Abstract
Background:
Articular cartilage lacks the ability for intrinsic repair after acute injury, and focal articular cartilage lesions cause significant morbidity worldwide. Arthroscopic debridement (chondroplasty) represents the majority of cartilage procedures of the knee; however, limited data exist regarding outcomes after chondroplasty performed in isolation of concurrent procedures or not as a primary treatment for osteoarthritis (OA).
Hypothesis:
Arthroscopic mechanical chondroplasty is beneficial for patients with a focal cartilage lesion of the knee in the absence of meniscal pathology or OA.
Study Design:
Case series; Level of evidence, 4.
Methods:
Potential participants were identified by querying billing data from a 3-year period in a single-surgeon practice, and eligible patients were verified to meet inclusion criteria through electronic medical record review. OA was quantified through Kellgren-Lawrence (KL) scoring. Subjective patient-reported outcome (PRO) scores, including International Knee Documentation Committee (IKDC), Knee injury and Osteoarthritis Outcome Score (KOOS), Western Ontario and McMaster Universities Arthritis Index (WOMAC), Tegner, Lysholm, and Veterans RAND 12-Item Health Survey (VR-12), were collected preoperatively and at follow-up intervals. International Cartilage Repair Society (ICRS) grade and lesion size were determined at arthroscopy. Linear regression was used to determine the effect of baseline score on final follow-up score. Correlated regression equations were used to assess the relationship of covariates and change in PRO scores.
Results:
Fifty-three of 86 (62%) eligible participants completed postoperative questionnaires at an average of 31.5 months (range, 11.5-57 months). The mean patient age was 37.3 ± 9.7 years and mean body mass index (BMI) was 27.7 ± 5.6 kg/m2; 33 (62%) participants were women. The mean treated lesion size was 3.3 ± 1.9 cm2, of these, 36 (68%) were ICRS grade 2 or 3, and 42 (79%) patients had a KL score of 0 to −2. On average, the cohort demonstrated significant improvement from baseline for almost all PRO scores. Regression analysis of change in score versus baseline indicated participants with lower preoperative scores gained more benefit from chondroplasty. Correlated regression equations showed KL score >0 and male sex had a consistent positive effect on change in PRO scores, high ICRS grade had a consistent negative effect, and lesion size, age, and obesity had no effect. Eight patients (15%) required further surgical intervention within the follow-up period.
Conclusion:
The clinical efficacy of chondroplasty for repair of focal cartilage defects of the knee has not been studied in isolation from concurrent orthopaedic procedures. Our data show that arthroscopic mechanical chondroplasty is beneficial to patients, and response to surgical intervention is correlated with baseline PRO scores, sex, ICRS grade, and KL score.
Keywords: chondroplasty, articular cartilage, cartilage repair, arthroscopy
Articular cartilage defects cause significant morbidity, as cartilage has limited ability for intrinsic repair or regeneration in adults.5 Patients with focal cartilage damage of the knee often present with pain and mechanical catching or popping symptoms, which collectively lead to substantial disability. Procedures to treat focal articular cartilage defects can be classified as palliative (lavage, chondroplasty), reparative (microfracture), or restorative (autologous chondrocyte implantation, osteochondral allograft, or osteochondral autograft).11,13 Arthroscopic debridement is a technique utilized to alleviate pain, mechanical symptoms, and recurrent effusions associated with symptomatic articular cartilage lesions.15 Currently, arthroscopic chondroplasty represents the vast majority of all cartilage procedures of the knee performed in the United States.13 Of the roughly 300,000 total patients who underwent surgery for focal knee cartilage disease in the United States in 2010, an estimated 220,000 patients underwent arthroscopic chondroplasty; however, the proportion of these patients who underwent a concomitant surgery at the time of chondroplasty or who had evidence of degenerative joint disease is unknown.11 Chondroplasty includes a spectrum of techniques specific to individual surgical practices, ranging from radiofrequency thermal ablation to abrasion chondroplasty or mechanical chondroplasty. The latter, and least invasive, consists of debriding the unstable cartilage tissue to a stable rim. No standard of care for chondroplasty exists, and surgical technique remains quite variable.
Despite the high prevalence of chondroplasty, limited outcomes data exist regarding short- and long-term results. To date, the procedure has only been characterized as a primary treatment for osteoarthritis (OA) or with concurrent surgical intervention, most commonly subtotal meniscectomy.1,4,8,12,14,17 Heavily cited studies have investigated the effect of debridement and lavage in patients with OA, demonstrating limited benefit.9,14 While investigation into efficacy of this procedure as a primary treatment for OA was warranted, a majority of patients receiving chondroplasty are between 40 and 59 years old, an age group that may or may not have degenerative changes in the setting of a focal chondral injury.13 The lack of outcomes data on isolated chondroplasty in patients without OA, meniscal injury, or concomitant ligament tear demands investigation to determine if chondroplasty is an efficacious therapeutic strategy.
The purpose of our study was to define the clinical outcomes of arthroscopic chondroplasty in isolation. We hypothesized patients undergoing chondroplasty would have a clinically meaningful improvement in patient-reported outcome (PRO) measures at minimum 2-year follow-up.
