Abstract
Background
Patients with Crohn’s disease (CD) frequently complain of cognitive difficulties such as problems with concentration and clouding of thought, yet this has scarcely been objectively defined and underlying mechanisms remain unknown.
Objective
The objective of this article is to objectively measure cognitive impairments in patients with CD compared with healthy controls, and if present, to identify potentially modifiable, contributing factors associated with cognitive impairment.
Methods
CD patients and healthy age-/sex-matched controls completed surveys encompassing clinical, demographic, psychiatric, fatigue and sleep parameters. Contemporaneously, disease activity assessment with serum CRP, faecal calprotectin, Harvey–Bradshaw Index and the Subtle Cognitive Impairment test (SCIT) were performed, with the primary measure of response time (SCIT-RT) compared between groups. Multiple linear regression assessed for factors associated with slower SCIT-RT, denoting subtle cognitive impairment.
Results
A total of 49 CD and 31 control individuals participated, with median age 44 years (range 22–65) and 43 years (21–63), respectively. Compared to controls, SCIT-RT was slower across all timepoints in CD patients (ANOVA p < 0.001). In multivariate analysis, serum CRP (standardised beta coefficient 0.27, 95% CI (0.02, 0.51)), abdominal pain (0.43 (0.16, 0.70)), plasma haemoglobin (1.55 (1.42, 1.68)), and concurrent fatigue (0.56 (0.25, 0.88)) were each independently associated with slower SCIT-RT in CD (each p < 0.05), with a trend for poorer sleep quality 0.54 (−0.03, 1.11) (p = 0.06), yet conversely, higher faecal calprotectin titres were associated with faster SCIT-RT (−1.77 (−1.79, −1.76), p < 0.01).
Conclusions
Patients with CD demonstrated subtle cognitive impairment utilising the objective SCIT, correlating with systemic inflammation and other disease burden measures, although higher faecal calprotectin titres were unexpectedly associated with less cognitive impairment.
Keywords: Cognition, inflammation, Crohn’s disease, fatigue
Introduction
Chronic diseases like Crohn’s disease (CD) have wide-ranging effects on health and normal function beyond classic symptoms like diarrhoea and abdominal pain. These include effects on the brain, as depicted by the increased incidence of psychological stress, mood disorder and neurological sequelae in CD.1–3 Moreover, patients with CD frequently complain of vague symptoms such as difficulty concentrating and clouding of thought, which align with the notion that cognitive dysfunction is a major concern for sufferers, yet it is often ignored by clinicians. Few studies have demonstrated that cognitive dysfunction is more prevalent in patients with chronic diseases like CD compared to healthy controls.4,5 Nonetheless, this is an important consideration, as even if the decrements in cognitive function in CD are subtle,6 such deficits can have serious consequences, such as an increased risk of motor vehicle or workplace accidents.7,8
The Subtle Cognitive Impairment Test (SCIT)9,10 is sufficiently sensitive to detect mild cognitive impairment in elderly individuals with Mini-Mental State Examination (MMSE) scores in the normal range (≥24 points) and has been used and validated in many healthy and diseased study populations, including coeliac disease.10–13 Based on our observations that those with active CD are more likely to report severe cognitive fatigue,14 plus the concept of a ‘cytokine hypothesis’ for depression in chronic disease,15 we hypothesised that systemic CD-related inflammation may be associated with cognitive impairment in patients with CD. To address this hypothesis, the SCIT was applied to address two aims: (i) to compare cognitive function in patients with CD with demographically matched healthy controls; and (ii) to separate disease-related and patient-related factors that might be associated with the cognitive dysfunction. It is anticipated that the identification of modifiable factors could provide potential pathways for therapeutic application.
Materials and methods
Setting and recruitment
A cross-sectional, observational study was performed in patients with a confirmed diagnosis of CD according to standard histological, endoscopic and/or imaging criteria. Patients were recruited from the Box Hill Hospital Inflammatory Bowel Disease (IBD) Clinic, a metropolitan outpatient clinic providing secondary and tertiary IBD care. Relevant clinical and demographic data were extracted from the patients’ medical records and the IBD clinic database. Healthy volunteers were recruited via advertisement in local hospital notices, the local community newspaper and university newsletters/bulk emails. All study participants were aged between 18 and 65 years, were able to understand English and give written, informed consent. Participants were excluded if they had any known major medical comorbidity that may potentially affect cognition, independent of their CD, including neurological/ neuromuscular disorders, advanced renal, hepatic, cardiac, pulmonary and/or thyroid disorders, were known to be pregnant and/or if they consumed ≥40 g of alcohol per day on average.
