Abstract
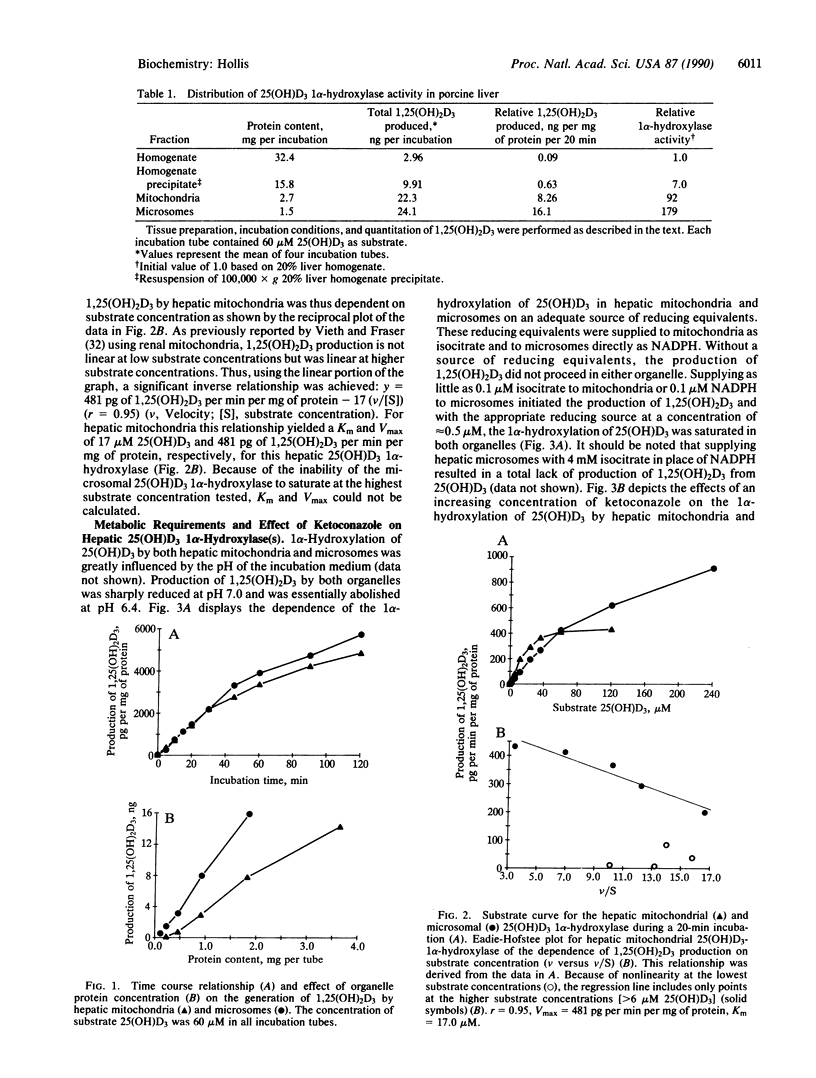
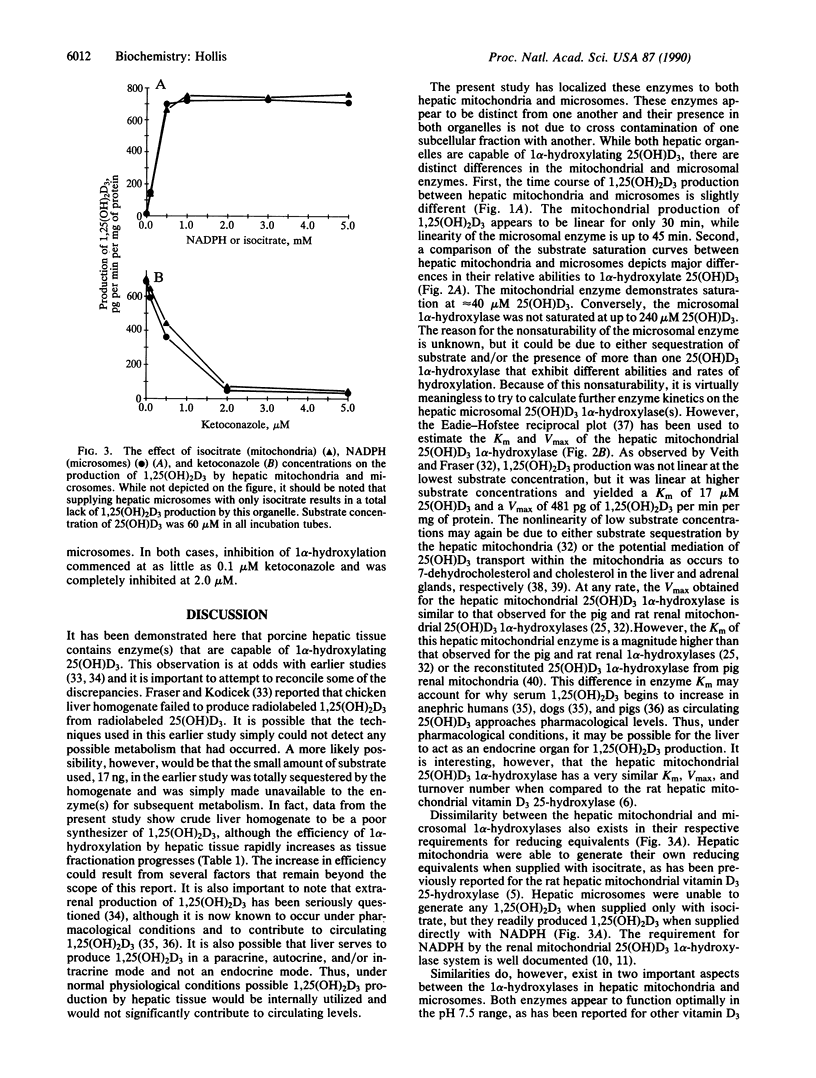
In vitro studies were performed to assess the ability of hepatic homogenates, mitochondria, and microsomes to 1 alpha-hydroxylate 25-hydroxyvitamin D3 [25(OH)D3]. Addition of 25(OH)D3 to either hepatic mitochondria or microsomes caused a concentration-dependent increase in the production of 1,25-dihydroxyvitamin D3 [1,25(OH)2D3]. Hepatic homogenates also produced purported 1,25(OH)2D3, although at a much reduced efficiency as compared with hepatic mitochondria or microsomes. Purported 1,25(OH)2D3 synthesized by hepatic mitochondria or microsomes was identified by its mobility on several high-performance liquid chromatographic systems and, ultimately, by its ability to interact with the bovine thymus 1,25(OH)2D3 receptor protein. Production of 1,25(OH)2D3 by hepatic mitochondria and microsomes was dependent on time of incubation, protein content, and pH of incubation medium, and it required an adequate source of reducing equivalents. Generation of 1,25(OH)2D3 by these organelles could be totally blocked by the cytochrome P-450 inhibitor ketoconazole. The microsomal 1 alpha-hydroxylase could not be saturated even at the highest concentration (240 microM) of 25(OH)D3 used. The mitochondrial 1 alpha-hydroxylase, however, displayed saturation at approximately 40 microM 25(OH)D3. Eadie-Hofstee reciprocal plot analysis of the hepatic mitochondrial 1 alpha-hydroxylase gave a Km of 17 microM 25(OH)D3 and a Vmax of 481 pg of 1,25(OH)2D3 per min per mg of protein. Because of its inability to achieve substrate saturation, meaningful kinetic parameters could not be calculated for the hepatic microsomal 1 alpha-hydroxylase. These data demonstrate the liver to be an even more dynamic organ than was previously believed with respect to vitamin D metabolism in that the liver has the potential to produce 1,25(OH)2D3 in situ by at least two separate mechanisms.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. S., Sharma O. P., Gacad M. A., Singer F. R. Metabolism of 25-hydroxyvitamin D3 by cultured pulmonary alveolar macrophages in sarcoidosis. J Clin Invest. 1983 Nov;72(5):1856–1860. doi: 10.1172/JCI111147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson S., Boström H., Danielsson H., Wikvall K. Purification from rabbit and rat liver of cytochromes P-450 involved in bile acid biosynthesis. Methods Enzymol. 1985;111:364–377. doi: 10.1016/s0076-6879(85)11023-2. [DOI] [PubMed] [Google Scholar]