Methods
Ethical approval was obtained from the institutional review board at Oregon Health & Science University before enrollment (IRB# 4745 and 11437). All participants who presented to the principal investigator’s clinic had consented to participate in a prospective longitudinal database (SOCRATES, Ortholink Pty Ltd) for use in any future retrospective studies. Potential participants were identified on querying billing data for chondroplasty codes (CPT [Current Procedural Terminology] 29877 and G0289) from January 2011 through December 2014, and evidence of a chondroplasty procedure was verified on review of electronic medical records (EMRs). Participants were excluded if additional CPT codes were listed with their index procedure, or if a concurrent procedure was identified within the EMR. Potential participants initially presented or were referred to the clinic for knee pain and/or mechanical symptoms and subsequently underwent arthroscopy for 1 or more identified chondral lesions. Patients were not excluded for previous surgical intervention that was at least 12 months prior on the ipsilateral knee. Patient demographics and baseline PRO data were collected preoperatively. PRO measures included the International Knee Documentation Committee (IKDC) subjective knee evaluation form, all 5 domains (symptoms, pain, activities of daily living [ADL], sports and recreation [Sports], and quality of life [QOL]) of the Knee injury and Osteoarthritis Outcome Score (KOOS), the Western Ontario and McMaster Universities Arthritis Index (WOMAC), the Veterans RAND 12-Item Health Survey (VR-12), the Lysholm Knee Scoring Scale, and the Tegner Activity Score. All patients underwent a preoperative history and physical examination and standard weightbearing knee radiographs. The degree of OA for both the overall operative knee and the operative compartment was quantified by a senior orthopaedic surgery resident using a standardized Kellgren-Lawrence (KL) scoring system: grade 0 showed no arthritis, grade 1 showed minimal osteophytes, grade 2 showed definite osteophytes and possible joint space narrowing, grade 3 showed definite joint space narrowing and subchondral sclerosis, and grade 4 showed bony deformity.
All procedures were performed by a fellowship-trained orthopaedic surgeon. Arthroscopic treatment was performed under general anesthesia and without a tourniquet. Ringer’s lactate solution was used to distend the joint during confirmatory examination and the procedure. Articular lesions in the medial, lateral, or patellofemoral compartments were graded according to the International Cartilage Repair Society (ICRS) classification.2 Lesion size was measured intraoperatively with an arthroscopic ruler. Damaged articular cartilage and/or associated flap(s) were debrided with a 4.5-mm oscillating shaver and a sharp curette to produce a stable tissue border at the minimum depth possible regardless of ICRS grade for the lesion. Also independent of ICRS grade, no subchondral abrasion was performed, and the calcified cartilage layer was left intact. On completion of the operation, local anesthetic (bupivacaine 0.5%, 10 mL) was injected into the dermis at incision sites, but no anesthetic was placed into the joint. Surgical compartment, size of lesion(s), and ICRS grade(s) were recorded in the EMR after surgery. For patients with more than 1 treated lesion, only a single index lesion was used for analysis. The index lesion happened to be unanimously the largest and the most severe based on ICRS grade for all these patients. Routine clinical follow-up was conducted at 2 and 6 weeks and at 3, 6, 12, and 24 months postoperatively at the discretion of the patient. PRO surveys were distributed to all patients 6 weeks after surgery and annually thereafter. If study participants did not complete PRO surveys on automatic distribution, investigators sent 2 reminder emails and 1 phone call to encourage participation before patients were considered lost to follow-up. No financial incentive was offered for participation.
Statistical analysis was performed with Stata v14 (Stata-Corp LP). Sample means, standard deviations, and 95% confidence intervals were calculated for PROs at baseline, final follow-up, and change from baseline. Comparison of patient demographics, lesion characteristics, and baseline PRO scores between the cohort lost to follow-up with the final cohort were performed using an unpaired t test to compare means and an F test to compare variance. A Mann-Whitney rank-sum test was used to compare nonnormal data. Differences in distribution between categorical data were tested using Fisher exact test. For the final cohort, clinical improvement was defined as an increase in mean change in PRO scores from baseline greater than would be expected for the population given the distribution of baseline scores. For each outcome measure, we calculated this expected amount of change through mathematical modeling based on multiple assumptions. We expected (1) near-universal improvement in PRO scores from baseline, (2) participants with lower baseline scores should improve more than those with higher scores due to the ceiling effect of outcome measures, and (3) the range of baseline scores should predictably influence mean improvement seen at final follow-up. For example, if baseline scores for an outcome were mostly small, then mean improvement should be larger than if baseline scores were spread evenly across the scale.
The amount of change for a PRO measure that would be predicted from these assumptions was labeled the minimal interesting change (MIC) and was calculated as follows. Baseline scores were converted to Z scores for normalization. The change from baseline was regressed against the normalized baseline score using errors-in-variables regression, which accounted for the test-retest reliability of the outcome measure. The slope of the regression was taken as the MIC, which represented the amount of shift in improvement induced by a standard deviation difference at baseline. A set of baseline scores that differed, on average, by 1 standard deviation was expected to yield a set of changes from baseline that differed, on average, by this slope. If mean improvement across the entire group was no larger than the MIC, then we concluded that average improvement was within expected bounds. If, on average, the group improved more than the MIC, it was considered clinically significant.