Survey
Prior to the study visit, all prospective participants were required to complete a survey either online or on paper sent via mail, comprising part of a larger study described elsewhere.14 The survey included the Hospital Anxiety Depression Scale (HADS) to assess for comorbid anxiety and/or depression. Initially validated in hospital clinics, it has since been used and validated in chronic diseases including IBD, with scores >7 indicating possible and >11 probable ‘caseness’ for anxiety and/or depression.16 The Pittsburgh Sleep Quality Index (PSQI) was used to assess participants’ sleep quality in the past month, providing a global sleep quality score ranging from 0 to 21 – a higher score equalling poorer sleep quality. An empirically derived cut-off score >5 indicates poor sleep quality.17 The PSQI has been validated in healthy and diseased populations,18,19 and used previously in IBD.20 Also the Fatigue Impact scale (FIS, 40 items), with particular reference to the cognitive fatigue dimension in this study (10 items), was applied as in multiple studies previously in IBD.5,21 The FIS asks the participant to rate his or her fatigue in the past month in reference to each item’s statement on a scale of 0 (no problem) to 4 (extreme problem). The cognitive fatigue subscore of the FIS has a maximum possible score of 40, indicating severe cognitive fatigue.22
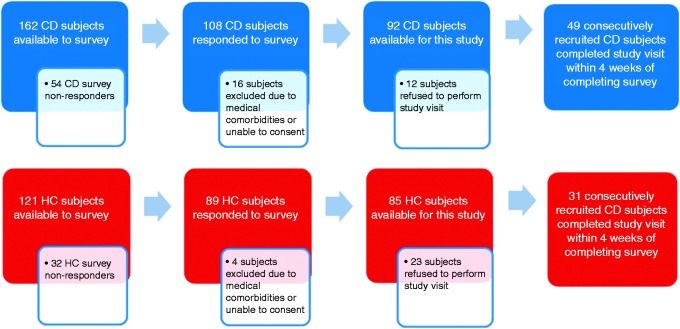
The survey also comprised demographic information, medical, psychiatric and medication history and, for patients with CD, detailed questioning on disease history including surgical, admission and treatment data. Initial non-responders were sent two reminders after six and 12 weeks, respectively, and were also contacted via phone to maximise the return of surveys and study participation (see Figure 1).
Figure 1.
Flowchart of participants (CD and HCs) completing the initial survey and study visit. CD: Crohn’s disease; HC: healthy control individuals.
Study visit
All recruited individuals completed a visit with the primary investigator (DvL) during which participants gave informed consent and further up-to-date demographic and disease-related information could be gleaned. In patients with CD, disease activity was assessed at the time of interview using the Harvey–Bradshaw Index (HBI)23 with active disease defined as an HBI ≥ 5. Participants provided a faecal sample for measurement of calprotectin concentration, and a sample of peripheral blood was taken for detailed pathology testing as outlined elsewhere.24 Individuals were excluded if any of the following tests/data were incomplete.
Cognitive testing
During the study visit above, each participant performed the SCIT.9,10 This test requires a participant to make a decision regarding which of two lines, presented very briefly on a computer screen, is the shorter. The visual stimulus is presented for varying durations, with the shorter side appearing randomly on the left or right side. Two psychometric measures are recorded: error rate (SCIT-ER) and response time (SCIT-RT). These are divided according to whether the stimulus was on the screen long enough for conscious inspection. Thus responses to stimulus durations of less than 65 ms are referred to as the ‘head’ of the data curve, while responses to stimulus durations of longer than 65 ms comprise the ‘tail’. The SCIT takes four minutes; it can detect small decrements in cognition and has no learning effect, or gender or cultural bias. The SCIT does not measure performance in specific cognitive domains, such as memory. Rather it is a global measure of cognition that is sensitive to decrements in signal processing that underpin most cognitive domains. The SCIT head component is a measure of conscious and subconscious signal processing speed, while the tail component is more concerned with the efficacy of conscious and subconscious decision making.25,26
Statistical analysis
Grouped data (between CD patients and healthy controls) were examined descriptively and normality was assessed with the Shapiro-Wilk test. Given that the SCIT-RT and SCIT-ER variables conformed to a Gaussian distribution, the mean with 95% confidence intervals (CIs) are presented, and one-way analysis of variance (ANOVA) tests were applied to compare SCIT-RT and SCIT-ER between the groups across all exposure durations of the SCIT. Also in the CD group, exploratory bivariate comparisons using Pearson correlations, then subsequently a multiple linear regression analysis were conducted with head and tail response (difference from the mean of healthy controls in each case) as the dependent variables. A stepwise model was used and variables were kept continuous wherever possible. Variables were included in the multivariate model on the basis of statistically significant (or trending towards) bivariate associations with SCIT-RTH (head) and SCIT-RTT (tail) as well as those variables considered to be of importance/relevance to cognitive dysfunction and/or CD pathogenesis (including age, symptom-based disease activity score (HBI, including abdominal pain and diarrhoea subscores), cognitive fatigue and duration since diagnosis of CD). A p value < 0.05 was deemed to be statistically significant in all analyses.