- Andersson S., Jörnvall H. Sex differences in cytochrome P-450-dependent 25-hydroxylation of C27-steroids and vitamin D3 in rat liver microsomes. J Biol Chem. 1986 Dec 25;261(36):16932–16936. [PubMed] [Google Scholar]
- Baran D. T., Kelly A. M. Lysophosphatidylinositol: a potential mediator of 1,25-dihydroxyvitamin D-induced increments in hepatocyte cytosolic calcium. Endocrinology. 1988 Mar;122(3):930–934. doi: 10.1210/endo-122-3-930. [DOI] [PubMed] [Google Scholar]
- Baran D. T., Milne M. L. 1,25 Dihydroxyvitamin D increases hepatocyte cytosolic calcium levels. A potential regulator of vitamin D-25-hydroxylase. J Clin Invest. 1986 May;77(5):1622–1626. doi: 10.1172/JCI112478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baran D. T., Sorensen A. M., Honeyman T. W. Rapid action of 1,25-dihydroxyvitamin D3 on hepatocyte phospholipids. J Bone Miner Res. 1988 Dec;3(6):593–600. doi: 10.1002/jbmr.5650030603. [DOI] [PubMed] [Google Scholar]
- Bell N. H. Vitamin D-endocrine system. J Clin Invest. 1985 Jul;76(1):1–6. doi: 10.1172/JCI111930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikle D. D., Nemanic M. K., Gee E., Elias P. 1,25-Dihydroxyvitamin D3 production by human keratinocytes. Kinetics and regulation. J Clin Invest. 1986 Aug;78(2):557–566. doi: 10.1172/JCI112609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björkhem I., Holmberg I. Assay and properties of a mitochondrial 25-hydroxylase active on vitamine D3. J Biol Chem. 1978 Feb 10;253(3):842–849. [PubMed] [Google Scholar]
- Björkhem I., Holmberg I., Oftebro H., Pedersen J. I. Properties of a reconstituted vitamin D3 25-hydroxylase from rat liver mitochondria. J Biol Chem. 1980 Jun 10;255(11):5244–5249. [PubMed] [Google Scholar]
- Björkhem I., Holmberg I. On the 25-hydroxylation of vitamin D3 in vitro studied with a mass fragmentographic technique. J Biol Chem. 1979 Oct 10;254(19):9518–9524. [PubMed] [Google Scholar]
- Chanderbhan R., Noland B. J., Scallen T. J., Vahouny G. V. Sterol carrier protein2. Delivery of cholesterol from adrenal lipid droplets to mitochondria for pregnenolone synthesis. J Biol Chem. 1982 Aug 10;257(15):8928–8934. [PubMed] [Google Scholar]
- Davis W. L., Matthews J. L., Goodman D. B. Glyoxylate cycle in the rat liver: effect of vitamin D3 treatment. FASEB J. 1989 Mar;3(5):1651–1655. doi: 10.1096/fasebj.3.5.2537775. [DOI] [PubMed] [Google Scholar]
- Duncan W. E., Whitehead D., Wray H. L. A 1,25-dihydroxyvitamin D3 receptor-like protein in mammalian and avian liver nuclei. Endocrinology. 1988 Jun;122(6):2584–2589. doi: 10.1210/endo-122-6-2584. [DOI] [PubMed] [Google Scholar]
