Abstract
Background
Gastric mucosa-associated lymphoid tissue lymphoma (gMALT) and gastric adenocarcinoma (GC) are long-term complications of chronic Helicobacter pylori (HP) gastritis. Treatment of HP infection induces remission in most patients with gMALT. Endoscopic follow-up is not currently endorsed after complete remission. However, the risk of GC in these patients is unclear.
Objective
The objective of this study is to estimate GC risk in gMALT patients.
Methods
The National Cancer Institute Surveillance, Epidemiology and End Results 13 (SEER) database-Nov 2014 Sub (1992–2012) was used to identify adult patients diagnosed with gMALT between 1992 and 2012. The standardized incidence ratio of second primary GC after a latency period of 12 months was calculated and compared to a reference SEER cohort of identical age, sex and time period. The risk of GC in these patients was also stratified by latency period (five years) and age.
Results
We identified 2195 cases of gMALT lymphoma, and 20 (0.91%) of them subsequently developed GC with a relative risk (RR) of 4.32 (95% CI 2.64–6.67) compared to the American population. The median latency time was five years and the risk was maintained afterward (RR 4.92, 95% CI 2.45–8.79). When stratified by age group the risk was highest for the 45–64 group (RR 14.04, 95% CI 5.64–28.93).
Conclusion
gMALT lymphoma is associated with an increased risk of metachronous gastric adenocarcinoma. The risk is still present after more than five years of follow-up. Further studies may clarify the most adequate follow-up strategy.
Keywords: Gastric MALT lymphoma, gastric adenocarcinoma, Helicobacter pylori, metachronous cancer risk, follow-up studies
Introduction
Adenocarcinomas represent the majority of gastric malignancies, whereas lymphomas constitute 1%–7% of all malignant tumors of the stomach.1,2 Gastric adenocarcinoma (GC) is the fourth most common cancer worldwide and the second cause of cancer-related death.1,3 It is often diagnosed at an advanced stage and has a low five-year survival rate.3 Gastric marginal zone lymphomas of mucosa-associated lymphoid tissue, usually named gastric MALT lymphomas (gMALTs), are indolent, low-grade B-cell non-Hodgkin’s lymphomas classified as independent entities and considered to be an infection-associated malignancy.4–6
Helicobacter pylori (HP) is the number one risk factor for GC, probably due to chronic inflammation of the mucosa, which leads to atrophic gastritis, intestinal metaplasia and ultimately dysplasia and adenocarcinoma.7,8 HP causes peptic ulcer, both intestinal and diffuse type GC and gMALT.9,10
The incidence of distal GC has been declining in high-income countries, which can be due to the declining prevalence of HP infection.11,12 Although gMALTs are also strongly associated with HP infection, the incidence of this disease has, in contrast to GC, been reported to increase, probably due to improved endoscopic and histological diagnostic procedures.13 Incidence of gMALT is relatively low in Western European countries, ranging from 0.21 (England) to 13 (Italy) per 100,000.8,14,15
The relationship between the two types of cancer and particularly whether patients with diagnosed gMALT have an increased risk of developing GC is controversial. Though that has been suggested by case series and small cohorts,4,16–19 other studies were not able to support these claims.13,20–22 A large study conducted in the Netherlands using a nationwide database found that patients with gMALT have a six-fold increased risk of GC when compared to the general population (p < 0.001), and a 16.6 times higher risk for patients aged 45 to 59 compared to the general population (p < 0.001).5
Current guidelines are contradictory regarding follow-up endoscopy in patients with localized gMALT in remission after HP eradication. Some guidelines recommend a follow-up based on clinical, and physical examination and laboratory assessment for five years. Endoscopy is not included in the assessment after complete remission is attained.23
With this study, we aim to evaluate the risk of GC in patients after a diagnosis of gMALT. Our hypothesis is that the risk of GC is increased in gMALT patients compared with an age- and sex-matched reference population.
Materials and methods
National Cancer Institute Surveillance, Epidemiology and End Results (SEER) database
We used the SEER Program (www.seer.cancer.gov) SEER*Stat Database: Incidence – SEER 13 Regs Research Data, Nov 2014 Sub (1992–2012) <Katrina/Rita Population Adjustment> linked to County Attributes – Total U.S., 1969–2013 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2015, based on November 2014 submission. The SEER database collects and maintains high-quality cancer data from hospitals and cancer treatment centers from the United States (US) with a 98% completeness rate. The SEER 13 database covers approximately 13.4% of the US population (2010 census) with a total of 4,057,213 tumors from 13 geographic areas: San Francisco-Oakland, Connecticut, Detroit (Metropolitan), Hawaii, Iowa, New Mexico, Seattle (Puget Sound), Utah, Atlanta (Metropolitan), San Jose-Monterey, Los Angeles, Alaska Natives and Rural Georgia.