Ethical considerations
Participants provided written informed consent for their involvement in all aspects of this study. This research was approved by the Eastern Health Research & Ethics Committee (E103/0809).
Results
Participant characteristics
Forty-nine patients with CD and 31 healthy controls were recruited, with these two groups being well matched demographically (Table 1). Compared with healthy controls, participants with CD had higher depression scores (as per HADS, median 8.0 vs 3.0) and poorer sleep quality (PSQI, median 7.0 vs 4.0) scores, the latter despite slightly higher median sleep duration per night reported by CD participants (median 8.5 vs 8.0 hours) (each p < 0.05). Further important disease-specific characteristics of the patients with CD are shown in Table 2.
Table 1.
Patients with CD vs healthy controls by group
| CD | Healthy controls | p valueb | |
|---|---|---|---|
| Number of participants | 49 | 31 | – |
| Age (range) ya | 44 (22–65) | 43 (21–63) | 0.98 |
| Female sex (%) | 58.3 | 64.5 | 0.64 |
| Completed secondary school (%) | 79.2 | 93.5 | 0.11 |
| Gainful employment (%) | 79.2 | 87.1 | 0.55 |
| Married/de facto (%) | 56.3 | 77.4 | 0.09 |
| Current smoker (%) | 16.7 | 3.2 | 0.08 |
| Alcohol ≥10 g per day (%) | 35.4 | 34.4 | 1.0 |
| Current psychotropic drug (%) | 10.2 | 4.1 | 0.70 |
| Sleep quality score – PSQIa | 7.0 (2–19) | 4.0 (0–12) | <0.001 |
| Sleep duration per night (hours) – PSQI | 8.5 (6–12) | 8.0 (6.5–11) | 0.047 |
| HADS depression scorea | 8.0 (3–13) | 3.0 (0–17) | <0.001 |
Percentages are shown as per number where data are available.
Medians shown.
Mann-Whitney or Fisher’s exact tests as appropriate.
CD: Crohn’s disease; y: years; PSQI: Pittsburgh Sleep Quality Index; HADS: Hospital Anxiety Depression Scale.
Table 2.
Relevant clinical characteristics of the 49 patients with CD
| Disease classification (as per Montreal criteria) | |
|---|---|
| Age at diagnosis | |
| A1 16 y or below | 5 (10%) |
| A2 17–40 y | 34 (71%) |
| A3 above 40 y | 9 (19%) |
| Location | |
| L1 ileal | 11 (23%) |
| L2 ileocolonic | 17 (35%) |
| L3 colonic | 20 (42%) |
| L4 upper GI | 3 (6%) |
| P perianal | 11 (23%) |
| Behavior | |
| B1 non-stricturing/penetrating | 29 (60%) |
| B2 stricturing | 11 (23%) |
| B3 penetrating | 8 (17%) |
| Median duration since CD diagnosis (range) y | 14 (1–46) |
| Active disease, as per: | |
| Disease activity score | |
| HBI ≥ 5 | 20 (42%) |
| Median HBI (range) | 4 (1–18) |
| Intestinal inflammation | |
| Faecal calprotectin ≥ 150 µg/g | 25 (52%) |
| Median calprotectin (range) µg/g | 255 (30–1800) |
| Systemic inflammation | |
| CRP ≥ 5 mg/l | 13 (27%) |
| Median CRP (range) mg/l | 2.4 (0.4–45) |
| Low iron status (serum ferritin ≤ 30 g/l) | 8 (17%) |
| Prior bowel resection for CD | 19 (40%) |
| Documented psychological comorbidity | 15 (31%) |
| Current therapy | |
| Corticosteroid use | 6 (13%) |
| Immunomodulator use | 26 (54%) |
| Anti-TNF use | 12 (25%) |
| Smoking status | |
| Current smoker | 8 (17%) |
| Ex-smoker | 19 (40%) |
| Never smoked | 21 (43%) |
CD: Crohn’s disease; y: years; TNF: tumour necrosis factor; GI: gastrointestinal; HBI: Harvey–Bradshaw Index; CRP: C-reactive protein.
SCIT outcomes
Response times (SCIT-RT) were significantly slower in the CD vs healthy control group across all the exposure durations (one-way ANOVA p < 0.001), alternatively expressed overall as mean SCIT-RTH for CD of 604.4 ms (95% CI, 574.6, 634.1) vs 551.1 ms (523.4, 578.8) for controls (two-tailed t-test, p = 0.01) and mean SCIT-RTT for CD of 571.0 (545.4, 596.6) vs 521.2 (500.3, 542.2) (t-test, p = 0.01) for controls (Figure 2). Error rates (SCIT-ER) were, however, not statistically different between the groups (Figure 3) with the mean SCIT-ERH for CD of 0.12 (0.10, 0.15) vs 0.13 (0.10, 0.16) (t-test, p = 0.7) for controls and mean SCIT-ERT for CD of 0.016 (0.01, 0.02) vs 0.018 (0.01, 0.03) for controls (p = 0.7).