A Student t test was used to compare mean change from baseline with MIC for all outcome measures, with significance set at P < .05. Potential factors (covariates) influencing change from baseline for PRO scores were selected for correlated regression analysis. These included baseline PRO score, KL score, ICRS grade, lesion size, age, body mass index (BMI), and sex. Correlated regressions were performed using the Zellner method of seemingly unrelated regression (SUR), which enabled simultaneous estimation of multiple related regressions within a unified framework.21 If the SUR suggested a net positive or negative effect for a covariate on a given outcome score when controlling for the other factors, univariate analysis (adjusted for baseline score) was used to confirm that this relationship was retained in the presence of confounding in the clinical cohort. If the coefficient estimate was similar for the correlated and univariate regressions, we considered it clinically relevant.
Results
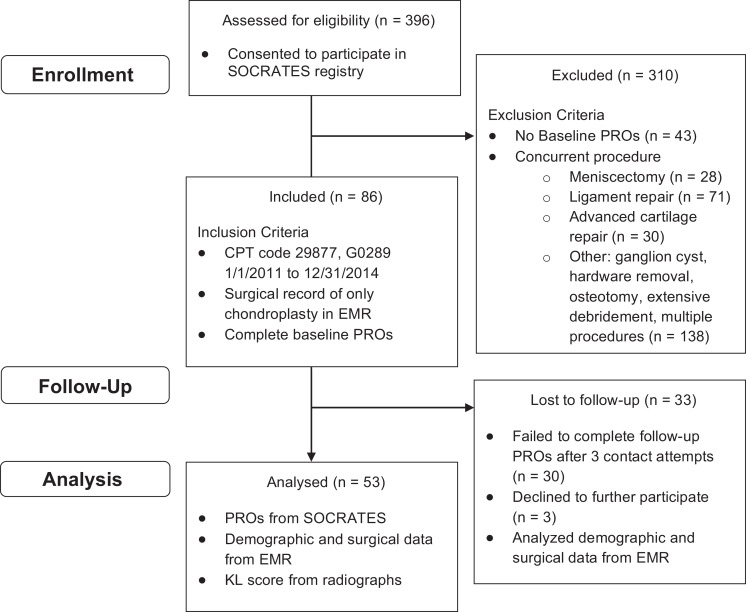
A total of 396 potential participants were identified from billing data, and 86 subjects met inclusion criteria (Figure 1). Of the 86 patients, 53 (62%) completed the postoperative questionnaires, with a final follow-up reported on average at 31.5 ± 13.9 months. The cohort that completed follow-up was characterized by baseline demographics and surgical findings (Table 1). Average age of the patients was 37.3 ± 9.7 years with an average BMI of 27.7 ± 5.6 kg/m2. All but 1 patient presented with subjective pain at baseline as reported in the KOOS pain questionnaire. Thirty-three (62%) participants were female, 35 (66%) had mechanical symptoms, and 24 (48%) had a flap tear, such that greater than 50% of the circumference was displaced to create an anchored chondral flap. Thirty (57%) patients presented with acute symptoms (less than 6 months). Thirty-one (58.5%) had a lesion in the patellofemoral joint, followed by 9 (17%) lateral compartment, 7 (13.2%) medial compartment, and 1 (1.9%) tibial plateau lesion. The remaining 5 (9.4%) patients did not have a specific compartment recorded in the surgical note. Average lesion size was 3.3 ± 1.9 cm2, and the highest percentage of patients had ICRS 3c lesions (41.5%). There were 2 (3.8%) ICRS grade 2 lesions, 34 (64.2%) ICRS grade 3 lesions, and 11 (20.8%) ICRS grade 4 lesions. In comparison with the final cohort, the cohort lost to follow-up was of similar mean age and BMI but had significantly smaller lesions (Table 2). Relative to the final cohort, this cohort also had mostly ICRS grade 3 lesions (21 patients, 63.6%) but contained a significantly different distribution regarding sex and compartment, with a predominance of males (20 of 33) and an increase in medial compartment disease (11 of 33). Eight study participants (15%) required further surgical intervention (2 total knee arthroplasties, 1 autologous chondrocyte implantation, 1 meniscectomy, 1 DeNovo [Zimmer], 2 anterior cruciate ligament reconstructions, and 1 tibial tubercle osteotomy). Comparison of the baseline scores for participants lost to follow-up with those for the final cohort indicated a significant mean difference for only WOMAC, although there was no difference in the variance of scores (Table 2).
Figure 1.
CONSORT (Consolidated Standards of Reporting Trials) diagram categorizing the cohort by patient enrollment, follow-up, and analysis. CPT, Current Procedural Terminology; EMR, electronic medical records; KL, Kellgren-Lawrence; PRO, patient-reported outcome.
TABLE 1.