The event variable was “Site recode B ICD-O-3/WHO 2008.”
Statistical analysis
We used the multiple primary standardized incidence ratio (MP-SIR) session of Surveillance Research Program, National Cancer Institute SEER*Stat software (seer.cancer.gov/seerstat) version 8.2.1 April 7, 2015, to calculate the standardized incidence ratio (SIR) and absolute excess risk (AER). The database used was the SEER.
The expected gastric cancer (GC) incidence was calculated for a reference SEER cohort of identical age, sex and time period. We also stratified the analysis by latency period (five years after the latency exclusion period equaling six years after gMALT recode in the database), by gender and by age (0–44, 45–64, 65–84 and > 84).
Continuous data are presented either as mean or median (interquartile range (IQR)) and dichotomous or categorical data as proportions. SIR or relative risk (RR) is presented as risk with 95% confidence intervals (CIs). A p value < 0.05 was considered statistically significant.
Patient selection
All patients with a histologically confirmed diagnosis of gMALT between 1992 and 2012 were included. We excluded patients who were diagnosed with gMALT diagnosis at autopsy or lost to follow-up. Patients with GC diagnosis within 12 months of gMALT lymphoma diagnosis were excluded as the GC was considered to be the prevalent disease.
The selection criteria, using first record matching selection criteria, were {Site and Morphology.Lymphoma subtype recode/WHO 2008}= (a)2.5.2 Extranodal MZL, MALT type' AND {Site and Morphology.Primary Site - labeled} = 'C16.0-Cardia, NOS','C16.1-Fundus of stomach','C16.2-Body of stomach','C16.3-Gastric antrum','C16.4-Pylorus','C16.5-Lesser curvature of stomach NOS','C16.6-Greater curvature of stomach NOS','C16.8-Overlapping lesion of stomach','C16.9-Stomach, NOS'.
Patients were followed from diagnosis of gMALT to the last known vital status, death or the last point of data collection. Within this cohort, all patients with a histologically confirmed diagnosis of GC were identified.
Primary endpoint
The primary endpoint was the development of metachronous GC at least 12 months after gMALT diagnosis.
Results
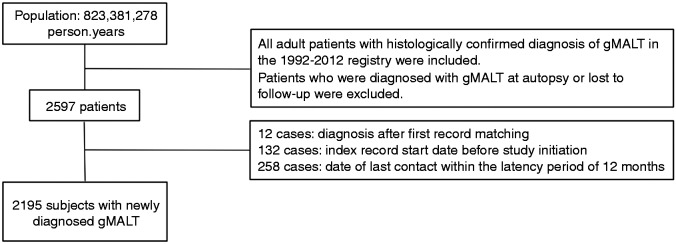
Between 1992 and 2012, 2597 patients with a gMALT diagnosis were identified from a population of 823,381,278 person-years. The final cohort included 2195 cases of newly diagnosed gMALT, after exclusion of 12 cases of diagnosis after first record matching, 132 cases with an index record start date before the study initiation and 258 cases in which the date of last contact fell within the latency exclusion criteria of 12 months (Figure 1). The overall incidence of gMALT in the SEER population was 2.81/1,000,000, the lowest incidence was 0 in 1992 and the highest was 4.43/1,000,000 in 2002. Median follow-up time of the entire cohort was five (IQR 3–10) years reaching 13,186.07 person-years at risk.
Figure 1.
Flowchart of the population selection gMALT: gastric mucosa-associated lymphoid tissue lymphoma.
Baseline characteristics are shown in Table 1.
Table 1.
Baseline characteristics of patients with a newly diagnosed gastric MALT (gMALT) lymphoma (n = 2195) and the subgroup of patients with a metachronous gastric cancer (GC) diagnosis after a diagnosis of gMALT (n = 20).
| Baseline characteristics | Gastric MALT lymphoma (n = 2195) | Subsequent metachronous GC (n = 20) |
|---|---|---|
| Male gender, n (%) | 1058 (48.2) | 12 (60) |
| Female gender, n (%) | 1137 (51.8) | 8 (40) |
| White race, n (%) | 1704 (77.6) | 15 (75) |
| Black race, n (%) | 209 (9.5) | 3 (15) |
| Other, n (%) | 282 (12.8) | 2 (10) |
| Median age at time of diagnosis, years (IQR) | 67 (61, 78) | 69 (58, 78) |
| Median latency time, months (IQR) | – | 63.5 (25, 89) |
MALT: mucosa-associated lymphoid tissue lymphoma; IQR: interquartile range.
