (−0.16, 0.044) | 0 (0.72) | 13 |
| Attention | −0.077 (−0.20, 0.047) | 0 (0.65) | 14 |
| Working memory | −0.011 (−0.14, 0.12) | 3 (0.42) | 14 |
| Executive function | 0.006 (−0.18, 0.19) | 32 (0.16) | 9 |
| Speed of processing | −0.015 (−0.14, 0.11) | 0 (0.98) | 13 |
| Verbal learning | −0.076 (−0.25, 0.096) | 16 (0.30) | 9 |
| Visual learning | −0.048 (−0.19, 0.092) | 0 (0.57) | 11 |
| Social cognition | 0.031 (−0.15, 0.21) | 0 (0.95) | 5 |
| Language | 0.12 (−0.49, 0.72) | 0 (0.38) | 2 |
Note: negative effect size favors drug.
4. Discussion
The main findings of our meta-analyses are that α7 nAChR agonists show large ES in commonly used preclinical behavioral tests of cognition in rodents, yet do not demonstrate commensurate large effects on cognition in humans with SCZ and AD. In most subdomains of cognition, including attention, α7 nAChR drugs across a range of tested doses showed extremely modest beneficial effects that did not approach statistical or clinical significance. Such results were consistent in subgroup analyses by diagnosis and when varenicline was excluded. Even when the most effective doses were entered into a subgroup meta-analysis, only small ES of uncertain clinical significance were found. The discrepancy between results of preclinical animal models demonstrating large ES and those of clinical trials demonstrating non-significant or small magnitude ES further illuminates the challenges of translating nAChR-based drugs for psychiatric and neurocognitive disorders.
4.1. Challenges of α7 nAChR agonists as human therapeutics and alternative pharmacological options
Our search strategy identified eight pharmacological compounds or combinations, all of which were used as exogenous partial or full agonists at α7 nAChRs. Multiple pharmacological properties of α7 nAChR agonists complicate their use as therapeutic compounds. Most importantly, the proclivity of α7 nAChRs to rapidly desensitize in the presence of agonists, including nicotine, is well described (Amar et al., 1993), which may interfere with repeated dosing, and some studies suggest that desensitization varies between agonists (Stevens et al., 1998). Furthermore, α7 nAChR agonists frequently demonstrate inverted U-shaped curves in cognitive tasks, exemplified in a recent study of working memory in non-human primates (Yang et al., 2013). These properties enhance the difficulty of dose choices in human trials. Finally, many of the agonists studied have activity at non-α7 nAChRs, especially 5-HT3, complicating the interpretation of their effects. An alternative strategy to target the α7 nAChR is through the use of PAMs (reviewed in (Uteshev, 2014)), which are compounds that do not activate the receptor on their own, but rather increase the peak current amplitude (type I PAM) or both increase the peak current amplitude and influence desensitization kinetics (type II PAM) in response to the endogenous agonists acetylcholine and choline. Thus, unlike α7 agonists, PAMs preserve the endogenous spatial and temporal properties of cholinergic signaling through α7 nAChRs and minimize off-target effects. Similar to α7 agonists, PAMs such as PNU-120596 can improve the auditory gating abnormality in rodents, yet do not cause desensitization and do not generally exhibit U-shaped dosing curves (Uteshev, 2014). Pharmaceutical development of PAMs for cognitive outcomes has not been as robust as for α7 nAChR agonists for cognition, though both galantamine monotherapy and JNJ-39393406 have been examined in small human studies with mixed results (reviewed in (Rowe et al., 2015)). Larger scale trials of α7 PAMs may overcome some of the challenges presented by α7 agonists for cognitive enhancement.
