Abstract
Plant-plant interactions that change along environmental gradients can be affected by different combinations of environmental characteristics, such as the species and planting density ratios. Suaeda salsa and Scirpus planiculumis are regionally dominant species in the Shuangtai estuarine wetland. Compared with non-clonal S. salsa, clonal S. planiculumis has competitive advantages because of its morphological plasticity. However, salt-tolerant S. salsa may grow faster than S. planiculumis in saline-alkali estuary soil. Whether the interactions between these two species along salinity gradients are affected by the level of salt stress and mixed planting density ratio remains unclear. Thus, to test the effects of salt stress and planting density ratios on the interactions between S. planiculumis and S. salsa in the late growing season, we conducted a greenhouse experiment consisting of 3 salinity levels (0, 8 and 15ppt) and 5 planting density ratios. Our results showed that the promotion of S. salsa growth and inhibition of S. planiculumis growth at low salinity levels (8 ppt) did not alter the interactions between the two species. Facilitation of S. salsa occurred at high salinity levels, and the magnitude of this net outcome decreased with increases in the proportion of S. salsa. These results suggest that competition and facilitation processes not only depend on the combinations of different life-history characteristics of species but also on the planting density ratio. These findings may contribute to the understanding of the responses of estuarine wetland plant-plant interactions to human modifications of estuarine salinity.
Introduction
Plant-plant interactions play an important role in determining population dynamics and structuring ecological communities [1–2]. In recent decades, numerous studies have focused on plant-plant interactions along environmental gradients [3–5], and researchers have postulated the stress-gradient hypothesis (SGH). This hypothesis predicts that facilitation and competition have simultaneous effects on neighbouring individual plants, and the net outcome of this interaction will shift from negative to positive with increasing environmental stress [6–7]. Although this is a well-supported hypothesis, it is still debated with respect to specific issues, such as species traits and stress types [7–11].
Certain scientists oppose the SGH because of the inconsistent results observed between different studies [5, 12]. Maestre et al. [13] extended the SGH and proposed that the interactions between a single competitive pair will frequently transition from facilitation to competition along abiotic stress gradients (i.e., gradients of water, nutrient and environmental stress). In addition, effects are not only influenced by the physiological characteristics, functional traits and stress tolerance of the species but also by the combinations of different life-history characteristics of the species being tested. Previous studies found that the trade-offs between competitive ability and stress tolerance are key factors driving zonation patterns along environmental gradients [14–15]. Clonal plants with strong spatial expansion capabilities can fully exploit heterogeneous resources and avoid abiotic stress via their morphological plasticity in natural habitats [16–18]. To our knowledge, however, there is little experimental evidence on the interactions between clonal plants and stress-tolerant species transitioning from facilitation to competition along non-resource stress gradients.
Most tests of the SGH have focused on interactions between a single pair or a few pairs of species at a planting density ratio of 1:1 [19–22]. However, the individual plants were grown with different numbers of neighbouring species in natural habitats, and a species growing in a stressful environment may have a neighbour that exhibits different interaction strengths that may partly depend on the planting ratio. Interaction strength can be driven by the number of competitive neighbours as well as the environmental stress. However, few studies have measured the extent to which the proportions of species within the same habitat affect their interaction strength along environmental gradients [23].
Estuarine wetland plant communities are characterized by striking zonation patterns across salt stress gradients [14, 24–26]. Salinization is occurring at an unprecedented geographic scale that far exceeds natural variation trends, and these changes have profound consequences for wetland ecosystems [25, 27], especially estuaries. Thus, the mechanisms underlying variations in the relationship between salinity stress tolerance and competitive ability must be determined [28–30].
In the Shuangtai estuarine wetland, clonal Scirpus planiculumis and non-clonal Suaeda salsa are the dominant species in the saline-alkali field, and both species can coexist across broad ranges, from the low marsh to the terrestrial border of the marsh. Using such a combination of species to examine different responses to salt stress can reduce the confounding effects of other traits and reveal the importance of strategic traits on species interactions. To better understand the impact of salt stress and planting density ratio on the interactions between these two species, we addressed the following questions: (1) Do salt stress and the planting density ratio affect the growth of S. planiculumis and S. salsa? (2) Does the interspecific interaction between the two species along salinity gradients change depending on the planting density ratio? To answer these questions, we conducted a greenhouse experiment consisting of three levels of salt stress (low and high stress treatments and a no-stress control) and five planting density ratios (4:0, 3:1, 2:2, 1:3 and 0:4). Determining the competitive relationships between regionally dominant species will provide valuable insights for restoration practices for estuarine wetlands.