Figure 2.
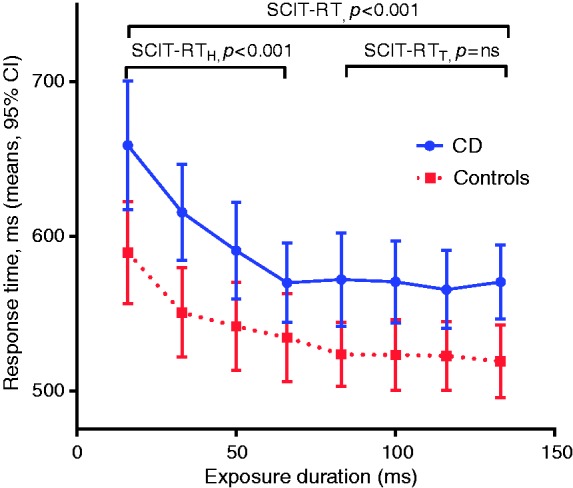
Mean response times per exposure duration (95% CI) for CD vs control groups showing significantly slower response times in the group with CD, across all exposure durations (SCIT-RT), head (SCIT-RTH), and tail responses (SCIT-RTT), each p < 0.001, one-way ANOVA test). CI: confidence interval; CD: Crohn’s disease; SCIT-RT: Subtle Cognitive Impairment test response time; ANOVA: analysis of variance.
Figure 3.
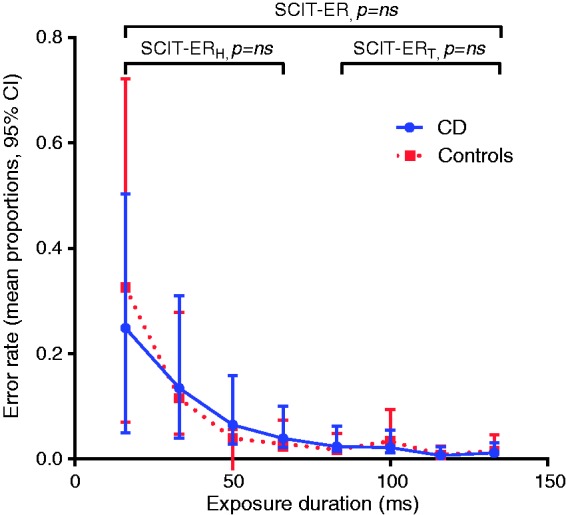
Mean error rates per exposure duration (95% CI) for CD vs control groups showing no significant difference between groups (across either all exposure durations or SCIT-ERH only, each p = ns, one-way ANOVA test). CI: confidence interval; CD: Crohn’s disease; SCIT-ERH: Subtle Cognitive Impairment test error rate (head); ANOVA: analysis of variance.
Factors associated with impaired SCIT-RT
On bivariate analysis, multiple variables were significantly associated with a slower SCIT-RTH, including total HBI (and including subscores HBI-well-being and HBI-abdominal pain) and fatigue (as per FIS, including cognitive fatigue subscore) (each p < 0.05). There were non-significant trends towards correlation between SCIT-RTH and serum high-sensitivity C-reactive protein (hsCRP), HADS anxiety score and disease duration since diagnosis. There was an unexpected inverse correlation between faecal calprotectin concentration and SCIT-RTH (r = −0.38, p < 0.01). Yet when assessing the subgroup only with ‘active disease’ defined by an HBI ≥ 5, this relationship between calprotectin and SCIT-RTH did not persist (r = −0.18, p = 0.5).
Regarding a slower SCIT-RTT, HBI-well-being and HBI-abdominal pain subscores, serum CRP and fatigue (relevantly the cognitive FIS subscore) were significantly correlated, but only non-significant trends existed for total HBI, HADS anxiety score and inversely for faecal calprotectin (Table 3).
Table 3.