Patient Demographics and Surgical Characteristicsa
| Variable | Mean ± SD or Fraction (%) |
|---|---|
| Age, y | 37.3 ± 9.7 |
| ≥40 y | 21/53 (40) |
| BMI | 27.7 ± 5.6 |
| Sex, male/female, n | 20/33 |
| Mechanical symptoms | 35/53 (66) |
| Acute (<6 mo) | 30/53 (57) |
| Preoperative Tegner ≥6 | 6/48 (13) |
| Follow-up, mo | 31.5 ± 13.9 |
| Flap tear | 24/50 (48) |
| Lesion size, cm2 | 3.3 ± 1.9 |
| Size >2 cm2 | 26/42 (62) |
| ICRS grade (N = 53) | |
| 2 | 2 (3.8) |
| 3 | 34 (64.2) |
| 3a | 7 |
| 3b | 4 |
| 3c | 22 |
| 3d | 1 |
| 4 | 11 (20.8) |
| 4a | 8 |
| 4b | 3 |
| Unassigned | 6 (11.3) |
| Compartment (N = 53) | |
| Medial | 7 (13.2) |
| Lateral | 9 (17.0) |
| Patellofemoral | 31 (58.5) |
| Other | 1 (1.9) |
| Unassigned | 5 (9.4) |
| KL grade (N = 53) | |
| 0 | 28 (52.8) |
| 1-2 | 14 (26.4) |
| 3-4 | 5 (9.4) |
| Unassigned | 6 (11.3) |
aBMI, body mass index; ICRS, International Cartilage Repair Society; KL, Kellgren-Lawrence.
TABLE 2.
Comparison of Final Cohort With Cohort Lost to Follow-up (Baseline Measurements)a
| Variable | Cohort | Lost to Follow-up | t Test P Valueb | F Test P Value |
|---|---|---|---|---|
| Patient and lesion characteristic | ||||
| Age, y, mean ± SD | 37.3 ± 9.7 | 34.9 ± 10.1 | .147c | .801 |
| BMI, kg/m2, mean ± SD | 27.7 ± 5.6 | 29.4 ± 6.4 | .211 | .381 |
| Lesion size, cm2, mean ± SD | 3.3 ± 1.9 | 2.4 ± 1.5 | .034 | .273 |
| Sex, male/female, n | 20/33 | 20/13 | .047 d | |
| Compartment, PF/M/L, n | 31/7/9 | 13/11/8 | .068 d | |
| ICRS grade 2/3/4, n | 2/34/11 | 2/21/8 | .852d | |
| Outcome measure, mean ± SD | ||||
| IKDC | 42.7 ± 16.4 | 42.6 ± 18.1 | .995 | .530 |
| KOOSSymptoms | 57.2 ± 20.2 | 51.9 ± 17.1 | .204 | .305 |
| KOOSPain | 61.8 ± 18.2 | 55.0 ± 19.6 | .112 | .626 |
| KOOSADL | 71.6 ± 19.8 | 66.6 ± 20.1 | .257 | .905 |
| KOOSSports | 36.4 ± 29.8 | 32.2 ± 26.2 | .498 | .454 |
| KOOSQOL | 26.0 ± 21.2 | 28.8 ± 17.7 | .322c | .276 |
| WOMAC | 27.8 ± 18.2 | 37.2 ± 17.5 | .023 | .831 |
| Lysholm | 56.4 ± 19.6 | 51.7 ± 19.6 | .306 | .960 |
| Tegner | 3.2 ± 2.1 | 3.2 ± 1.5 | .680c | .063 |
| VR-12PCS | 34.6 ± 10.6 | 33.8 ± 10.5 | .723 | .967 |
| VR-12MCS | 52.7 ± 10.0 | 51.2 ± 11.5 | .547 | .364 |
aADL, activities of daily living; BMI, body mass index; ICRS, International Cartilage Repair Society; IKDC, International Knee Documentation Committee; KOOS, Knee injury and Osteoarthritis Outcome Score; L, lateral; M, medial; MCS, mental component score; PCS, physical component score; PF, patellofemoral; QOL, quality of life; VR-12, Veterans RAND 12-Item Health Survey; WOMAC, Western Ontario and McMaster Universities Arthritis Index.
bBoldfaced values are statistically significant for P < .05 with trends identified for P < .1.
cMann-Whitney rank sum.
dFisher exact test.
