ABSTRACT
Mitochondrial autophagy (mitophagy) is thought to be a multi-step pathway wherein mitochondria are first divided into small fragments, which are subsequently recognized by the phagophore. DNM1L (dynamin 1 like) plays a pivotal role in mitochondrial division; however, its role in mitophagy remains controversial. In our recent study, we examined the contribution of DNM1L to mitophagy and showed that mitophagy and mitochondrial division occur even in DNM1L-defective cells. Furthermore, time-lapse imaging of mitophagy showed that DNM1L-independent mitochondrial division occurs concomitantly with autophagosome formation. Upstream factors of autophagosome formation, i.e., RB1CC1/FIP200, ATG14, and WIPIs, are required for mitochondrial division, whereas ATG5 and ATG3 are dispensable. These results indicate that a portion of the tubular mitochondria is first recognized and then divided into small fragments by a phagophore-mediated event, independently of DNM1L. This autophagic process suggests that autophagy has the potential to degrade substrates larger than autophagosomes.
KEYWORDS: autophagy, Dnm1, DNM1L, mitochondria, mitochondrial division, mitophagy
Mitochondria have several important functions, including ATP synthesis, calcium buffering, apoptosis regulation, and thermogenesis, and play a role in numerous cellular events. The morphology of mitochondria is maintained by balancing their division and fusion, and correct mitochondrial morphology is critical for proper cellular function. Mitochondrial division is coordinated by several factors including DNM1L (dynamin 1 like), MFF (mitochondrial fission factor), MIEF1 (mitochondrial elongation factor 1) and MIEF2. When mitochondrial division occurs, cytoplasmic DNM1L is recruited on the mitochondrial outer membrane by interaction with adaptor proteins such as MFF, MIEF1, or MIEF2. In mitochondrial division-defective cells, the mitochondrial morphology becomes largely clustered or highly elongated.
Mitochondria can be degraded by mitochondria-specific autophagy, i.e., mitophagy. It has been suggested that mitophagy contributes to the maintenance of mitochondrial quality and quantity. With respect to mitochondrial morphology during mitophagy, it is widely accepted that the damaged portion of the mitochondrion is first separated from the healthy mitochondrial network and divided into small fragments, and the phagophore for autophagic degradation subsequently recognizes the fragment. In this model, it seems that canonical DNM1L-dependent mitochondrial division plays an important role in mitophagy. However, whether DNM1L is required for mitophagy is debatable. To elucidate the regulation of mitochondrial morphology on mitophagy, we analyzed mitochondrial division in DNM1L-defective cells under mitophagic conditions.
We first investigated the contribution of Dnm1, a yeast homolog of DNM1L, to mitochondria degradation in budding yeast. Mitophagy induced by nitrogen starvation slightly decreased, but still occurred at a significant level in dnm1Δ cells compared with that in WT cells. We further analyzed the mitochondria within the autophagic body using electron microscopy and found that only small fragmented mitochondria of < 600 nm in diameter were present in the autophagic bodies of both WT and dnm1Δ cells, although almost all mitochondria in the cytoplasm were largely clustered or highly elongated in dnm1Δ cells. We then analyzed the contribution of DNM1L to mitophagy in DNM1L-defective mammalian cells. Mitophagy induced by hypoxic and iron-depletion conditions was not significantly suppressed in DNM1L knockout (KO) or knockdown HeLa, MEF, or SH-SY5Y cells compared with that in WT. Small fragmented mitochondria enwrapped by autophagosomes were observed in both DNM1L KO and WT cells in the presence of bafilomycin A1. These results suggest that mitochondria are divided into small fragments under mitophagic conditions in a DNM1L/Dnm1-independent manner.
Time-lapse imaging of mito-mCherry and EGFP-LC3B to visualize mitochondria and phagophores, respectively, clearly revealed the mitochondrial fragmentation process during mitophagy. Following the induction of mitophagy in WT cells, a phagophore membrane first appeared on the tubular mitochondria and then a portion of the mitochondria started budding at the same site. The budded portion of mitochondria was enwrapped by the phagophore and eventually separated from the tubular mitochondria concomitant with autophagosome formation. The phagophore membrane recognizes the portion of the tubular mitochondria destined for degradation before mitochondrial division. Similarly, mitochondrial division concomitant with autophagosome formation was observed even in DNM1L KO cells, suggesting that mitochondrial division during mitophagy occurs independently of DNM1L. Interestingly, DNM1L-independent mitochondrial division during mitophagy requires RB1CC1/FIP200, ATG14, and WIPIs, which are essential for the formation and extension of the phagophore membrane, whereas ATG5 or ATG3, which are required for the closure of the phagophore, are not always required for mitochondrial division. These results suggest that an elongation of the phagophore membrane is required for DNM1L-independent mitochondrial division during mitophagy.
From these findings, we propose a novel model of mitochondrial division for mitophagy, which occurs concomitant with autophagosome formation (Fig. 1A). When mitophagy is induced, phagophore membranes emerge on the mitochondria and extend along the mitochondrial surface. The mitochondria make a bud at or close to the site of formation of the phagophore membranes, which then enwrap the budded portion of the mitochondria. The budded portion of the mitochondria is divided simultaneously with phagophore closure. Finally, the complete sealing of the phagophore occurs to form the mitophagosome. This model is completely different from the widely accepted model wherein mitochondrial fragmentation occurs first and the fragmented mitochondria are then enwrapped by phagophores (Fig 1A).
Figure 1.
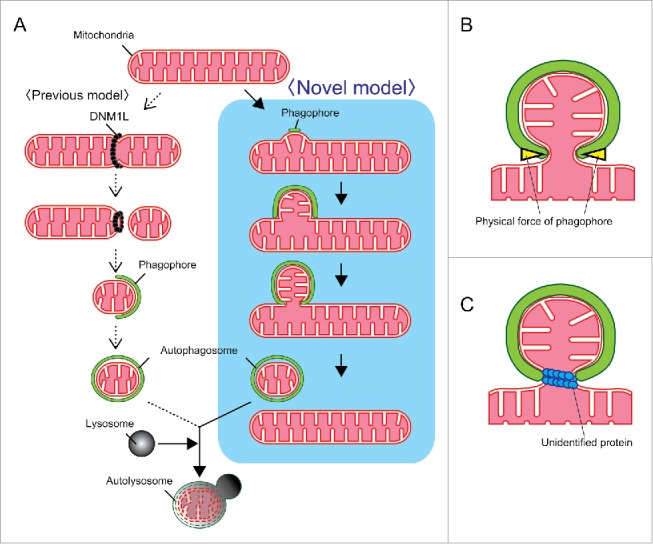
Models for mitochondrial division during mitophagy. (A) Mitochondrial division occurs before (previous model, left) or concomitant with (novel model, right) autophagosome formation. (B, C) The 2 possibilities show how mitochondria are divided independently of DNM1L. One model is that a phagophore membrane directly constricts the mitochondrial bud by physical force (B). Another model is that an unidentified protein drives the division (C).
So far, it is unclear how mitochondria are divided without DNM1L during mitophagy or the force driving such a division. The most plausible explanation is that mitochondria are constricted and directly divided by an extended phagophore membrane (Fig. 1B). In this case, the energetic difference between the partially bended form and almost closed form of the phagophore membrane may be the physical force driving mitochondrial division. However, it is also possible that an unidentified protein functions in the mitochondrial division step (Fig. 1C). Further studies are required to clarify this issue.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by JSPS KAKENHI Grant Numbers 26291039 (TK), 16H01384 (TK), 16H01198 (TK), and 15K18501 (SY).