Exploratory bivariate correlations and final multivariate linear regression analysis assessing for variables associated with slower than average (a) SCIT head, and (b) SCIT tail response times, in patients with CD
| Variable | Bivariate Pearson’s r | p value | Multivariate standardised beta coefficient (95% CI) | p value |
|---|---|---|---|---|
| (a) Head response time | ||||
| Age (y) | 0.09 | ns | ||
| Duration since diagnosis (y) | 0.25 | ns | 0.28 (–0.03, 0.59) | 0.10 |
| HBI abdominal pain (subscore) | 0.37 | 0.02 | 0.43 (0.16, 0.70) | 0.02 |
| HBI diarrhoea (subscore) | 0.15 | ns | ||
| hsCRP (mg/l) | 0.27 | ns | 0.27 (0.02, 0.51) | 0.05 |
| Faecal calprotectin (log10, µg/g) | −0.38 | <0.01 | −1.77 (–1.79, –1.76) | 0.001 |
| Haemoglobin (g/l) | −0.01 | ns | 1.55 (1.42, 1.68) | 0.004 |
| Ferritin (mg/l) | 0.10 | ns | ||
| Albumin mg/l) | −0.09 | ns | ||
| Sleep quality (PSQI) | 0.18 | ns | 0.54 (–0.03, 1.11) | 0.06 |
| Cognitive fatigue (FIS subscore) | 0.30 | 0.03 | 0.56 (0.25, 0.88) | 0.02 |
| Anxiety score (HADS) | 0.27 | ns | ||
| Depression score (HADS) | 0.18 | ns | ||
| (b) Tail response time | ||||
| Age (y) | −0.03 | ns | ||
| Duration since diagnosis (y) | 0.18 | ns | ||
| HBI score (total) | 0.27 | ns | ||
| HBI abdominal pain subscore | 0.33 | 0.02 | 0.19 (–0.08, 0.46) | 0.18 |
| HBI diarrhoea subscore | 0.06 | ns | ||
| hsCRP (mg/l) | 0.34 | 0.02 | 0.36 (0.11, 0.62) | 0.01 |
| Faecal calprotectin (log10, µg/g) | −0.25 | ns | −0.24 (–0.50, 0.03) | 0.08 |
| Haemoglobin (g/l) | 0.02 | ns | ||
| Ferritin (mg/l) | 0.06 | ns | ||
| Albumin mg/l) | −0.11 | ns | ||
| Sleep quality (PSQI) | 0.18 | ns | ||
| Cognitive fatigue (FIS subscore) | 0.28 | 0.05 | 0.26 (–0.29, 0.37) | 0.79 |
| Anxiety score (HADS) | 0.27 | ns | 0.25 (–0.01, 0.51) | 0.06 |
| Depression score (HADS) | 0.19 | ns | ||
CD: Crohn’s disease; y: years; PSQI: Pittsburgh Sleep Quality Index; HADS: Hospital Anxiety Depression Scale; FIS: Fatigue Impact scale; hsCRP: high-sensitivity C-reactive protein; SCIT: Subtle Cognitive Impairment test.
Incorporating the above variables and others putatively important in cognitive dysfunction in CD, the multivariate linear regression analyses for each dependent variable are also shown in Table 3. Factors independently and significantly associated with slower SCIT-RTH included a higher serum CRP, more severe abdominal pain (as per HBI subscore), poorer sleep quality (PSQI score) and a higher cognitive FIS fatigue score (each p < 0.05). Interestingly, a higher plasma haemoglobin (Hb) was positively associated, yet faecal calprotectin level was again inversely associated with SCIT-RTH (each p < 0.01).
The only factor significantly associated with a slower SCIT-RTT when correcting for other variables in the multivariate analysis was serum CRP, whereas there were non-significant trends for HADS anxiety score (p = 0.06) and once again inversely for faecal calprotectin (log concentration, p = 0.08).
Discussion
Response times, as measured by the computer-based SCIT, were slower across all exposure durations in patients with CD compared to well-matched healthy controls. These results objectively and definitively demonstrate the presence of subtle cognitive impairment in patients with CD. The impairment was limited to response times as there was no discernible difference between CD and control groups in the rates of errors made during the SCIT. The present results support the anecdotal observations of difficulties with concentration, clouding of thought and memory lapses that are frequently reported to clinicians by patients with CD.
The presence of deficits in response time but not in error rates indicates that patients with CD have impairments in the speed of signal processing rather than in the efficiency of processing or decision making.25 Importantly, this decrement in the speed of processing was equivalent for exposure durations in both the head and tail of the response curve, suggesting that the impairment is due to problems in rapid automatic processes that are involved in detecting, coding and integrating perceptual information. It is noteworthy that the slowed response times were significantly correlated with clinical indices of inflammation, such as the HBI scores and serum titres of CRP. This finding is consistent with recent reports that elevations in serum titres of cytokines, including CRP, are associated with significant increases in response times on cognitive tasks in middle-aged and elderly populations.27,28 While the basis of this effect is not fully understood, experiments on rodents have shown that inflammation of the colon results in an upregulation of inflammatory activity in microglia of the hippocampus that in turn causes profound impairments in post-synaptic responses.29 If such decrements in post-synaptic responsiveness occur in humans with CD, they could readily account for the slower response times observed in the present study.
The strong association of poorer sleep quality with cognitive dysfunction is another salient finding of this study, given the prevalence of sleep disturbance in CD patients and the potentially cumulative effects of poor sleep quality on cognition.30 Overall self-reported, nightly sleep durations were generally longer in CD than control participants in this study, thus implying a problem of greater sleep disturbance in CD within sleep, rather than the overall amount of sleep per se.