Materials and methods
Sampling sites
Plant samples were collected from the Shuangtai estuarine wetland, which is located at the north latitudes 40°45′ − 41°10′ and east longitudes 121°30′ − 122°00′ in Panjin, Liaoning Province of China. This wetland covers an area of 1579 km2 and experiences a semi-humid temperate monsoon climate, with a mean annual temperature of 8.4°C and mean annual precipitation ranging from 611.6 mm to 640.0 mm. In this ecosystem, the salinity values range from 0 to 34.7 ppt (parts per thousand, soil pore water salinity measured as micrograms NaCl per gram of water) in different areas, which affect the distribution and performance of halophytic plants [31–32].
Species and propagation
The investigated plants were S. salsa (L.) and S. planiculumis Fr. Schmidt, which are dominant species in the Shuangtai estuarine wetland. S. salsa is an annual non-clonal halophyte in the family Chenopodiaceae, which is widely distributed along northern coasts consisting of saline alkali land. NaCl can accumulate in the vacuoles of this species to ensure plant survival under high salt stress [33]. S. planiculumis is a perennial, herbaceous clonal plant in the family Cyperaceae. In nature, the tubers of S. planiculumis can vegetatively produce new ramets to increase the plant’s ability to reproduce [34]. Both species commonly co-occur in the Shuangtai estuarine wetland.
The plants were collected from a single location in the wetland in the summer of 2015. Interactions between species are often affected by life-history stage, for instance, adult plants often compete as a consequence of facilitation of juvenile plants [11, 35]. To exclude temporal effects, we collected over 200 single adult plants, instead of juveniles, per species and grew them in a greenhouse for 10 days. This greenhouse located in the Wildlife Rescue & Rehabilitation Center, Beijing, China (the center is a protected area and specific permission should be issued by Beijing Municipal Bureau of Landscape and Forestry ahead of time). The plants were watered with tap water (no salt) to allow for acclimation to the indoor conditions before exposing them to the experimental salinity treatments. We discarded plants that exhibited any transplanting stress during these 10 days and then collected 150 single plants of S. salsa and 150 single ramets of S. planiculumis. We measured the average total dry mass and the average height of both species, and the initial respective measurements were 5.71 ± 0.93 g and 25.22 ± 5.22 cm for S. salsa and 3.24 ± 0.72 g and 38.99 ± 7.04 cm for S. planiculumis.
Experimental design
Standard replacement series experiments have been widely used to evaluate interspecific interactions of mixed species, especially when studying interactions that involve only two species [36–37]. We grew S. salsa and S. planiculumis under three salt stress treatments: low salt stress (8 ppt), high salt stress (15 ppt) and a control treatment (no salt added). The salinity gradient was based on field measurements from related research [30, 38]. S. salsa and S. planiculumis were set up in mixtures and monocultures with five different planting density ratios: 0:4, 1:3, 2:2, 3:1 and 4:0. We set up 6 replicates for each treatment, and a total of 90 containers were used.
On 6 August 2015, according to the previously described cultivation density ratios, four plants of each species were transplanted in a vertical position into each experimental container. The containers were dark plastic barrels that measured 23 cm in diameter and 25.5 cm in depth, and they were filled with 12 cm of substrate, which was a 1:1 (v/v) mixture of sand and soil, which was obtained from the bank of an artificial lake in the Beijing Wildlife Rescue & Rehabilitation Center. The mixed substrate contained 0.31 (0.01) mg total N g-1 dry mass of soil (mean [SE]; N = 3), 0.55 (0.03) mg total P g-1, 1.33 (0.09) mg K g-1, and 8.66 (0.71) mg organic matter g-1 based on analysis conducted at the Institute of Botany at the Chinese Academy of Sciences in Beijing.