4.2. Translational barriers between rodent and human studies
4.2.1. Dosing paradigms between rodent preclinical studies and clinical trials
The choice of dose and dosing frequency are critical to the demonstration of therapeutic effects in clinical trials. Many of the rodent studies included in our meta-analysis employed acute or sub-acute dosing of α7 nAChR agonists, in stark contrast to many of the larger clinical trials, which were conducted over months. As noted above, repeated doses of α7 nAChR agonists can induce receptor desensitization, potentially even resulting in functional antagonism. Thus, the results of acute dosing in rodent behavioral tasks of many α7 nAChR agonists may not accurately predict their effects in chronic dosing paradigms. Furthermore, the specific choice of dose in clinical trials is extremely challenging given the wide range of receptor activation in conjunction with the demonstration of the inverted U-shape curve. These challenges are mitigated in rodent tasks, especially tasks with acute administration, as such studies can relatively quickly generate dose-response curves using numerous doses. Additionally, such acute studies are not hampered by drug intolerances or side effects that might develop over time. For logistical and financial reasons and to maintain statistical power, clinical trials can at most study a few doses, with doses frequently chosen from earlier tolerability data, cognitive findings in healthy subjects, or from in vitro studies using binding and activation data with human α7 nAChR. For instance, the doses of TC-5619 were chosen based on phase I tolerability data, improvement in attentional measures in healthy controls, and oocyte electrophysiological studies expressing human α7 nAChR (Lieberman et al., 2013). The exquisite sensitivity of α7 agonists to dose for cognitive outcomes is illustrated by two subgroup meta-analyses. Meta-analysis incorporating the highest dose of α7 agonist within each trial failed to demonstrate any significant drug effects on overall cognitive index or attention. The sub-group analysis incorporating only the most effective doses, which can be considered an estimate of the upper bound of ES, calculated only small effect sizes on overall cognitive index or attention (ES = −0.12 to −0.13). These ES are of unclear clinical significance and differ substantially from the large ES found in rodent cognitive studies (ES = −1.18 to −0.73). The results of these analyses clearly illustrate that dose selection is a difficult barrier to overcome in the translation of α7 nAChR agonists into human treatments.
4.2.2. Relevance and predictive validity of rodent models and tasks to human cognitive enhancement
Many preclinical studies performed the NOR task, a test of nonspatial learning and memory, and/or the Morris WM or related tests of spatial learning and memory. Other tasks more specifically examining attention, social interaction, executive function, or processing, represented a minority. Both the NOR and WM map onto multiple domains of human cognition in addition to learning and memory, recruiting attentional processes as well as executive functioning and working memory. We thus reasoned that focusing on the NOR and WM were appropriate rodent paradigms to include in a meta-analysis not only because of the quantity of published studies reporting these tests, but also because they involve similar cognitive processes as tested in humans. It should be noted that the NOR and WM have predictive validity for deficits in cognition in humans (Cohen and Stackman, 2015; Terry, 2009), but it is poorly understood whether predictive validity in these tasks is bidirectional, whereby improved performance predicts improvement in human cognition, either in healthy controls or in clinical populations. As opposed to the rather narrowly focused set of behavioral tasks, we identified a large range of pharmacological, environmental, and genetic preclinical models of cognitive deficits. Most pharmacological models involved acute or sub-chronic systemic administration of muscarinic AChR or NMDA receptor antagonists. In especially the case of glutamate receptor-antagonists such as PCP, MK-801, and ketamine, these compounds can mimic some of the positive and cognitive symptoms of psychotic illness and model pathological network effects that arise within patients with SCZ through changes in excitatory-inhibitory balance (Meltzer et al., 2013). However, unlike genetic models and environmental models with early-life insults, acute and sub-chronic pharmacological models do not model deficits in neural development or migration, a critical aspect of SCZ pathogenesis. Thus, difficulty in recapitulating critical aspects of underlying pathophysiology might represent an additional roadblock to translation.
4.2.3. α7 nAChR genetic, pharmacological, and expression differences between rodent and human
Important differences exist in the genetics, pharmacology, biophysical properties, and neuronal localization of the α7 nAChR between rodents and humans, some of which might mediate the observed differences between preclinical studies and clinical trials. In humans but not rodents, CHRNA7, the gene coding for α7 nAChR protein, is partially duplicated and known as CHRFAM7A (Bertrand et al., 2015). The partially duplicated gene can coassemble with full-length α7 nAChR to potentially regulate its expression and function, which might differentially influence agonist effects between human and rodent. Additional complexities of the human genetics of CHRNA7 include a highly unstable localization on chromosome 15 that predisposes to inversions, deletions, and duplications, as well as polymorphisms in the promoter region resulting in differential expression levels of the protein (Bertrand et al., 2015). The complexity of genetics of CHRNA7 in humans as compared to the analogous gene in rodents might influence the findings in clinical trials. Along similar lines, the pharmacology of specific agonists differs between human and rodents sequences coding for α7 nAChRs. For example, the partial agonist GTS-21 activates the rat α7 nAChR to a maximal response greater than twice that of the human α7 nAChR, and the Ki of GTS-21 at the rat receptor is roughly an order of magnitude less than at the human receptor (Meyer et al., 1998), suggesting that similar serum levels might have strongly disparate effects between species. Finally, localization differences of α7 nAChRs may differ between rodents and humans, as suggested by a recent study in non-human primates demonstrating postsynaptic localization of α7 nAChRs in glutamatergic synapses of layer III dorsolateral prefrontal cortex (Yang et al., 2013). The authors suggest that higher evolved organisms with enhanced cognitive capacity, such as primates, may place increasing importance on α7 nAChRs localized to the post-synaptic spine as compared to rodents. Thus, the mechanism of functional interaction with other receptors critical for cognition, such as NMDA receptors, may differ between rodents and humans.