Perhaps more intriguing, however, was the positive association between higher Hb titres and slower SCIT-RTH. In this cohort very few participants were anaemic with Hb < 100 g/l, precluding the opportunity to assess for lower Hb effects on cognition, which are well described,31 but there are also studies demonstrating that higher Hb titres may be associated with cognitive dysfunction. These include a study by Shah et al.32 which demonstrated a link between higher Hb and slower perceptual speed, which is a similar construct to that measured by SCIT-RTH.
The inverse correlation of faecal calprotectin with cognitive function might appear counterintuitive, but we were anticipating no relationship since systemic inflammation is often discordant with intestinal inflammation. For instance in this cohort, there were no significant correlations between faecal calprotectin and HBI or CRP (each p > 0.05), whereas CRP significantly correlated both with HBI and cognitive function. As a quantitative global measure of intestinal inflammation at one point in time, calprotectin is unhelpful for comparing the degree of inflammation between individuals given its concentration is dependent on multiple factors including the location and extent of inflammation and may be confounded by concurrent rectal bleeding, medications and gastrointestinal (GI) infection.
The present study is the first to use an objective measure to demonstrate that patients with CD have impaired cognition compared to healthy controls. Our team previously showed that patients with untreated celiac disease exhibit impaired performance on the SCIT that improves when the patients adhere to a gluten-free diet.12 Similarly, Hollerbach and colleagues (published only in abstract form) reported that electrophysiological testing showed that patients with IBD had higher mean peak P300 latencies, suggestive of short-term memory disturbances, compared to controls.33 Other studies have used a semi-objective approach, utilising multiple questionnaires or paper-based test including the Wechsler Abbreviated Scale of Intelligence (WASI) to assess cognitive function in patient groups with IBD, irritable bowel syndrome (IBS) and healthy controls.34,35 Dancey et al. demonstrated a deficit in verbal intelligence quotient (IQ) as a measure of higher cognitive processing in the IBD group compared to both the IBS and control groups. Kennedy et al. showed cognitive deficits in both their CD and IBS groups compared to controls, though comparatively CD patients had less memory disturbance, but similar to our study, exhibited more abnormal executive functioning, particularly slower response times (i.e. Stroop interference effect).29,30,36,37 Thus it may be postulated that cognitive dysfunction in IBD and IBS may share some underlying pathophysiology (such as cofactors of abdominal symptom burden and psychiatric comorbidity) but also exhibit disorder-specific mechanisms, which may include effects of Crohn’s-related systemic inflammation.
Prior studies in IBD and those in other chronic diseases may have been stymied by the fact that the differences between normal and impaired cognition are typically subtle. The main question then is whether the subtle cognitive impairments in individuals with CD are clinically or functionally meaningful. Although definitively answering this question is beyond the scope of the current study, a mean difference of over 50 ms in SCIT-RTH scores between the CD and healthy control groups is of a greater magnitude than the change in the same measure in healthy volunteers after consuming sufficient alcohol to reach a mean peak blood alcohol concentration of just above 0.05 g/100 ml (over the legal limit for driving a motor vehicle in Australia and most European Union (EU)-zone countries). Although many people with CD function effectively in their chosen work or study, it remains uncertain whether a patient who reports decreased cognitive performance should, from a societal perspective, continue in performance-critical roles such as driving a bus, working as an air traffic controller or as a surgeon operating during a 24-hour on-call shift.
The current study has limitations. The cross-sectional, observational design does not enable assessment of causality or of longitudinal changes in cognition, including treatment effects. Second, the relatively small sample size prevented a more detailed assessment of the relationship between cognitive impairment and CD-related inflammation. Third, there is the potential for selection bias given the ‘captive’ recruitment of consecutive CD patients from a specialist clinic vs our ‘open’ recruitment of healthy controls who volunteered in response to advertisements. Fourth, the anomalous inverse relationship between faecal calprotectin and cognitive function cannot be easily explained in this study from a clinical nor a theoretical perspective and warrants further investigation. Nevertheless, strengths of this study include the quality of matching between CD and control groups in terms of relevant confounders (such as age, sex, educational attainment and employment status). Even more important is the use of an entirely objective, operator-dependent test that enabled the detection of cognitive impairment that may have gone undetected by standard pencil and paper tests.25
In summary, this study has objectively demonstrated the presence of subtle cognitive impairment in a clinic-based cohort of CD patients compared with well-matched controls using the SCIT computer-based test. The results suggest that CD-related cognitive impairment is strongly linked with systemic inflammation yet is inversely related to intestinal mucosal inflammation, and might be a consequence of an amalgam of ‘indirect’ disease effects, including the cumulative burden and duration of CD, comorbid anxiety and sleep disturbance. While further research is required to define the impact of this cognitive impairment on normal activities of daily living, the results of this study reinforce the long-held notion that CD is a multisystemic disease with wide-ranging consequences.