On 13 August 2015, which represented one week of establishment, we slowly added an NaCl solution to the containers. According to the volume of the substrate in a container, we added 40.57 g and 76.06 g sodium chloride (99.59% purity) into separate containers for the low and high salt treatments, respectively, and we prepared 1 L of sodium chloride solution and added this to each treatment container. We added 1 L of tap water to the control containers. The NaCl solution was only added at the beginning of the experiment. During the experiment, the containers were arranged in blocks and were watered twice a week to minimize growth limitations caused by water availability. During the experiment, the mean temperature in the greenhouse was 21.2°C and the relative humidity was 75.5%.
On 22 October 2015, we harvested the experiment. The 10-week experimental duration was sufficient for these two species to reach maturity and exhibit peak biomass because both species had blossomed before the end of the experiment, and all plants survived. We measured the heights of the plants, and then each plant was divided into above- and belowground parts, dried at 70°C for over 72 hours and weighed because biomass is an important growth index to measure interspecific competition.
Data analysis
We analysed the growth of S. salsa and S. planiculumis and performed two-way ANOVAs to test the effects of the different levels of salt stress (no-salt control, 8 ppt low salt and 15 ppt high salt) and planting density ratios of these species (4:0, 3:1, 2:2, 1:3 and 0:4) on the total biomass, aboveground biomass, belowground biomass and average height. Differences were tested using Tukey’s post hoc honest significant difference test. In the ANOVA models, salt stress and the planting density ratio were treated as fixed effects. The mean values of all plants in a container were used in these analyses.
Based on the total biomass data of S. salsa and S. planiculumis, we analysed the competitive response using the relative interaction index (RII). The RII is suitable for calculating the positive and negative interactions between plants because this index can compare the performance of each species grown in a mixture to the performance of the species grown in a monoculture. In addition, the competition intensity between two species can effectively be measured because the RII often better meets the assumptions for statistical analysis compared with alternative competitive models [39–40]. The calculation formulae are as follows:
where Y is the total biomass per plant in each experimental container; a and b represent the two species; Ya is the total biomass of species a when grown alone; Yb is the total biomass of species b when grown alone; Yab is the total biomass of species a when grown with species b; and Yba is the total biomass of species b when grown with species a. If the RII value is 0, there are no significant differences between the mixtures and the monoculture; if the RII value is positive; then the interaction is facilitative; and if the RII value is negative, the interaction is competitive. Significant deviations of RII values from zero at the P = 0.05 level were determined using a t-test. All analyses were conducted using SPSS 20.0 (Statistical Product and Services Solutions, version 20.0; SPSS Inc., Chicago, IL).
Results
Effects of salt stress and planting density ratio on the growth of the two species
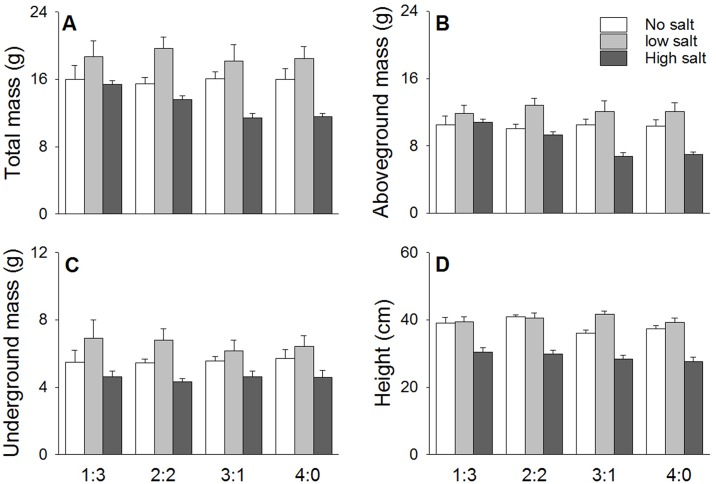
As predicted, the biomass of S. salsa decreased significantly with increasing salt stress (P < 0.001, Table 1 and Fig 1). The low salt treatment resulted in the highest biomass value of S. salsa, which was approximately 15% higher than that in the control treatment and 40% higher than that in the high salt stress treatment. The treatments with different mixed planting density ratios did not affect the total biomass (F3, 60 = 1.06, P = 0.372) or height (F3, 60 = 2.08, P = 0.112) of S. salsa (Table 1).