4.3. Study limitations
Our human meta-analysis, despite studying 18 trials, synthesized heterogeneous trial designs and was of modest overall subject size, which precluded the possibility of performing many meaningful subgroup analyses that may be informative as to which groups of patients may truly benefit from α7 agonists. Studies of cognitive enhancement require very large sample sizes to find small ES, and thus there exists the possibility of type II error in our clinical trial meta-analysis. Furthermore, only three of 18 studies enrolled subjects with AD, with the two largest AD trials reporting only composite cognitive outcomes but not cognitive subdomains, limiting our power to assess α7 drugs in this clinical population. We nonetheless felt it was important to perform meta-analysis combining SCZ and AD populations because postmortem evidence suggests similar loss of α7 nAChR expression in cortical and other brain regions (Burghaus et al., 2000; Marutle et al., 2001). It is unknown however to what extent this misregulation plays a causal role in the pathophysiology of these two disorders. We were unable to include a robust dataset of a phase II trial of EVP-6124 for cognition as an adjunct to cholinesterase inhibitors for subjects with mild-moderate AD because to our knowledge this study was not published or publicly available (Anon, 2015). Subsequent phase III studies were unfortunately put on hold due to gastrointestinal side effects. Thus, it is possible that α7 agonists and/or PAMs may indeed be more effective for AD than SCZ, but additional data is needed to support this assertion.
4.4. Future directions and conclusions
Closing the translational gap that appears to exist between animal studies and human clinical trials is challenging. One first step that stems from our findings is the more consistent use of chronic dosing paradigms in animal studies to parallel chronic administration in clinical populations. Along similar lines, successful translation of α7 nAChR agonists might be facilitated by the incorporation of intermediate stages of testing that employ human paradigms with increased face validity to animal paradigms, including virtual WM (Astur et al., 2002) or human object recognition tasks (Raber, 2015). These human studies could be performed after both acute and chronic dosing, and only if improvements are found on chronic dosing would the compound progress to larger scale clinical trials using more traditional cognitive outcomes for SCZ and AD cohorts.
A small subset of the included clinical trials performed subgroup analyses to further pinpoint the clinical characteristics of patients who might benefit preferentially from these agents. Given the relatively limited numbers of patients within these subgroups and post-hoc nature of some of these analyses, we did not perform meta-analysis using these subgroups, however, certain themes arise. Smoking status may play an important mediating role in the effects of α7 agonists, though the direction of its effect seems to be compound-specific, with some studies finding no effect of smoking. In addition to cognitive outcomes, negative symptoms were often a primary or secondary outcome, with some trials, including GTS-21 (Freedman et al., 2008), encenicline (Keefe et al., 2015), and RG3487 (Umbricht et al., 2014) showing some efficacy in secondary outcome or post-hoc subgroup analyses. Similar to cognitive dysfunction, negative symptoms of SCZ are disabling without any highly effective pharmacological treatments, and thus further investigation into the possible use of α7 nAChR-targeting compounds for this constellation of symptoms is warranted. Finally, most compounds were well tolerated in humans, which may allow for further trials in these subgroups or for other indications. The preclinical efficacy of compounds targeting α7 nAChRs is encouraging. However, the aggregate lack of effect in clinical trials reinforces the importance of identifying the major translational roadblocks, which may occur at multiple steps in the drug development pipeline.
Supplementary Material
Acknowledgments
Funding
This work was supported by Autism Speaks Grant # 9699 (ASL). The funding agency had no influence on data collection, analysis, or reporting.