Declaration of conflicting interests
DR van Langenberg reports speaker funding and/or acting as a consultant for AbbVie, Janssen, Shire, Ferring, Fresenius Kabi and Takeda. He has received research funding from Abbvie, Shire, Orphan Australia and Ferring during the conduct of this study. GW Yelland and SR Robinson hold five patents for the Subtle Cognitive Impairment Test (SCIT) from which neither receives any royalties. They are co-directors of NeuroTest Pty Ltd, the company that distributes the SCIT. They receive no payment from NeuroTest Pty Ltd, and the SCIT was provided for free for use in this study.
PR Gibson reports grants from Gastroenterological Society of Australia, during the conduct of the study; grants, personal fees and non-financial support from AbbVie, grants, personal fees and non-financial support from Janssen, personal fees from Takeda, grants, personal fees and other from Ferring, personal fees from Fresenius Kabi, research funding from Shire, research funding from Falk Pharma GmbH, research funding and personal fees from Danone, personal fees from Abbott, and personal fees from Nestle, outside the submitted work.
Funding
This work was funded in full by a Gastroenterological Society of Australia IBD clinical research grant, and DR van Langenberg’s salary was funded in part by a National Health and Medical Research Council (NHMRC) Medical Postgraduate Scholarship. No writing or other data support was received for this manuscript.
References
- 1.Tache Y, Bernstein CN. Evidence for the role of the brain-gut axis in inflammatory bowel disease: Depression as cause and effect? Gastroenterol 2009; 136: 2058–2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mawdsley JE, Rampton DS. Psychological stress in IBD: New insights into pathogenic and therapeutic implications. Gut 2005; 54: 1481–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Casella G, Tontini GE, Bassotti G, et al. Neurological disorders and inflammatory bowel diseases. World J Gastroenterol 2014; 20: 8764–8782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banovic I, Gilibert D, Cosnes J. Crohn’s disease and fatigue: Constancy and co-variations of activity of the disease, depression, anxiety and subjective quality of life. Psychol Health Med 2010; 15: 394–405. [DOI] [PubMed] [Google Scholar]
- 5.van Langenberg DR, Gibson PR. Systematic review: Fatigue in inflammatory bowel disease. Aliment Pharm Ther 2010; 32: 131–143. [DOI] [PubMed] [Google Scholar]
- 6.Martinić-Popović I, Lovrencić-Huzjan A, Demarin V. Assessment of subtle cognitive impairment in stroke-free patients with carotid disease. Acta Clin Croat 2009; 48: 231–240. [PubMed] [Google Scholar]
- 7.Fletcher A, McCulloch K, Baulk SD, et al. Countermeasures to driver fatigue: A review of public awareness campaigns and legal approaches. ANZ J Pub Health 2005; 29: 471–476. [DOI] [PubMed] [Google Scholar]
- 8.Swaen GM, Van, Amelsvoort LG, Bültmann U, et al. Fatigue as a risk factor for being injured in an occupational accident: Results from the Maastricht Cohort Study. Occup Environ Med 2003; 60(Suppl 1): i88–i92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yelland G, Robinson SR, Friedman T, et al. Assessment of cognitive impairment. Monash University. Australian Official Journal of Patents. Australia 2004. Available at: http://pericles.ipaustralia.gov.au (accessed 31 August 2015). [Google Scholar]
- 10.Friedman T, Yelland G, Robinson SR, et al. SCIT: A novel test that is sensitive to subtle deficits in cognitive performance in the elderly. Aust J Psychol 2005; 57: 10–10. [Google Scholar]
- 11.Friedman TW, Robinson SR, Yelland GW. Impaired perceptual judgment at low blood alcohol concentrations. Alcohol 2011; 45: 711–718. [DOI] [PubMed] [Google Scholar]
- 12.Lichtwark IT, Newnham ED, Robinson SR, et al. Cognitive impairment in coeliac disease improves on a gluten-free diet and correlates with histological and serological indices of disease severity. Aliment Pharmacol Ther 2014; 40: 160–170. [DOI] [PubMed] [Google Scholar]
- 13.Friedman TW, Yelland GW, Robinson SR. Subtle cognitive impairment in elders with Mini-Mental State Examination scores within the ‘normal’ range. Int J Geriatr Psych 2012; 27: 463–471. [DOI] [PubMed] [Google Scholar]
- 14.van, Langenberg DR, Gibson PR. Factors associated with physical and cognitive fatigue in patients with Crohn’s disease: A cross-sectional and longitudinal study. Inflamm Bowel Dis 2014; 20: 115–125. [DOI] [PubMed] [Google Scholar]
- 15.Maes M. The cytokine hypothesis of depression: Inflammation, oxidative & nitrosative stress (IO&NS) and leaky gut as new targets for adjunctive treatments in depression. Neuro Endocrinol Lett 2008; 29: 287–291. [PubMed] [Google Scholar]