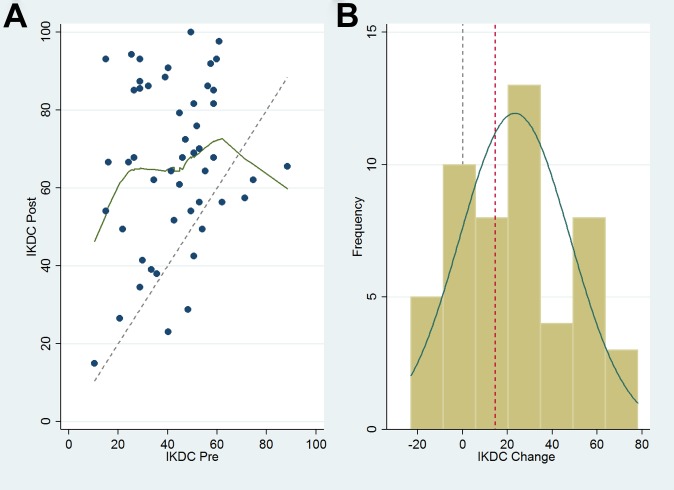
Overall, the final cohort demonstrated improvement from baseline to final follow-up for all PRO outcomes scores except the mental component of the VR-12 (Table 3). The IKDC, WOMAC, Lysholm, and VR-12physical function scores all improved more than the minimal clinically important difference (MCID) reported in the literature.3,6,20 All KOOS subsets improved more than 15 points, with the KOOSSports and KOOSQOL subsets showing the greatest improvement (25.5 and 30.6, respectively). Data for the IKDC are given as an example of trends seen in the pre- and postsurgical outcome measures (Figure 2). Linear regression models found statistically significant associations between the change from baseline and the standardized baseline scores for all outcome measures (P < .001). The strongest relationships (R 2 > 0.5) were in KOOSADL, KOOSQOL, KOOSSports, and WOMAC (R 2 = 0.57, 0.61, 0.81, and 0.58, respectively). Regression analysis of change in score versus baseline score showed that for all outcome measures, patients with lower preoperative scores gained more benefit from surgery than those with higher preoperative scores. Data for the IKDC are given as an example of trends seen in change in outcome measures (Figure 3). Importantly, the IKDC and VR-12physical function scores showed significant improvement based on the MIC (Table 3). KOOSSymptoms, KOOSPain, and WOMAC scores also improved more than the MIC, but not significantly. Correlated regression equations showed that lower baseline score, KL score greater than 0, and male sex had the most consistent positive effect on change in outcome scores, while ICRS score of 3 or 4 had the most consistent negative effect. For our study population, lesion size, age, and obesity (BMI ≥30 kg/m2) did not have a net positive or negative effect on change in outcome scores (Table 4).
TABLE 3.
Patient-Reported Outcome (PRO) Scoresa
| Outcome Measure | Baseline PRO Score, Mean ± SD | Change From Baseline, Mean (95% CI) | MIC | P Value | MCID From Literature3,6,20 |
|---|---|---|---|---|---|
| IKDC | 42.7 ± 16.4 | 23.3 (16.4 to 30.3) | 14.6 | .007b | 3.19-16.7 |
| KOOSSymptoms | 57.2 ± 20.2 | 15.8 (10.5 to 21.2) | 15.6 | .465 | NA |
| KOOSPain | 61.8 ± 18.2 | 19.7 (14.1 to 25.3) | 15.6 | .075 | NA |
| KOOSADL | 71.6 ± 19.8 | 16.7 (10.9 to 22.5) | 17.9 | .660 | NA |
| KOOSSports | 36.4 ± 29.8 | 25.5 (15.6 to 35.3) | 49.1 | >.999 | NA |
| KOOSQOL | 26.0 ± 21.2 | 30.6 (22.0 to 39.2) | 33.1 | .719 | NA |
| WOMACc | 27.8 ± 18.2 | 16.0 (10.6 to 21.3) | 14.2 | .258 | 11.5 |
| Lysholm | 56.4 ± 19.6 | 17.4 (11.0 to 23.8) | 18.2 | .600 | 10.1 |
| Tegner | 3.2 ± 2.1 | 1.8 (1.0 to 2.7) | 2.0 | .667 | NA |
| VR-12PCS | 34.6 ± 10.6 | 13.1 (9.6 to 16.5) | 8.1 | .003b | 6.5 |
| VR-12MCS | 52.7 ± 10.0 | −0.2 (−3.6 to 3.2) | 9.4 | >.999 | 7.9 |
aValues are reported as mean ± SD or mean (95% CI) for interpretation of spread of scores or comparison to MIC, respectively. MIC is calculated by standardizing the baseline scores (converting to Z scores) and regressing the change in score on the baseline score. The slope of the regression line, accounting for reliability of the outcome measure, represents the MIC for the outcome score of interest. If the mean change was significantly greater than MIC, as assessed by a Student t test, the result was considered statistically significant. ADL, activities of daily living; IKDC, International Knee Documentation Committee; KOOS, Knee injury and Osteoarthritis Outcome Score; MCID, minimal clinically important difference; MCS, mental component score; MIC, minimal interesting change; NA, not available; PCS, physical component score; QOL, quality of life; VR-12, Veterans RAND 12-Item Health Survey; WOMAC, Western Ontario and McMaster Universities Arthritis Index.
bP < .05.
cWOMAC score was reversed, so larger (rather than smaller) values would be considered improvements.
Figure 2.
(A) Scatterplot of International Knee Documentation Committee (IKDC) final values (post) and baseline values (pre). The dashed line represents no change in final score from baseline score for all possible values. Data points above the line represent an improvement from baseline. (B) Histogram plot of improvement from baseline (change) for final follow-up scores. Dashed gray vertical line represents no change. On average, the group improved 23.3 points. The minimal interesting change for the IKDC from our model was 14.6 (dashed red line).