Table 1. ANOVA results for the effects of salt stress and interspecific competition on the growth of S. salsa and S. planiculumis.
| Response variables | Salt stress (S) | Competition (C) | S * C | |||
|---|---|---|---|---|---|---|
| F2, 60 | P | F3, 60 | P | F6, 60 | P | |
| Suaeda salsa | ||||||
| Total mass | 23.12 | < 0.001 | 1.06 | 0.372 | 0.86 | 0.530 |
| Aboveground mass | 22.44 | < 0.001 | 2.04 | 0.118 | 2.16 | 0.060 |
| Belowground mass* | 13.41 | < 0.001 | 0.08 | 0.969 | 0.21 | 0.973 |
| Height | 94.58 | < 0.001 | 2.08 | 0.112 | 1.34 | 0.252 |
| Scirpus planiculumis | ||||||
| Total mass | 163.46 | < 0.001 | 0.91 | 0.440 | 0.28 | 0.945 |
| Aboveground mass | 149.20 | < 0.001 | 0.96 | 0.418 | 0.37 | 0.895 |
| Belowground mass* | 97.19 | < 0.001 | 0.34 | 0.796 | 0.18 | 0.962 |
| Height | 55.79 | < 0.001 | 3.17 | 0.031 | 1.24 | 0.297 |
*These data were transformed to meet the requirements for homoscedasticity and normality. Bold type indicates a significant difference (P < 0.05).
Fig 1. Effects of salt stress (no salt, low salt and high salt) and planting density ratio (proportions of S. salsa and S. planiculumis were 1:3, 2:2, 3:1 and 4:0) on the (A) total mass, (B) aboveground mass, (C) belowground mass and (D) height (mean + SE) of S. salsa.
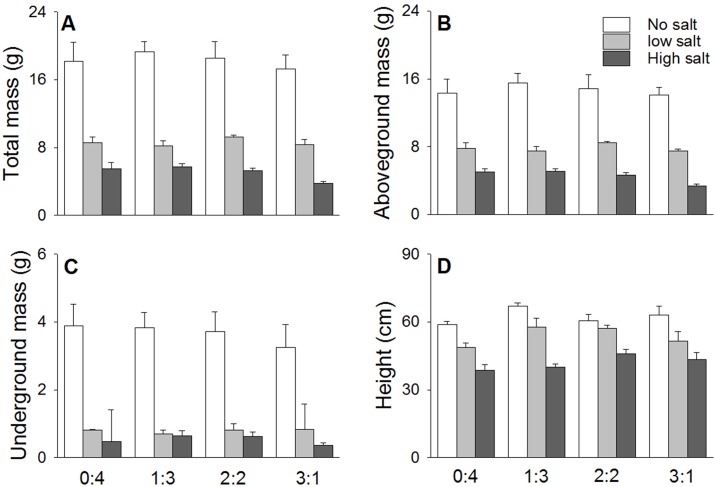
Similar to the results for S. salsa, the salt stress treatments also had an obviously negative effect on the growth of S. planiculumis. The control treatment resulted in the highest biomass value of S. planiculumis (Table 1 and Fig 2), which was approximately 60% lower in the low salt treatment and 80% lower in the high salt treatment. Significant differences were not observed in the total biomass (F3, 60 = 0.91, P = 0.440), aboveground mass (F3, 60 = 0.96, P = 0.418) and belowground mass (F3, 60 = 0.34, P = 0.796) of S. planiculumis across the different planting density ratios, although the presence of S. salsa significantly enhanced the height of S. planiculumis (F3, 60 = 3.71, P = 0.031). No interactions were observed between the treatments.
Fig 2. Effects of salt stress (no salt, low salt and high salt) and density ratio (proportions of S. salsa and S. planiculumis were 0:4, 1:3, 2:2 and 3:1) on the (A) total mass, (B) aboveground mass, (C) belowground mass and (D) height (mean + SE) of S. planiculumis.