- Dusso A., Lopez-Hilker S., Rapp N., Slatopolsky E. Extra-renal production of calcitriol in chronic renal failure. Kidney Int. 1988 Sep;34(3):368–375. doi: 10.1038/ki.1988.190. [DOI] [PubMed] [Google Scholar]
- Engstrom G. W., Horst R. L., Reinhardt T. A., Littledike E. T. 25-Hydroxyvitamin D 1 alpha- and 24-hydroxylase activities in pig kidney homogenates: effect of vitamin D deficiency. J Nutr. 1984 Jan;114(1):119–126. doi: 10.1093/jn/114.1.119. [DOI] [PubMed] [Google Scholar]
- Ethier C., Kestekian R., Beaulieu C., Dubé C., Havrankova J., Gascon-Barré M. Vitamin D depletion retards the normal regeneration process after partial hepatectomy in the rat. Endocrinology. 1990 Jun;126(6):2947–2959. doi: 10.1210/endo-126-6-2947. [DOI] [PubMed] [Google Scholar]
- Fraser D. R., Kodicek E. Unique biosynthesis by kidney of a biological active vitamin D metabolite. Nature. 1970 Nov 21;228(5273):764–766. doi: 10.1038/228764a0. [DOI] [PubMed] [Google Scholar]
- Fraser D. R., Kodicek E. Unique biosynthesis by kidney of a biological active vitamin D metabolite. Nature. 1970 Nov 21;228(5273):764–766. doi: 10.1038/228764a0. [DOI] [PubMed] [Google Scholar]
- Ghazarian J. G., DeLuca H. F. 25-Hydroxycholecalciferol-1-hydroxylase: a specific requirement for NADPH and a hemoprotein component in chick kidney mitochondria. Arch Biochem Biophys. 1974 Jan;160(1):63–72. doi: 10.1016/s0003-9861(74)80009-3. [DOI] [PubMed] [Google Scholar]
- Gray R. W., Ghazarian J. G. Solubilization and reconstitution of kidney 25-hydroxyvitamin D3 1 alpha- and 24-hydroxylases from vitamin D-replete pigs. Biochem J. 1989 Apr 15;259(2):561–568. doi: 10.1042/bj2590561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A. K., Sexton R. C., Rudney H. Effect of vitamin D3 derivatives on cholesterol synthesis and HMG-CoA reductase activity in cultured cells. J Lipid Res. 1989 Mar;30(3):379–386. [PubMed] [Google Scholar]
- Henry H. L., Norman A. W. Studies on calciferol metabolism. IX. Renal 25-hydroxy-vitamin D3-1 hydroxylase. Involvement of cytochrome P-450 and other properties. J Biol Chem. 1974 Dec 10;249(23):7529–7535. [PubMed] [Google Scholar]
- Hollis B. W., Frank N. E. Solid phase extraction system for vitamin D and its major metabolites in human plasma. J Chromatogr. 1985 Sep 13;343(1):43–49. doi: 10.1016/s0378-4347(00)84566-1. [DOI] [PubMed] [Google Scholar]