Figure 3.
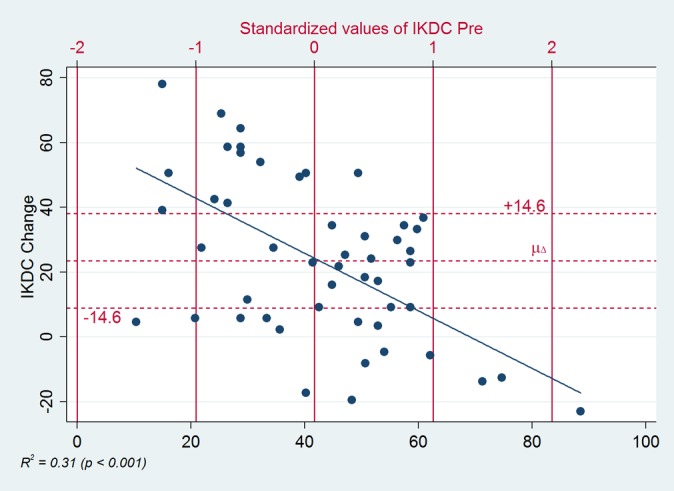
Regression of change in International Knee Documentation Committee (IKDC) score versus the standardized baseline score. There was a statistically significant but weak correlation between IKDC change and baseline score (R 2 = 0.31, P < .001). Vertical red bars indicate mean (42.7) and standard deviation units (approximately ± 20) of the baseline IKDC scores. Horizontal red lines indicate the average change from baseline (µΔ = 23.3) and the minimal interesting change (MIC = 14.6). MIC is based on the slope of the regression line and represents the expected change induced by standard deviation differences in baseline score. Note: if you follow the regression line from right to left starting at where it crosses 0, the dots progressively cluster higher for each standard deviation block; this is visual evidence that the IKDC changes tended to be larger than we would have predicted from baseline scores alone.
TABLE 4.
Correlated Regression Resultsa
| Outcome Measure | Higher Baseline Score | KL Score >0 | ICRS Grade >2 | Lesion Size >2 cm2 | Age > Mean | Obesity (BMI ≥30 kg/m2) | Male Sex |
|---|---|---|---|---|---|---|---|
| IKDC | 0/– | + | – | 0 | 0 | 0 | + |
| KOOSSymptoms | – | + | – | 0 | + | 0 | + |
| KOOSPain | – | + | – | 0 | 0 | – | + |
| KOOSADL | – | + | – | 0 | 0 | 0/– | 0/+ |
| KOOSSports | – | + | – | 0 | + | – | + |
| KOOSQOL | – | 0 | – | 0 | 0/+ | 0 | + |
| WOMAC | – | 0/+ | – | 0 | 0 | 0 | + |
| Lysholm | – | + | – | 0 | + | – | + |
| Tegner | – | 0 | – | 0 | 0 | 0 | + |
| VR-12PCS | – | 0/+ | – | 0 | 0 | 0 | + |
| VR-12MCS | – | – | 0 | – | 0 | – | 0/– |
aImprovement more than expected is noted with a “+” sign, a decline as “–”, and negligible or ambiguous effect on the change value as “0.” Correlated regressions were internally validated by comparing the coefficient estimates to those obtained in one-by-one individual regressions. To receive a “+” or a “–” in the table, the coefficient had to be of consistent sign and magnitude in both regressions and have P ≤ .15 in the correlated regressions. ADL, activities of daily living; BMI, body mass index; ICRS, International Cartilage Repair Society; IKDC, International Knee Documentation Committee; KL, Kellgren-Lawrence; KOOS, Knee injury and Osteoarthritis Outcome Score; MCS, mental component score; PCS, physical component score; QOL, quality of life; VR-12, Veterans RAND 12-Item Health Survey; WOMAC, Western Ontario and McMaster Universities Arthritis Index.
Discussion
Limited data exist on the clinical outcomes after isolated chondroplasty in patients with focal cartilage lesions. Historically, debridement of cartilage lesions has been shown to be successful in resolving symptoms for elite soccer players.10 This procedure, however, involved removal of the calcified cartilage through abrasion; therefore, it is not directly comparable to our surgical technique. More recently, a retrospective review showed that 67% of athletes returned to professional football after chondroplasty; however, most patients received concomitant procedures, and the specific effect of the chondroplasty could not be evaluated. For these athletes, postoperative activity did not depend on age, lesion size, grade, or location.15 These results are in partial concordance with our data, which showed that postoperative improvement is independent of age and size of lesion. We found, however, that lesion severity correlated with improvement, as patients with ICRS grade 3 and 4 lesions improved less on average. Additionally, we found male sex and lower baseline PRO scores were positive predictors of patient-reported response to surgery. Our data also indicated that participants with KL score >0 in the affected compartment improved more than would be expected, which was surprising after randomized clinical trials that showed negligible benefit to a patient population with KL score ≥2.4,9,14 We were unable, however, to distinguish among discreet KL scores with the small sample size of patients with arthritis (8 with KL score ≥2). Based on this limitation and the lack of a control group in our study, we are hesitant to generalize these results to a larger population with OA. The correlated regression model was limited by small sample sizes and the need to restrict the estimation sample to participants with complete observations on all outcomes and covariates. Although this restriction was necessary for direct comparability of the outcome effects, the ability to detect small effects was decreased, and decisive conclusions cannot be made about the magnitude of an effect. Therefore, we only made conclusions about the direction of an effect (positive, negative, or neutral).