The authors would like to acknowledge Marina R. Picciotto, PhD for helpful comments on the manuscript.
Abbreviations
- nAChR
nicotinic acetylcholine receptor
- NOR
novel object recognition
- WM
water maze
- ES
effect size
- CI
confidence interval
- SCZ
schizophrenia
- AD
Alzheimer’s disease
- PAM
positive allosteric modulator
Appendix A. Supplementary data
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.pnpbp.2017.01.001.
References
- Anonymous. [Access date: 10/02/2016];Rare but Severe Side Effects Sideline Some Phase 3 Encenicline Trials Alzforum. 2015 http://www.alzforum.org/news/research-news/rare-severe-side-effects-sideline-some-phase-3-encenicline-trials.
- Adler LE, Hoffer LD, Wiser A, Freedman R. Normalization of auditory physiology by cigarette smoking in schizophrenic patients. Am J Psychiatry. 1993;150:1856–1861. doi: 10.1176/ajp.150.12.1856. [DOI] [PubMed] [Google Scholar]
- Amar M, Thomas P, Johnson C, Lunt GG, Wonnacott S. Agonist pharmacology of the neuronal alpha7 nicotinic receptor expressed in Xenopus oocytes. FEBS Lett. 1993;327:284–288. doi: 10.1016/0014-5793(93)81005-k. [DOI] [PubMed] [Google Scholar]
- Astur RS, Taylor LB, Mamelak AN, Philpott L, Sutherland RJ. Humans with hippocampus damage display severe spatial memory impairments in a virtual Morris water task. Behav Brain Res. 2002;132:77–84. doi: 10.1016/s0166-4328(01)00399-0. [DOI] [PubMed] [Google Scholar]
- Bertrand D, Lee CH, Flood D, Marger F, Donnelly-Roberts D. Therapeutic potential of alpha7 nicotinic acetylcholine receptors. Pharmacol Rev. 2015;67:1025–1073. doi: 10.1124/pr.113.008581. [DOI] [PubMed] [Google Scholar]
- Bitner RS, Bunnelle WH, Anderson DJ, Briggs CA, Buccafusco J, Curzon P, et al. Broad-spectrum efficacy across cognitive domains by alpha7 nicotinic acetylcholine receptor agonism correlates with activation of ERK1/2 and CREB phosphorylation pathways. J Neurosci. 2007;27:10578–10587. doi: 10.1523/JNEUROSCI.2444-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie CR, Harvey PD. Cognition in schizophrenia: impairments, determinants, and functional importance. Psychiatr Clin North Am. 2005;28(26):613–633. doi: 10.1016/j.psc.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Burghaus L, Schutz U, Krempel U, de Vos RA, Jansen Steur EN, Wevers A, et al. Quantitative assessment of nicotinic acetylcholine receptor proteins in the cerebral cortex of Alzheimer patients. Brain Res Mol Brain Res. 2000;76:385–388. doi: 10.1016/s0169-328x(00)00031-0. [DOI] [PubMed] [Google Scholar]
- Chu LW, Ma ES, Lam KK, Chan MF, Lee DH. Increased alpha 7 nicotinic acetylcholine receptor protein levels in Alzheimer’s disease patients. Dement Geriatr Cogn Disord. 2005;19:106–112. doi: 10.1159/000082661. [DOI] [PubMed] [Google Scholar]
- Coe JW, Brooks PR, Vetelino MG, Wirtz MC, Arnold EP, Huang J, et al. Varenicline: an alpha4beta2 nicotinic receptor partial agonist for smoking cessation. J Med Chem. 2005;48:3474–3477. doi: 10.1021/jm050069n. [DOI] [PubMed] [Google Scholar]
- Cohen SJ, Stackman RW., Jr Assessing rodent hippocampal involvement in the novel object recognition task. A review. Behav Brain Res. 2015;285:105–117. doi: 10.1016/j.bbr.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch SI, Schwartz BL, Schooler NR, Brown CH, Rosse RB, Rosse SM. Targeting alpha-7 nicotinic neurotransmission in schizophrenia: a novel agonist strategy. Schizophr Res. 2013;148:138–144. doi: 10.1016/j.schres.2013.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunbar GC, Kuchibhatla RV, Lee G, Group T-ACS. A randomized double-blind study comparing 25 and 50 mg TC-1734 (AZD3480) with placebo, in older subjects with age-associated memory impairment. J Psychopharmacol. 2011;25:1020–1029. doi: 10.1177/0269881110367727. [DOI] [PubMed] [Google Scholar]