In total, 20 (0.91%) gMALT patients (12 males, eight females) were diagnosed with metachronous gastric adenocarcinoma (GC) at a mean age of 69.71 years. The RR for GC after gMALT was 4.05 (95% CI 2.09–7.08) in males and 4.79 (95% CI 2.07–9.44) in females. When stratified by age group the risk was highest for the 45–64 group (RR 14.04, 95% CI 5.64–28.93) (Table 2).
Table 2.
The relative risk of gastric cancer (GC) in patients with gastric MALT lymphoma (gMALT) as compared to the general population, overall and stratified by age.
| Age (years) | GC in patients with gMALT | Expected GC incidence | Relative risk (RR) (95% CI) | Person-years at risk |
|---|---|---|---|---|
| 0–44 | 0 | 0 | n/a | 843.25 |
| 45–64 | 7 | 0.50 | 14.04a, (5.64–28.93) | 4060.55 |
| 65–84 | 10 | 2.97 | 3.36a, (1.61–6.18) | 6553.41 |
| >84 | 3 | 1.14 | 2.63, (0.54–7.69) | 1728.85 |
| Overall | 20 | 4.63 | 4.32a, (2.64–6.67) | 13,186.07 |
p < 0.05. MALT: mucosa-associated lymphoid tissue lymphoma; CI: confidence interval.
The average number of GC diagnosis was two cases per year, and the age standardized incidence rate was 0.002 per 100,000 per year. The proportions according to ethnicity were 75% white, 15% black and 10% other. The median interval between GC and gMALT in patients with GC development after diagnosis of gMALT was 63.5 (IQR 25-89) months (Table 1).
Overall, patients with a diagnosis of gMALT were at a 4.32 times (95% CI 2.64–6.67) higher risk of developing GC compared to the general population of identical age, sex and time period. The RR was increased even after five years from gMALT diagnosis with an RR for GC of 4.92 (95% CI 2.45–8.79, p < 0.05) (Table 3).
Table 3.
The relative risk of gastric cancer (GC) in patients with gastric MALT lymphoma (gMALT) as compared to the general population, overall and stratified by latency period (five years).
| GC in patients with gMALT | Expected GC incidence | Relative risk (RR) | 95% CI | Person-years at risk | |
|---|---|---|---|---|---|
| Overall | 20 | 4.63 | 4.32a | 2.64–6.67 | 13,186.07 |
| <5 years of latency | 9 | 2.39 | 3.76a | 1.72–7.14 | 6915.77 |
| ≥5 years of latency | 11 | 2.24 | 4.92a | 2.45–8.79 | 6270.30 |
p < 0.05. MALT: mucosa-associated lymphoid tissue lymphoma; CI: confidence interval.
Discussion
This study provides long-term data on a relevant national database representative of a Western population and confirms the suggestion from previous reports that gMALT patients have a considerably higher GC risk than the general population. We have shown that this risk seems to peak at five years after gMALT diagnosis. Our study also allowed us to see a difference across age groups with the highest risk for metachronous GC in those aged 45 to 64 years.
Several small studies observed either the occurrence of synchronous or metachronous GC in gMALT patients or progression from premalignant states to gastric carcinoma in such patients.2,5,10,18,24 In these studies, the majority of lymphomas was larger than adenocarcinoma, and additionally most of the GC reported were early cancers, suggesting lymphoma developed before carcinoma.25
A long-term nationwide study of a Dutch population suggested that gMALT patients had an important increased risk for developing GC when compared to the general population (six times higher risk), especially for female and younger patients (45–59 years).5 Furthermore, the authors also reported that GC risk was independent of the gMALT grade.5 Similarly, a German prospective multicenter trial showed an 8.6 times higher morbidity ratio from GC compared with the general German population and a 18.6 times higher morbidity ratio from non-Hodgkin’s lymphoma.4
Our results showed a 4.3 times higher risk of developing GC compared to the general American population of identical age, sex and time period. The median latency time for development of GC after gMALT diagnosis was 63.5 months. These results show a longer latency period compared to a previous case review on metachronous occurrence of adenocarcinoma in gMALT patients, which reported latencies from six months to five years.17 Our study, as in the Dutch national study, also supports the hypothesis that the risk is higher at younger ages (45–64) but failed to demonstrate the association with gender.