- 16.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983; 67: 361–370. [DOI] [PubMed] [Google Scholar]
- 17.Buysse DJ, Reynolds CF, 3rd, Monk TH, et al. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res 1989; 28: 193–213. [DOI] [PubMed] [Google Scholar]
- 18.Beck SL, Schwartz AL, Towsley G, et al. Psychometric evaluation of the Pittsburgh Sleep Quality Index in cancer patients. J Pain Sympt Manage 2004; 27: 140–148. [DOI] [PubMed] [Google Scholar]
- 19.Carpenter JS, Andrykowski MA. Psychometric evaluation of the Pittsburgh Sleep Quality Index. J Psychosom Res 1998; 45: 5–13. [DOI] [PubMed] [Google Scholar]
- 20.Ali T, Madhoun MF, Orr WC, et al. Assessment of the relationship between quality of sleep and disease activity in inflammatory bowel disease patients. Inflamm Bowel Dis 2013; 19: 2440–2443. [DOI] [PubMed] [Google Scholar]
- 21.Castillo-Cejas MD, Robles V, Borruel N, et al. Questionnaries [sic] for measuring fatigue and its impact on health perception in inflammatory bowel disease. Revista Esp Enferm Digest 2013; 105: 144–153. [DOI] [PubMed] [Google Scholar]
- 22.Fisk JD, Ritvo PG, Ross L, et al. Measuring the functional impact of fatigue: Initial validation of the fatigue impact scale. Clin Infect Dis 1994; 18(Suppl 1): S79–S83. [DOI] [PubMed] [Google Scholar]
- 23.Harvey RF, Bradshaw JM. A simple index of Crohn’s-disease activity. Lancet 1980; 1: 514–514. [DOI] [PubMed] [Google Scholar]
- 24.van, Langenberg DR, Della Gatta P, Warmington SA, et al. Objectively measured muscle fatigue in Crohn’s disease: Correlation with self-reported fatigue and associated factors for clinical application. J Crohn Colitis 2014; 8: 137–146. [DOI] [PubMed] [Google Scholar]
- 25.Bruce KM, Robinson SR, Smith JA, et al. Validity of a screening tool for detecting subtle cognitive impairment in the middle-aged and elderly. Clin Interv Aging 2014; 9: 2165–2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Speirs SJ, Rinehart NJ, Robinson SR, et al. Efficacy of cognitive processes in young people with high-functioning autism spectrum disorder using a novel visual information-processing task. J Autism Dev Disord 2014; 44: 2809–2819. [DOI] [PubMed] [Google Scholar]
- 27.Windham BG, Simpson BN, Lirette S, et al. Associations between inflammation and cognitive function in African Americans and European Americans. J Am Geriatr Soc 2014; 62: 2303–2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heringa SM, Walraven I, Moll AC, et al. Vascular retinopathy in relation to cognitive functioning in an older population – the Hoorn Study. J Am Geriatr Soc 2014; 62: 977–979. [DOI] [PubMed] [Google Scholar]
- 29.Riazi K, Galic MA, Kentner AC, et al. Microglia-dependent alteration of glutamatergic synaptic transmission and plasticity in the hippocampus during peripheral inflammation. J Neurosci 2015; 35: 4942–4952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yaffe K, Falvey CM, Hoang T. Connections between sleep and cognition in older adults. Lancet Neurol 2014; 13: 1017–1028. [DOI] [PubMed] [Google Scholar]
- 31.Denny SD, Kuchibhatla MN, Cohen HJ. Impact of anemia on mortality, cognition, and function in community-dwelling elderly. Am J Med 2006; 119: 327–334. [DOI] [PubMed] [Google Scholar]
- 32.Shah RC, Wilson RS, Tang Y, et al. Relation of hemoglobin to level of cognitive function in older persons. Neuroepidemiol 2009; 32: 40–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hollerbach SH, Kullmann F, Geissler A, et al. Impairment of short-term memory function and morphologic brain abnormalities in inflammatory bowel disease (IBD). Gastroenterol 2000; 118: A313–A313. [Google Scholar]
- 34.Dancey CP, Attree EA, Stuart G, et al. Words fail me: The verbal IQ deficit in inflammatory bowel disease and irritable bowel syndrome. Inflamm Bowel Dis 2009; 15: 852–857. [DOI] [PubMed] [Google Scholar]
- 35.Attree EA, Dancey CP, Keeling D, et al. Cognitive function in people with chronic illness: Inflammatory bowel disease and irritable bowel syndrome. Appl Neuropsychol 2003; 10: 96–104. [DOI] [PubMed] [Google Scholar]
- 36.Denney DR, Lynch SG. The impact of multiple sclerosis on patients’ performance on the Stroop Test: Processing speed versus interference. J Int Neuropsychol Soc 2009; 15: 451–458. [DOI] [PubMed] [Google Scholar]
- 37.Kennedy PJ, Clarke G, O’Neill A, et al. Cognitive performance in irritable bowel syndrome: Evidence of a stress-related impairment in visuospatial memory. Psychol Med 2014; 44: 1553–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]