- Florian H, Meier A, Gauthier S, Lipschitz S, Lin Y, Tang Q, et al. Efficacy and safety of ABT-126 in subjects with mild-to-moderate Alzheimer’s disease on stable doses of acetylcholinesterase inhibitors: a randomized, double-blind, placebo-controlled study. J Alzheimers Dis. 2016;51:1237–1247. doi: 10.3233/JAD-150978. [DOI] [PubMed] [Google Scholar]
- Freedman R. Alpha7-nicotinic acetylcholine receptor agonists for cognitive enhancement in schizophrenia. Annu Rev Med. 2014;65:245–261. doi: 10.1146/annurev-med-092112-142937. [DOI] [PubMed] [Google Scholar]
- Freedman R, Hall M, Adler LE, Leonard S. Evidence in postmortem brain tissue for decreased numbers of hippocampal nicotinic receptors in schizophrenia. Biol Psychiatry. 1995;38:22–33. doi: 10.1016/0006-3223(94)00252-X. [DOI] [PubMed] [Google Scholar]
- Freedman R, Olincy A, Ross RG, Waldo MC, Stevens KE, Adler LE, et al. The genetics of sensory gating deficits in schizophrenia. Curr Psychiatry Rep. 2003;5:155–161. doi: 10.1007/s11920-003-0032-2. [DOI] [PubMed] [Google Scholar]
- Freedman R, Olincy A, Buchanan RW, Harris JG, Gold JM, Johnson L, et al. Initial phase 2 trial of a nicotinic agonist in schizophrenia. Am J Psychiatry. 2008;165:1040–1047. doi: 10.1176/appi.ajp.2008.07071135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frolich L, Ashwood T, Nilsson J, Eckerwall G, Sirocco I. Effects of AZD3480 on cognition in patients with mild-to-moderate Alzheimer’s disease: a phase IIb dose-finding study. J Alzheimers Dis. 2011;24:363–374. doi: 10.3233/JAD-2011-101554. [DOI] [PubMed] [Google Scholar]
- Gault LM, Ritchie CW, Robieson WZ, Pritchett Y, Othman AA, Lenz RA. A phase 2 randomized, controlled trial of the α7 agonist ABT-126 in mild-to-moderate Alzheimer’s dementia. Alzheimers Dement Transl Res Clin Interv. 2015;1:81–90. doi: 10.1016/j.trci.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Z, Yakel JL. Timing-dependent septal cholinergic induction of dynamic hippocampal synaptic plasticity. Neuron. 2011;71:155–165. doi: 10.1016/j.neuron.2011.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haig GM, Bain EE, Robieson WZ, Baker JD, Othman AA. A randomized trial to assess the efficacy and safety of ABT-126, a selective alpha7 nicotinic acetylcholine receptor agonist, in the treatment of cognitive impairment in schizophrenia. Am J Psychiatry. 2016 doi: 10.1176/appi.ajp.2015.15010093. (appiajp201515010093) [DOI] [PubMed] [Google Scholar]
- Hong LE, Thaker GK, McMahon RP, Summerfelt A, Rachbeisel J, Fuller RL, et al. Effects of moderate-dose treatment with varenicline on neurobiological and cognitive biomarkers in smokers and nonsmokers with schizophrenia or schizoaffective disorder. Arch Gen Psychiatry. 2011;68:1195–1206. doi: 10.1001/archgenpsychiatry.2011.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe RS, Silva SG, Perkins DO, Lieberman JA. The effects of atypical antipsychotic drugs on neurocognitive impairment in schizophrenia: a review and meta-analysis. Schizophr Bull. 1999;25:201–222. doi: 10.1093/oxfordjournals.schbul.a033374. [DOI] [PubMed] [Google Scholar]
- Keefe RS, Meltzer HA, Dgetluck N, Gawryl M, Koenig G, Moebius HJ, et al. Randomized, double-blind, placebo-controlled study of encenicline, an alpha7 nicotinic acetylcholine receptor agonist, as a treatment for cognitive impairment in schizophrenia. Neuropsychopharmacology. 2015;40:3053–3060. doi: 10.1038/npp.2015.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Choi SH, Rollema H, Schwam EM, McRae T, Dubrava S, et al. Phase II crossover trial of varenicline in mild-to-moderate Alzheimer’s disease. Dement Geriatr Cogn Disord. 2014;37:232–245. doi: 10.1159/000355373. [DOI] [PubMed] [Google Scholar]