An important question is the pathogenicity of the two different malignancies. HP infection is thought to be a common etiological agent for both cancers.25 On the one hand, HP infection induces chronic gastritis with activation of neutrophils that produce nitric oxide and superoxides, resulting in DNA damage and eventually gastric carcinogenesis.26 On the other hand, HP induces T-cell activation, lymphoid follicle formation and B-cell hyperproliferation, eventually leading to gMALT lymphoma development.27 The common finding in the background of the carcinomatous and lymphomatous lesions is severe infiltration of lymphocytes.
There have been some reports of GC arising in the same anatomic localization of gMALT, most of them showing residual lymphoma cells in the gastrectomy specimen.16,28 Ioachim et al.29 suggested that an immunological imbalance caused by the lymphoma could contribute to the development of dysplastic epithelial cells. Furthermore, even if the GC arises in a different anatomical location, a previous gMALT may constitute a marker of chronic inflammation, a premalignant lesion similar to atrophy and/or intestinal metaplasia.
Currently, it is established that HP eradication is not sufficient to prevent GC in all cases. HP infection must be cured early in order to prevent development of atrophy and/or intestinal metaplasia, precancerous conditions that might not be reversible after HP eradication.25,30 In the setting of gMALT lymphoma, HP eradication is widely recognized as the initial treatment.21 A further problem is that even after successful HP eradication and gMALT remission, the risk of developing GC might still be increased.16,25 Wündisch et al.4 reported on three of 120 patients (3%) with HP-associated gMALT lymphoma who developed early GC four to five years after complete lymphoma remission following HP eradication. In this study endoscopic controls were carried out at monthly intervals, and after gMALT remission, endoscopy was performed every six to 12 months. All three cases were managed successfully using mucosectomy, strongly supporting the need for long-term endoscopic follow-up in responding patients with gMALT.4
Current guidelines are contradictory regarding follow-up endoscopies in patients with localized gMALT in remission. The National Comprehensive Cancer Network (NCCN)23 advises endoscopy revision three months after HP eradication, and in cases of HP-negative gMALT remission, maintaining only clinical follow-up, reserving endoscopy for symptomatic patients. On the contrary, European Society for Medical Oncology (ESMO) guidelines31 advise follow-up endoscopies every six months for two years. The American Society for Gastrointestinal Endoscopy (ASGE) guidelines recommend extended surveillance both with endoscopy and tissue sampling after successful HP eradication.32 The rationale for this recommendation is that complete regression of gMALT may require a long period of time and there is also a risk of recurrence, even without HP reinfection.32 In a large international series by Fischbach et al.,13 a watch-and-wait follow-up strategy using endoscopy and mucosal sampling every three to six months for the first two years after HP eradication with extension to every six to 12 months thereafter identified a low rate of gMALT progression, based on a median follow-up of 42.2 months. Therefore, the long-term follow-up is uncertain. In this study, we realized that patients with a prior gMALT more than five years before were still at increased risk for GC. This may be taken into account for the follow-up of these patients, lowering the threshold for endoscopic examinations.
One of the main limitations of this study is the lack of comparison of our cohort with HP-positive patients.5,33 Previous studies showed that HP-positive individuals have an estimated lifetime risk for GC of 1%,34 and that premalignant conditions associated with HP infections are associated with a risk of 2% to 3% of developing GC in a 10-year period,33 but direct comparisons are lacking. Capelle et al.5 showed that the risk for GC in gMALT patients was very similar to patients with atrophic gastritis and intestinal metaplasia. Additionally, it would be important to know whether such precancerous lesions were present in the histological samples surrounding gMALT lymphoma. To overcome this limitation we used a 12-month latency period.
Similarly, it would have been important to access the type of treatment gMALT patients underwent since there is a previous study that showed that patients treated with radiotherapy and/or chemotherapy had an increased risk of GC.35 However, in the last 15 years the majority of gMALT patients have been treated almost exclusively by HP eradication with antibiotics.
In conclusion, although HP infection is decreasing in prevalence with high response to eradication treatment, this bacterium is associated with higher risk for gMALT and GC. Currently there is debate regarding the optimal endoscopic follow-up in this setting. We have shown that the risk is higher for those aged 45–64 and that it peaks at five years after gMALT diagnosis but is still increased afterward. Our findings reinforce the necessity of not only short- but also long-term follow-up after the diagnosis of gMALT, with meticulous endoscopic and histological re-evaluations. In the future, guidelines should take into account not only the risk of gMALT lymphoma relapse/non-response but also the risk of GC. A large prospective study of patients with gMALT is essential to evaluate patients at high risk of developing GC, and define the optimal follow-up timing.