- Hollis B. W., Iskersky V. N., Chang M. K. In vitro metabolism of 25-hydroxyvitamin D3 by human trophoblastic homogenates, mitochondria, and microsomes: lack of evidence for the presence of 25-hydroxyvitamin D3-1 alpha- and 24R-hydroxylases. Endocrinology. 1989 Sep;125(3):1224–1230. doi: 10.1210/endo-125-3-1224. [DOI] [PubMed] [Google Scholar]
- Horsting M., DeLuca H. F. In vitro production of 25-hydroxycholecalciferol. Biochem Biophys Res Commun. 1969 Jul 23;36(2):251–256. doi: 10.1016/0006-291x(69)90322-2. [DOI] [PubMed] [Google Scholar]
- Littledike E. T., Horst R. L. Metabolism of vitamin D3 in nephrectomized pigs given pharmacological amounts of vitamin D3. Endocrinology. 1982 Dec;111(6):2008–2013. doi: 10.1210/endo-111-6-2008. [DOI] [PubMed] [Google Scholar]
- Madhok T. C., DeLuca H. F. Characteristics of the rat liver microsomal enzyme system converting cholecalciferol into 25-hydroxycholecalciferol. Evidence for the participation of cytochrome p-450. Biochem J. 1979 Dec 15;184(3):491–499. doi: 10.1042/bj1840491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masumoto O., Ohyama Y., Okuda K. Purification and characterization of vitamin D 25-hydroxylase from rat liver mitochondria. J Biol Chem. 1988 Oct 5;263(28):14256–14260. [PubMed] [Google Scholar]
- Noland B. J., Arebalo R. E., Hansbury E., Scallen T. J. Purification and properties of sterol carrier protein2. J Biol Chem. 1980 May 10;255(9):4282–4289. [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. I. EVIDENCE FOR ITS HEMOPROTEIN NATURE. J Biol Chem. 1964 Jul;239:2370–2378. [PubMed] [Google Scholar]
- Pahuja D. N., Deshpande U. R., Soman C. S., Nadkarni G. D. Altered hepatic function in vitamin D-deprived rats. J Hepatol. 1989 Sep;9(2):209–216. doi: 10.1016/0168-8278(89)90053-6. [DOI] [PubMed] [Google Scholar]
- Paulson S. K., Phelps M., DeLuca H. F. Assay and properties of rat yolk sac 25-hydroxyvitamin D3 1 alpha-hydroxylase. Biochemistry. 1986 Nov 4;25(22):6821–6826. doi: 10.1021/bi00370a014. [DOI] [PubMed] [Google Scholar]
- Pierce J., Suelter C. H. An evaluation of the Coomassie brillant blue G-250 dye-binding method for quantitative protein determination. Anal Biochem. 1977 Aug;81(2):478–480. doi: 10.1016/0003-2697(77)90723-0. [DOI] [PubMed] [Google Scholar]
- Puzas J. E., Turner R. T., Howard G. A., Brand J. S., Baylink D. J. Synthesis of 1,25-dihydroxycholecalciferol and 24,25-dihydroxycholecalciferol by calvarial cells. Characterization of the enzyme systems. Biochem J. 1987 Jul 15;245(2):333–338. doi: 10.1042/bj2450333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt T. A., Horst R. L., Orf J. W., Hollis B. W. A microassay for 1,25-dihydroxyvitamin D not requiring high performance liquid chromatography: application to clinical studies. J Clin Endocrinol Metab. 1984 Jan;58(1):91–98. doi: 10.1210/jcem-58-1-91. [DOI] [PubMed] [Google Scholar]