- Levin ED. Alpha7-nicotinic receptors and cognition. Curr Drug Targets. 2012;13:602–606. doi: 10.2174/138945012800398937. [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Dunbar G, Segreti AC, Girgis RR, Seoane F, Beaver JS, et al. A randomized exploratory trial of an alpha-7 nicotinic receptor agonist (TC-5619) for cognitive enhancement in schizophrenia. Neuropsychopharmacology. 2013;38:968–975. doi: 10.1038/npp.2012.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsey MW, Wilson DB. Practical Meta-Analysis. Sage Publications; Thousand Oaks, Calif: 2001. [Google Scholar]
- Marutle A, Zhang X, Court J, Piggott M, Johnson M, Perry R, et al. Laminar distribution of nicotinic receptor subtypes in cortical regions in schizophrenia. J Chem Neuroanat. 2001;22:115–126. doi: 10.1016/s0891-0618(01)00117-x. [DOI] [PubMed] [Google Scholar]
- Meltzer HY, Rajagopal L, Huang M, Oyamada Y, Kwon S, Horiguchi M. Translating the N-methyl-D-aspartate receptor antagonist model of schizophrenia to treatments for cognitive impairment in schizophrenia. Int J Neuropsychopharmacol. 2013;16:2181–2194. doi: 10.1017/S1461145713000928. [DOI] [PubMed] [Google Scholar]
- Meyer EM, Kuryatov A, Gerzanich V, Lindstrom J, Papke RL. Analysis of 3-(4-hydroxy, 2-methoxybenzylidene)anabaseine selectivity and activity at human and rat alpha-7 nicotinic receptors. J Pharmacol Exp Ther. 1998;287:918–925. [PubMed] [Google Scholar]
- Moran TP, Schroder HS, Kneip C, Moser JS. Meta-analysis and psychophysiology: a tutorial using depression and action-monitoring event-related potentials. Int J Psychophysiol. 2016 doi: 10.1016/j.ijpsycho.2016.07.001. [DOI] [PubMed] [Google Scholar]
- Olincy A, Harris JG, Johnson LL, Pender V, Kongs S, Allensworth D, et al. Proof-of-concept trial of an alpha7 nicotinic agonist in schizophrenia. Arch Gen Psychiatry. 2006;63:630–638. doi: 10.1001/archpsyc.63.6.630. [DOI] [PubMed] [Google Scholar]
- Preskorn SH, Gawryl M, Dgetluck N, Palfreyman M, Bauer LO, Hilt DC. Normalizing effects of EVP-6124, an alpha-7 nicotinic partial agonist, on event-related potentials and cognition: a proof of concept, randomized trial in patients with schizophrenia. J Psychiatr Pract. 2014;20:12–24. doi: 10.1097/01.pra.0000442935.15833.c5. [DOI] [PubMed] [Google Scholar]
- Raber J. Novel images and novel locations of familiar images as sensitive translational cognitive tests in humans. Behav Brain Res. 2015;285:53–59. doi: 10.1016/j.bbr.2015.01.046. [DOI] [PubMed] [Google Scholar]
- Roh S, Hoeppner SS, Schoenfeld D, Fullerton CA, Stoeckel LE, Evins AE. Acute effects of mecamylamine and varenicline on cognitive performance in non-smokers with and without schizophrenia. Psychopharmacology. 2014;231:765–775. doi: 10.1007/s00213-013-3286-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe AR, Mercer L, Casetti V, Sendt KV, Giaroli G, Shergill SS, et al. Dementia praecox redux: a systematic review of the nicotinic receptor as a target for cognitive symptoms of schizophrenia. J Psychopharmacol. 2015;29:197–211. doi: 10.1177/0269881114564096. [DOI] [PubMed] [Google Scholar]
- Samochocki M, Hoffie A, Fehrenbacher A, Jostock R, Ludwig J, Christner C, et al. Galantamine is an allosterically potentiating ligand of neuronal nicotinic but not of muscarinic acetylcholine receptors. J Pharmacol Exp Ther. 2003;305:1024–1036. doi: 10.1124/jpet.102.045773. [DOI] [PubMed] [Google Scholar]