Declaration of conflicting interests
None declared.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Note
Patients were not required to give informed consent for this study because the analysis used anonymous registry data.
References
- 1.Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin 2005; 55: 74–108. [DOI] [PubMed] [Google Scholar]
- 2.Nakamura S, Aoyagi K, Iwanaga S, et al. Synchronous and metachronous primary gastric lymphoma and adenocarcinoma: A clinicopathological study of 12 patients. Cancer 1997; 79: 1077–1085. [PubMed] [Google Scholar]
- 3.Fox JG, Wang TC. Inflammation, atrophy, and gastric cancer. J Clin Invest 2007; 117: 60–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wündisch T, Thiede C, Morgner A, et al. Long-term follow-up of gastric MALT lymphoma after Helicobacter pylori eradication. J Clin Oncol 2005; 23: 8018–8024. [DOI] [PubMed] [Google Scholar]
- 5.Capelle LG, de Vries AC, Looman CW, et al. Gastric MALT lymphoma: Epidemiology and high adenocarcinoma risk in a nation-wide study. Eur J Cancer 2008; 44: 2470–2476. [DOI] [PubMed] [Google Scholar]
- 6.Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: Rationale and important changes. Blood 2009; 114: 937–951. [DOI] [PubMed] [Google Scholar]
- 7.Correa P. Human gastric carcinogenesis: A multistep and multifactorial process—First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res 1992; 52: 6735–6740. [PubMed] [Google Scholar]
- 8.Farinha P, Gascoyne RD. Helicobacter pylori and MALT lymphoma. Gastroenterology 2005; 128: 1579–1605. [DOI] [PubMed] [Google Scholar]
- 9.Isaacson P, Wright DH. Malignant lymphoma of mucosa-associated lymphoid tissue. A distinctive type of B-cell lymphoma. Cancer 1983; 52: 1410–1416. [DOI] [PubMed] [Google Scholar]
- 10.Wotherspoon AC, Ortiz-Hidalgo C, Falzon MR, et al. Helicobacter pylori-associated gastritis and primary B-cell gastric lymphoma. Lancet 1991; 338: 1175–1176. [DOI] [PubMed] [Google Scholar]
- 11.de Vries AC, Meijer GA, Looman CW, et al. Epidemiological trends of pre-malignant gastric lesions: A long-term nationwide study in the Netherlands. Gut 2007; 56: 1665–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Post PN, Kuipers EJ, Meijer GA. Declining incidence of peptic ulcer but not of its complications: A nation-wide study in The Netherlands. Aliment Pharmacol Ther 2006; 23: 1587–1593. [DOI] [PubMed] [Google Scholar]
- 13.Fischbach W, Goebeler ME, Ruskone-Fourmestraux A, et al. Most patients with minimal histological residuals of gastric MALT lymphoma after successful eradication of Helicobacter pylori can be managed safely by a watch and wait strategy: Experience from a large international series. Gut 2007; 56: 1685–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doglioni C, Wotherspoon AC, Moschini A, et al. High incidence of primary gastric lymphoma in northeastern Italy. Lancet 1992; 339: 834–835. [DOI] [PubMed] [Google Scholar]
- 15.Ullrich A, Fischbach W, Blettner M. Incidence of gastric B-cell lymphomas: A population-based study in Germany. Ann Oncol 2002; 13: 1120–1127. [DOI] [PubMed] [Google Scholar]
- 16.Morgner A, Miehlke S, Stolte M, et al. Development of early gastric cancer 4 and 5 years after complete remission of Helicobacter pylori associated gastric low grade marginal zone B cell lymphoma of MALT type. World J Gastroenterol 2001; 7: 248–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamaloglu E, Topaloglu S, Ozdemir A, et al. Synchronous and metachronous occurrence of gastric adenocarcinoma and gastric lymphoma: A review of the literature. World J Gastroenterol 2006; 12: 3564–3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goteri G, Ranaldi R, Rezai B, et al. Synchronous mucosa-associated lymphoid tissue lymphoma and adenocarcinoma of the stomach. Am J Surg Pathol 1997; 21: 505–509. [DOI] [PubMed] [Google Scholar]