Of most interest, we found that for all outcome measures, baseline score had a large and unambiguous effect on patient response at follow-up. Through regression modeling, we showed that baseline score predicted a quantifiable effect in the change from baseline. However, when we compared mean change with the MIC, we found that only 2 outcome measures were significantly greater. We conclude that improvement from baseline was clear for most outcome measures. For many measures, however, this change was not more than what would be expected based on observed variation in baseline presentation. Our method of calculating the MIC is unique and has not been reported previously in the literature. This method was preferred over the MCID because it is based on the distribution of our population and not on historical norms. Previously reported MCIDs have wide-ranging values, which limit interpretation in a specific population.7 Furthermore, our method allows for a critical interpretation of clinically significant response that can be consistently applied even when the MCID is not available for a given outcome score (eg, KOOS). Importantly, we were able to interpret the response of the cohort to treatment across multiple PROs using a standardized system with the MIC, and this analysis was previously not possible using historically reported MCIDs for each PRO.
The strength of our study lies in the single-surgeon practice, which allowed for surgical indication and technique to be standardized for all participants in the study. Among the wide variety of techniques that are categorized as arthroscopic chondroplasty, our study benefits from clear definition of interventions performed. This is in contrast to other short- to long-range studies that have compared mechanical chondroplasty with radiofrequency ablation. In these studies, concurrent subtotal meniscectomy was performed at a higher rate in the group receiving mechanical chondroplasty as compared with radiofrequency ablation, thus limiting the direct comparison of the groups.17,18 We excluded concomitant pathologies, including meniscal tears, to remove confounding variables and to define the efficacy of mechanical chondroplasty in isolation.
Limitations of our study include loss to follow-up, lack of a control group, and lack of blinding. We know that the cohort lost to follow-up had equivalent baseline scores for all but 1 PRO; however, this group contained half of the total eligible males. Since being male had a net positive effect on outcome measures, our cohort may have been weighted toward high responders after losing half of the male population. The group lost to follow-up also had significantly smaller lesions on average. Losing this cohort may have diminished the effect of lesion size on outcome measures, such that there was no influence in the final cohort.
We know from previous randomized controlled trials that arthroscopic lavage can have a substantial placebo effect in patients with arthritis.9,14 In our cohort, there was a trend for longer follow-up times to be associated with larger changes from baseline for most PRO scores. A trend of sustained benefit would argue against a pure placebo effect for surgical intervention, as we would expect the effect to diminish over a finite period, but we cannot discount the possibility of temporal improvement due exclusively to the natural history of an untreated chondral injury that does not present with mechanical symptoms.16 Additionally, since we are not treating patients with diffuse arthritis, our study population differs from previous widely cited studies. Future investigation with a larger prospective randomized trial for patients with focal cartilage lesions in the absence of concurrent pathology may allow surgeons to better predict the magnitude of change that can be expected based on patient characteristics. Along with a larger cohort, additional follow-up intervals and a longer total follow-up period may elucidate trends in patient-reported outcomes not captured at an average 31-month follow-up. Specifically, more invasive cartilage repair and restoration procedures such as microfracture and autologous chondrocyte implantation show gradual improvement in PROs over the first few years followed by a subsequent plateau or decline,19 which are trends not afforded by the current study design.
With inherent limitations of the current study, future studies with larger populations and increased follow-up duration may better inform clinical decisions regarding repair of focal cartilage defects of the knee and help to define the role of chondroplasty in current clinical practice. Importantly, this is the first study to investigate the efficacy of chondroplasty absent of potentially confounding surgical interventions. It is also important to note that three-quarters of our initial patient population were excluded from the final cohort because they received another intervention at the time of chondroplasty. The exclusion of these participants allowed interpretation of subjective clinical outcomes exclusive to chondroplasty, and this protocol allowed us to define outcomes that have not previously been defined despite the widespread use of mechanical chondroplasty worldwide. Based on our clinical experience, we expect that the majority of patients undergoing chondroplasty receive a concomitant surgical intervention, but we can now better define the contribution of chondroplasty to clinical outcomes. Our data showed significant improvements in most PROs in patients undergoing mechanical chondroplasty as a primary treatment for focal cartilage lesions in knees with minimal degenerative disease. These improvements were present up to 31 months after the chondroplasty. The improvement in PROs was highest in patients with greater subjective disability at baseline.
Footnotes
The authors declared that they have no conflicts of interest in the authorship and publication of this contribution.
Ethical approval for this study was obtained from the Oregon Health & Science University (IRB IDs: IRB00011437 and IRB00004745).