- Shiina A, Shirayama Y, Niitsu T, Hashimoto T, Yoshida T, Hasegawa T, et al. A randomised, double-blind, placebo-controlled trial of tropisetron in patients with schizophrenia. Ann General Psychiatry. 2010;9:27. doi: 10.1186/1744-859X-9-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim JC, Jung DU, Jung SS, Seo YS, Cho DM, Lee JH, et al. Adjunctive varenicline treatment with antipsychotic medications for cognitive impairments in people with schizophrenia: a randomized double-blind placebo-controlled trial. Neuropsychopharmacology. 2012;37:660–668. doi: 10.1038/npp.2011.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RC, Amiaz R, Si TM, Maayan L, Jin H, Boules S, et al. Varenicline effects on smoking, cognition, and psychiatric symptoms in schizophrenia: a double-blind randomized trial. PLoS One. 2016;11:e0143490. doi: 10.1371/journal.pone.0143490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens KE, Kem WR, Mahnir VM, Freedman R. Selective alpha7-nicotinic agonists normalize inhibition of auditory response in DBA mice. Psychopharmacology. 1998;136:320–327. doi: 10.1007/s002130050573. [DOI] [PubMed] [Google Scholar]
- Terry AV., Jr . Spatial Navigation (Water Maze) Tasks. In: Buccafusco JJ, editor. Methods of Behavior Analysis in Neuroscience. 2. Boca Raton (FL): 2009. [PubMed] [Google Scholar]
- Umbricht D, Keefe RS, Murray S, Lowe DA, Porter R, Garibaldi G, et al. A randomized, placebo-controlled study investigating the nicotinic alpha7 agonist, RG3487, for cognitive deficits in schizophrenia. Neuropsychopharmacology. 2014;39:1568–1577. doi: 10.1038/npp.2014.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uteshev VV. The therapeutic promise of positive allosteric modulation of nicotinic receptors. Eur J Pharmacol. 2014;727:181–185. doi: 10.1016/j.ejphar.2014.01.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velligan D, Brenner R, Sicuro F, Walling D, Riesenberg R, Sfera A, et al. Assessment of the effects of AZD3480 on cognitive function in patients with schizophrenia. Schizophr Res. 2012;134:59–64. doi: 10.1016/j.schres.2011.10.004. [DOI] [PubMed] [Google Scholar]
- Wallace BC, Dahabreh IJ, Trikalinos TA, Lau J, Trow P, Schmid CH. Closing the gap between methodologists and end-users: R as a computational back-end. J Stat Softw. 2012;49:1–15. [Google Scholar]
- Walling D, Marder SR, Kane J, Fleischhacker WW, Keefe RS, Hosford DA, et al. Phase 2 trial of an alpha-7 nicotinic receptor agonist (TC-5619) in negative and cognitive symptoms of schizophrenia. Schizophr Bull. 2016;42:335–343. doi: 10.1093/schbul/sbv072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DB. Practical Meta-Analysis Effect Size Calculator. 2010 http://www.campbellcollaboration.org/escalc/html/EffectSizeCalculator-SMD-main.php.
- Wonnacott S, Barik J, Dickinson J, Jones IW. Nicotinic receptors modulate transmitter cross talk in the CNS: nicotinic modulation of transmitters. J Mol Neurosci. 2006;30:137–140. doi: 10.1385/JMN:30:1:137. [DOI] [PubMed] [Google Scholar]
- Yang Y, Paspalas CD, Jin LE, Picciotto MR, Arnsten AF, Wang M. Nicotinic alpha7 receptors enhance NMDA cognitive circuits in dorsolateral prefrontal cortex. Proc Natl Acad Sci U S A. 2013;110:12078–12083. doi: 10.1073/pnas.1307849110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XY, Liu L, Liu S, Hong X, Chen da C, Xiu MH, et al. Short-term tropisetron treatment and cognitive and P50 auditory gating deficits in schizophrenia. Am J Psychiatry. 2012;169:974–981. doi: 10.1176/appi.ajp.2012.11081289. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.