- 19.Arista-Nasr J, Jiménez-Rosas F, Uribe-Uribe N, et al. Pathological disorders of the gastric mucosa surrounding carcinomas and primary lymphomas. Am J Gastroenterol 2001; 96: 1746–1750. [DOI] [PubMed] [Google Scholar]
- 20.Bayerdörffer E, Miehlke S, Neubauer A, et al. Gastric MALT-lymphoma and Helicobacter pylori infection. Aliment Pharmacol Ther 1997; 11(Suppl 1): 89–94. [DOI] [PubMed] [Google Scholar]
- 21.Bayerdörffer E, Neubauer A, Rudolph B, et al. Regression of primary gastric lymphoma of mucosa-associated lymphoid tissue type after cure of Helicobacter pylori infection. MALT Lymphoma Study Group. Lancet 1995; 345: 1591–1594. [DOI] [PubMed] [Google Scholar]
- 22.Au WY, Gascoyne RD, Le N, et al. Incidence of second neoplasms in patients with MALT lymphoma: No increase in risk above the background population. Ann Oncol 1999; 10: 317–321. [DOI] [PubMed] [Google Scholar]
- 23.Zelenetz AD, Gordon LI, Wierda WG, et al. Non-Hodgkin’s lymphomas, version 4.2014. J Natl Compr Canc Netw 2014; 12: 1282–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chan AO, Chu KM, Yuen ST, et al. Synchronous gastric adenocarcinoma and mucosa-associated lymphoid tissue lymphoma in association with Helicobacter pylori infection: Comparing reported cases between the East and West. Am J Gastroenterol 2001; 96: 1922–1924. [DOI] [PubMed] [Google Scholar]
- 25.Gisbert JP, Calvet X. Review article: Common misconceptions in the management of Helicobacter pylori-associated gastric MALT-lymphoma. Aliment Pharmacol Ther 2011; 34: 1047–1062. [DOI] [PubMed] [Google Scholar]
- 26.Saito M, Suzuki K, Maeda T, et al. The accumulation of DNA demethylation in Sat alpha in normal gastric tissues with Helicobacter pylori infection renders susceptibility to gastric cancer in some individuals. Oncol Rep 2012; 27: 1717–1725. [DOI] [PubMed] [Google Scholar]
- 27.Isaacson PG, Du MQ. MALT lymphoma: From morphology to molecules. Nat Rev Cancer 2004; 4: 644–653. [DOI] [PubMed] [Google Scholar]
- 28.Copie-Bergman C, Locher C, Levy M, et al. Metachronous gastric MALT lymphoma and early gastric cancer: Is residual lymphoma a risk factor for the development of gastric carcinoma? Ann Oncol 2005; 16: 1232–1236. [DOI] [PubMed] [Google Scholar]
- 29.Ioachim HL, Hajdu C, Giancotti FR, et al. Lymphoid proliferations and lymphomas associated with gastric metaplasia, dysplasia, and carcinoma. Hum Pathol 1999; 30: 833–842. [DOI] [PubMed] [Google Scholar]
- 30.Wong BC, Lam SK, Wong WM, et al. Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: A randomized controlled trial. JAMA 2004; 291: 187–194. [DOI] [PubMed] [Google Scholar]
- 31.Zucca E, Copie-Bergman C, Ricardi U, et al. Gastric marginal zone lymphoma of MALT type: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013; 24(Suppl 6): vi144–vi148. [DOI] [PubMed] [Google Scholar]
- 32.ASGE Standards of Practice Committee, Evans JA, Chandrasekhara V, et al. The role of endoscopy in the management of premalignant and malignant conditions of the stomach. Gastrointest Endosc 2015; 82: 1–8. [DOI] [PubMed] [Google Scholar]
- 33.de Vries AC, van Grieken NC, Looman CW, et al. Gastric cancer risk in patients with premalignant gastric lesions: A nationwide cohort study in the Netherlands. Gastroenterology 2008; 134: 945–952. [DOI] [PubMed] [Google Scholar]
- 34.Huang JQ, Zheng GF, Sumanac K, et al. Meta-analysis of the relationship between cagA seropositivity and gastric cancer. Gastroenterology 2003; 125: 1636–1644. [DOI] [PubMed] [Google Scholar]
- 35.Zauber NP, Berman EL. Synchronous and metachronous primary gastric lymphoma and adenocarcinoma: A clinicopathologic study of 12 patients. Cancer 1998; 82: 226–227. [DOI] [PubMed] [Google Scholar]