RII results
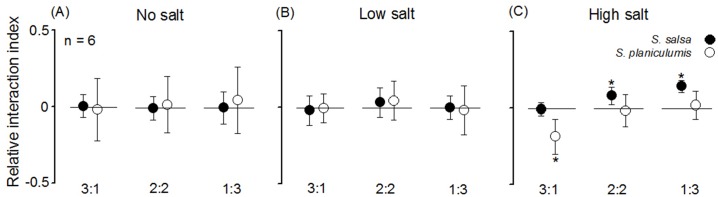
In the no salt and low salt treatments, the RII values were close to zero for both S. salsa and S. planiculumis (P > 0.05, Table 2). In the high salt treatments, the RII values of S. salsa were significantly greater than zero when the proportions of S. salsa and S. planiculumis were 2:2 and 1:3 (Table 2; Fig 3), which indicates that the salt-tolerant species S. salsa were benefactor and clonal S. planiculumis were beneficiary in this case. The RII values of S. planiculumis were strongly negative when the planting density ratio was 3:1, indicating that the effects of S. salsa on S. planiculumis were competitive (Table 2; Fig 3).
Table 2. One sample t-test results for S. salsa and S. planiculumis under different density ratios and salt stress treatments.
P values are shown.
| Species | df | No salt | Low salt | High salt | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 3:1 | 2:2 | 1:3 | 3:1 | 2:2 | 1:3 | 3:1 | 2:2 | 1:3 | ||
| S. salsa | 5 | 0.83 | 0.86 | 0.63 | 0.88 | 0.42 | 0.77 | 0.01 | 0.67 | 0.66 |
| S. planiculumis | 5 | 0.88 | 0.72 | 0.87 | 0.62 | 0.42 | 0.96 | 0.60 | 0.02 | <0.01 |
Bold type indicates a significant difference (P < 0.05).
Fig 3. One sample t-test results for the differences between the RII values and zero.
The results are for S. salsa and S. planiculumis under different density ratios (proportions of S. salsa and S. planiculumis were 3:1, 2:2 and 1:3) and salt treatments: (A) no salt, (B) low salt and (C) high salt. The mean values and 95% confidence intervals are shown, and * indicates a significant difference (P < 0.05).
Discussion
Salt stress is one of the main environmental factors that affect plant growth and morphology in estuaries [24]. High salt concentrations (15 ppt) significantly decreased the growth of the two studied species, and the osmotic effect of the salt damaged the roots of both species [41], which subsequently inhibited the growth of the plants. Photosystem II in S. salsa showed high resistance to low salinity [42], which makes the plant more suitable for growth under low salinity environments (8 ppt) than S. planiculumis. Thus, S. salsa reached its maximum biomass in the low salt treatment. These results are consistent with previous studies on the effects of salt stress on plants.
Different planting density ratios of S. salsa and S. planiculumis did not significantly affect the biomasses of the two species in any of the salinity treatments. However, the presence of S. salsa greatly increased the average height of S. planiculumis (P = 0.031, Table 1), which may be related to the morphological plasticity of clonal plants such as S. salsa, which can absorb more light and take up more space under competition. Many studies have also shown that clonal reproduction appears to select for escape strategies when plants experience competition [43–44].
The RII results for S. salsa and S. planiculumis along the salinity stress gradient and under the different planting density ratios were complex. Both competitive and facilitative processes were asymmetrical for S. salsa and S. planiculumis across the salinity stress gradients. In fresh water and low salinity water across all density ratios, plants interactions tended to be less facilitative relative to the high stress treatments (Fig 3), these results support the SGH. One possible explanation for these results may be related to the intensity of interspecific interaction between these two species, which was equal to their intraspecific interaction under low abiotic stress conditions. Another explanation may be that the fresh water and low salinity stress gradients were too weak to constitute a major proportion of the species’ niches. In other words, these species were not sensitive to these two levels [7, 10]. Moreover, He et al. [15] found that the stress-tolerant and competitively inferior S. salsa did not benefit from T. chinensis amelioration of abiotic stress, which is partly consistent with our findings. The duration of the growing season may also be a reasonable explanation for the almost non-existent interaction under low abiotic stress conditions.