References
- 1. Barber FA, Iwasko NG. Treatment of grade III femoral chondral lesions: mechanical chondroplasty versus monopolar radiofrequency probe. Arthroscopy. 2006;22:1312–1317. [DOI] [PubMed] [Google Scholar]
- 2. Brittberg M, Aglietti P, Gambardella R, et al. ICRS Cartilage Injury Evaluation Package. Wetzikon, Switzerland: International Cartilage Repair Society; 2000. [Google Scholar]
- 3. Busija L, Pausenberger E, Haines TP, Haymes S, Buchbinder R, Osborne RH. Adult measures of general health and health-related quality of life. Arthritis Care Res (Hoboken). 2011;63(suppl 11):S383–S412. [DOI] [PubMed] [Google Scholar]
- 4. Dervin GF, Stiell IG, Rody K, Grabowski J. Effect of arthroscopic débridement for osteoarthritis of the knee on health-related quality of life. J Bone Joint Surg Am. 2002;85-A:10–19. [DOI] [PubMed] [Google Scholar]
- 5. Gilbert JE. Current treatment options for the restoration of articular cartilage. Am J Knee Surg. 1998;11:42–46. [PubMed] [Google Scholar]
- 6. Irrgang J. Summary of Clinical Outcome Measures for Sports-Related Knee Injuries. Baltimore, MD: AOSSM Outcomes Task Force; 2012. [Google Scholar]
- 7. Irrgang JJ, Anderson AF, Boland AL, et al. Development and validation of the International Knee Documentation Committee Subjective Knee Form. Am J Sport Med. 2001;29:600–613. [DOI] [PubMed] [Google Scholar]
- 8. Jackson RW. Arthroscopic surgery and a new classification system. Am J Knee Surg. 1998;11:51–54. [PubMed] [Google Scholar]
- 9. Kirkley A, Birmingham TB, Litchfield RB, et al. A randomized trial of arthroscopic surgery for osteoarthritis of the knee. N Engl J Med. 2009;359:1097–1107. [DOI] [PubMed] [Google Scholar]
- 10. Levy AS, Lohnes J, Sculley S, LeCroy M, Garrett W. Chondral delamination of the knee in soccer players. Am J Sport Med. 1996;24:634–639. [DOI] [PubMed] [Google Scholar]
- 11. McCormick F, Harris JD, Abrams GD, et al. Trends in the surgical treatment of articular cartilage lesions in the United States: an analysis of a large private-payer database over a period of 8 years. Arthroscopy. 2014;30:222–226. [DOI] [PubMed] [Google Scholar]
- 12. Merchan EC, Galindo E. Arthroscope-guided surgery versus nonoperative treatment for limited degenerative osteoarthritis of the femorotibial joint in patients over 50 years of age: a prospective comparative study. Arthroscopy. 1993;9:663–667. [DOI] [PubMed] [Google Scholar]
- 13. Montgomery SR, Foster BD, Ngo SS, et al. Trends in the surgical treatment of articular cartilage defects of the knee in the United States. Knee Surg Sports Traumatol Arthrosc. 2013;22:2070–2075. [DOI] [PubMed] [Google Scholar]
- 14. Mosely BJ, O’Malley K, Petersen NJ, et al. A controlled trial of arthroscopic surgery for osteoarthritis of the knee. N Engl J Med. 2002;347:81–88. [DOI] [PubMed] [Google Scholar]
- 15. Scillia AJ, Aune KT, Andrachuk JS, et al. Return to play after chondroplasty of the knee in National Football League athletes. Am J Sport Med. 2015;43:663–668. [DOI] [PubMed] [Google Scholar]
- 16. Shelburne KD, Jari S, Gray T. Outcome of untreated traumatic articular cartilage defects of the knee. J Bone Joint Surg. 2003;85-A(suppl 2):8–16. [DOI] [PubMed] [Google Scholar]
- 17. Spahn G, Hofmann GO, von Engelhardt LV. Mechanical debridement versus radiofrequency in knee chondroplasty with concomitant medial meniscectomy: 10-year results from a randomized controlled study. Knee Surg Sports Traumatol Arthrosc. 2016;24:1560–1568. [DOI] [PubMed] [Google Scholar]
- 18. Spahn G, Klinger HM, Mückley T, Hofmann GO. Four-year results from a randomized controlled study of knee chondroplasty with concomitant medial meniscectomy: mechanical debridement versus radiofrequency chondroplasty. Arthroscopy. 2010;26(suppl 9):S73–S80. [DOI] [PubMed] [Google Scholar]
- 19. Vasiliadis HS, Wasiak J. Autologous chondrocyte implantation for full thickness articular cartilage defects of the knee. Cochrane Database Syst Rev. 2010;10:CD003323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ware JE, Bayliss MS, Rogers WH, Kosinski M, Tarlov AR. Differences in 4-year health outcome for elderly and poor, chronically ill patients treated in HMO and fee-for-service systems. JAMA. 1996;276:1039–1047. [PubMed] [Google Scholar]
- 21. Zellner A. An efficient method of estimating seemingly unrelated regressions and tests for aggregation bias. J Am Stat Assoc. 1962;57:348–368. [Google Scholar]