- Rixon R. H., Isaacs R. J., Whitfield J. F. Control of DNA polymerase-alpha activity in regenerating rat liver by calcium and 1 alpha,25(OH)2D3. J Cell Physiol. 1989 May;139(2):354–360. doi: 10.1002/jcp.1041390218. [DOI] [PubMed] [Google Scholar]
- Shultz T. D., Fox J., Heath H., 3rd, Kumar R. Do tissues other than the kidney produce 1,25-dihydroxyvitamin D3 in vivo? A reexamination. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1746–1750. doi: 10.1073/pnas.80.6.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sietsema W. K., DeLuca H. F. Retinoic acid 5,6-epoxidase. Properties and biological significance. J Biol Chem. 1982 Apr 25;257(8):4265–4270. [PubMed] [Google Scholar]
- Sikorska M., de Belle I., Whitfield J. F., Walker P. R. Regulation of the synthesis of DNA polymerase-alpha in regenerating liver by calcium and 1,25-dihydroxyvitamin D3. Biochem Cell Biol. 1989 Jul;67(7):345–351. doi: 10.1139/o89-054. [DOI] [PubMed] [Google Scholar]
- Simpson E. R., Miller D. A. Cholesterol side-chain cleavage, cytochrome P450, and iron-sulfur protein in human placental mitochondria. Arch Biochem Biophys. 1978 Oct;190(2):800–808. doi: 10.1016/0003-9861(78)90340-5. [DOI] [PubMed] [Google Scholar]
- Vieth R., Fraser D. Kinetic behavior of 25-hydroxyvitamin D-1-hydroxylase and -24-hydroxylase in rat kidney mitochondria. J Biol Chem. 1979 Dec 25;254(24):12455–12460. [PubMed] [Google Scholar]
- Whitsett J. A., Ho M., Tsang R. C., Norman E. J., Adams K. G. Synthesis of 1,25-dihydroxyvitamin D3 by human placenta in vitro. J Clin Endocrinol Metab. 1981 Sep;53(3):484–488. doi: 10.1210/jcem-53-3-484. [DOI] [PubMed] [Google Scholar]
- Wilkinson C. F., Hetnarski K., Cantwell G. P., Di Carlo F. J. Structure-activity relationships in the effects of 1-alkylimidazoles on microsomal oxidation in vitro and in vivo. Biochem Pharmacol. 1974 Sep 1;23(17):2377–2386. doi: 10.1016/0006-2952(74)90227-5. [DOI] [PubMed] [Google Scholar]
- Youdale T., Whitfield J. F., Rixon R. H. 1 alpha,25-Dihydroxyvitamin D3 enables regenerating liver cells to make functional ribonucleotide reductase subunits and replicate DNA in thyroparathyroidectomized rats. Can J Biochem Cell Biol. 1985 May;63(5):319–324. doi: 10.1139/o85-047. [DOI] [PubMed] [Google Scholar]