The balance between competition and facilitation was disturbed when the salinity was increased to 15 ppt according to the RII results (Fig 3C). S. salsa benefitted from the interaction under increased salt stress because of its high salt tolerance, especially when this species was present in the minority (S. salsa: S. planiculumis density ratio of 2:2 or 1:3). As predicted by Maestre et al. [13], plant-plant interactions will shift from competition to facilitation with increased abiotic stress for plant-plant combinations with dissimilar competitive abilities and stress tolerances, and this shift has been observed in previous studies [6, 22, 45–46]. However, the facilitative effects disappeared with increasing numbers of S. salsa because the intraspecific interactions between S. salsa plants inhibited their growth. This result suggests that the planting density ratio affects the interactions between the two species as they shift from competition to facilitation under high salinity.
Conclusions
Our study demonstrated that (1) the growth of S. planiculumis was inhibited under both low and high salinity conditions, whereas the growth of S. salsa was only inhibited under high salinity. The planting density ratios did not alter the growth of either S. planiculumis or S. salsa. (2) The promotion of S. salsa growth and the inhibition of S. planiculumis growth at low salinity (8 ppt) did not alter their interactions. (3) Facilitation occurred at high salinity, and the magnitude of this net outcome decreased with increases in the proportion of S. salsa. These results provide insights into the organization and assembly of estuarine wetland plant communities and may have important implications for our understanding of the responses of estuarine wetland plant communities to human modifications of estuarine salinity. However, the results may depend on a variety of biotic and abiotic environmental stress factors and complex combinations of these factors in natural habitats. Further studies should examine shifts between competition and facilitation under multiple types of environmental stresses in estuarine wetland plant communities.
Acknowledgments
We thank the Beijing Wildlife Rescue & Rehabilitation Center for providing the experimental space, and we are grateful to two anonymous reviewers for their valuable comments.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was funded by the Forestry Nonprofit Industry Scientific Research Special Project “The research of ecosystem service and evaluation techniques of coastal wetlands, China” (No.201404305). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Tilman D. Plant strategies and the dynamics and structure of plant communities. Princeton University Press; 1988. [Google Scholar]
- 2.Grant K, Kreyling J, Heilmeier H, Beierkuhnlein C, Jentsch A. Extreme weather events and plant–plant interactions: shifts between competition and facilitation among grassland species in the face of drought and heavy rainfall. Ecol Res. 2014; 29: 991–1001. [Google Scholar]
- 3.Bertness MD. Interspecific interactions among high marsh perennials in a New England salt marsh. Ecology. 1991; 72: 125–137. [Google Scholar]
- 4.Weigelt A, Steinlein T, Beyschlag W. Does plant competition intensity rather depend on biomass or on species identity? Basic Appl Ecol. 2002; 3: 85–94. [Google Scholar]
- 5.Wang C, Li B. Salinity and disturbance mediate direct and indirect plant–plant interactions in an assembled marsh community. Oecologia. 2016; 182: 139–152. 10.1007/s00442-016-3650-1 [DOI] [PubMed] [Google Scholar]
- 6.Bertness MD, Callaway R. Positive interactions in communities. Trends Ecol Evol. 1994; 9: 191–193. 10.1016/0169-5347(94)90088-4 [DOI] [PubMed] [Google Scholar]
- 7.He Q, Bertness MD, Altieri AH. Global shifts towards positive species interactions with increasing environmental stress. Ecol Lett. 2013; 16: 695–706. 10.1111/ele.12080 [DOI] [PubMed] [Google Scholar]
- 8.Liancourt P, Callaway RM, Michalet R. Stress tolerance and competitive-response ability determine the outcome of biotic interactions. Ecology. 2005; 86: 1611–1618. [Google Scholar]
- 9.Maestre FT, Valladares F, Reynolds JF. Is the change of plant–plant interactions with abiotic stress predictable? A meta-analysis of field results in arid environments. J Ecol. 2005; 93: 748–757. [Google Scholar]
- 10.Lortie CJ, Callaway RM. Re-analysis of meta-analysis: support for the stress-gradient hypothesis. J Ecol. 2006; 94: 7–16. [Google Scholar]
- 11.He Q, Bertness MD. Extreme stresses, niches, and positive species interactions along stress gradients. Ecology. 2014; 95: 1437–1443. [DOI] [PubMed] [Google Scholar]
- 12.Brooker RW, Kikvidze Z. Importance: an overlooked concept in plant interaction research. J Ecol. 2008; 96: 703–708. [Google Scholar]
- 13.Maestre FT, Callaway RM, Valladares F, Lortie CJ. Refining the stress-gradient hypothesis for competition and facilitation in plant communities. J Ecol. 2009; 97: 199–205. [Google Scholar]
- 14.Pennings SC, Grant MB, Bertness MD. Plant zonation in low-latitude salt marshes: disentangling the roles of flooding, salinity and competition. J Ecol. 2005; 93: 159–167. [Google Scholar]
- 15.He Q, Cui B, Bertness MD, An Y. Testing the importance of plant strategies on facilitation using congeners in a coastal community. Ecology. 2012; 93: 2023–2029. [DOI] [PubMed] [Google Scholar]
- 16.Alpert P, Holzapfel C, Slominski C. Differences in performance between genotypes of Fragaria chiloensis with different degrees of resource sharing. J Ecol. 2003; 91: 27–35. [Google Scholar]
- 17.Yu FH, Wang N, He WM, Dong M. Effects of clonal integration on species composition and biomass of sand dune communities. J Arid Environ. 2010; 74: 632–637. [Google Scholar]
- 18.Zhou J, Dong BC, Alpert P, Li HL, Zhang MX, Lei GC, Yu FH. Effects of soil nutrient heterogeneity on intraspecific competition in the invasive, clonal plant Alternanthera philoxeroides. Ann Bot. 2011; 109: 813–818. 10.1093/aob/mcr314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greenlee JT, Callaway RM. Abiotic stress and the relative importance of interference and facilitation in montane bunchgrass communities in western Montana. Am Nat. 1996; 148: 386–396. [Google Scholar]
- 20.Donovan LA, Richards JH. Juvenile shrubs show differences in stress tolerance, but no competition or facilitation, along a stress gradient. J Ecol. 2000; 88: 1–16. [Google Scholar]
- 21.Maestre FT, Cortina J. Do positive interactions increase with abiotic stress? A test from a semi-arid steppe. P Roy Soc Lond B Bio. 2004; 271: S331–S333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noto AE, Shurin JB. Population variation affects interactions between two California salt marsh plant species more than precipitation. Oecologia. 2016; 180: 499–506. 10.1007/s00442-015-3473-5 [DOI] [PubMed] [Google Scholar]
- 23.Čuda J, Skálová H, Janovský Z, Pyšek P. Competition among native and invasive Impatiens species: the roles of environmental factors, population density and life stage. AoB Plants. 2015; 7: plv033 10.1093/aobpla/plv033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crain CM, Silliman BR, Bertness SL, Bertness MD. Physical and biotic drivers of plant distribution across estuarine salinity gradients. Ecology. 2004; 85: 2539–2549. [Google Scholar]
- 25.Herbert ER, Boon P, Burgin AJ, Neubauer SC, Franklin RB, Ardon M, et al. A global perspective on wetland salinization: ecological consequences of a growing threat to freshwater wetlands. Ecosphere. 2015; 6: 1–43. [Google Scholar]
- 26.Liu G, Gao Y, Huang FF, Yuan MY, Peng SL. The invasion of coastal areas in south China by Ipomoea cairica may be accelerated by the ecotype being more locally adapted to salt stress. PloS One. 2016; 11: e0149262 10.1371/journal.pone.0149262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cañedo-Argüelles M, Kefford BJ, Piscart C, Prat N, Schafer RB, Schulz C. Salinisation of rivers: an urgent ecological issue. Environ Pollut. 2013; 173: 157–167. 10.1016/j.envpol.2012.10.011 [DOI] [PubMed] [Google Scholar]
- 28.Emery NC, Ewanchuk PJ, Bertness MD. Competition and salt-marsh plant zonation: stress tolerators may be dominant competitors. Ecology. 2001; 82: 2471–2485. [Google Scholar]
- 29.Guo H, Pennings SC. Mechanisms mediating plant distributions across estuarine landscapes in a low-latitude tidal estuary. Ecology. 2012; 93: 90–100. [DOI] [PubMed] [Google Scholar]
- 30.Morzaria-Luna HN, Zedler JB. Competitive interactions between two salt marsh halophytes across stress gradients. Wetlands. 2014; 34: 31–42. [Google Scholar]
- 31.Liu BL, Hu K, Jiang ZL, Qu FG, Su X. A 50-year sedimentary record of heavy metals and their chemical speciations in the Shuangtaizi River estuary (China): implications for pollution and biodegradation. Front Env Sci Eng. 2011; 5: 435–444. [Google Scholar]
- 32.Zhang ZS, Song XL, Lu XG, Xue ZS. Ecological stoichiometry of carbon, nitrogen, and phosphorus in estuarine wetland soils: influences of vegetation coverage, plant communities, geomorphology, and seawalls. J Soil Sediment. 2013; 13: 1043–1051. [Google Scholar]
- 33.Li PH, Wang ZL, Zhang H, Wang BS. Cloning and expression analysis of the B subunit of V-H+-ATPase in leaves of halophyte Suaeda salsa under salt stress. Acta Botanic Sinica. 2004; 46: 93–99. [Google Scholar]
- 34.Peng YK, Luo FL, Li HL, Yu FH. Growth responses of a rhizomatous herb Bolboschoenus planiculmis to scale and contrast of soil nutrient heterogeneity. J Plant Ecol. 2013; 37: 335–43. [Google Scholar]
- 35.Holzapfel C, Mahall BE. Bidirectional facilitation and interference between shrubs and annuals in the Mojave Desert. Ecology. 1999; 80: 1747–1761. [Google Scholar]
- 36.Gibson DJ, Connolly J, Hartnett DC, Weidenhamer JD. Designs for greenhouse studies of interactions between plants. J Ecol. 1999; 87: 1–16. [Google Scholar]
- 37.Jolliffe PA. The replacement series. J Ecol. 2000; 88: 371–385. [Google Scholar]
- 38.Tang L, Gao Y, Li B, Wang Q, Wang CH, Zhao B. Spartina alterniflora with high tolerance to salt stress changes vegetation pattern by outcompeting native species. Ecosphere. 2014; 5: 1–18. [Google Scholar]
- 39.Weigelt A, Jolliffe P. Indices of plant competition. J Ecol. 2003; 91: 707–720. [Google Scholar]
- 40.Armas CR, Pugnaire FI. Measuring plant interactions: A new comparative index. Ecology. 2004; 85: 2682–2686. [Google Scholar]
- 41.Cramer GR, Läuchli A, Polito VS. Displacement of Ca2+ by Na+ from the plasmalemma of root cells a primary response to salt stress? Plant physiol. 1985; 79: 207–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu C, Qiu N, Wang B, Zhang J. Salinity treatment shows no effects on photosystem II photochemistry, but increases the resistance of photosystem II to heat stress in halophyte Suaeda salsa. J Exp Bot. 2003; 54: 851–860. [DOI] [PubMed] [Google Scholar]
- 43.Aarssen LW. Hypotheses for the evolution of apical dominance in plants: implications for the interpretation of overcompensation. Oikos. 1995; 149–156. [Google Scholar]
- 44.Puijalon S, Bouma TJ, van Groenendael J, Bornette G. Clonal plasticity of aquatic plant species submitted to mechanical stress: escape versus resistance strategy. Ann Bot. 2008; 102: 989–996. 10.1093/aob/mcn190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Badano EI, Villarroel E, Bustamante RO, Marquet PA, Cavieres LA. Ecosystem engineering facilitates invasions by exotic plants in high-Andean ecosystems. J Ecol. 2007; 95: 682–688. [Google Scholar]
- 46.Michalet R, Bagousse-Pinguet YL, Maalouf JP, Lortie CJ. Two alternatives to the stress-gradient hypothesis at the edge of life: the collapse of facilitation and the switch from facilitation to competition. J Veg Sci. 2014; 25: 609–613. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.