Abstract
Background
Discrepancies are often noted between management of perihilar cholangiocarcinoma (PHC) in regional hospitals and the eventual treatment plan in specialized centers.
Objective
The objective of this article is to evaluate whether regional centers adhere to guideline recommendations following implementation in 2013.
Methods
Data were analyzed from all consecutive patients with suspected PHC referred to our academic center between June 2013 and December 2015. Frequency and quality of biliary drainage and imaging at referring centers were assessed as well as the impact of inadequate initial drainage.
Results
Biliary drainage was attempted at regional centers in 83 of 158 patients (52.5%), with a technical and therapeutic success rate of 79.5% and 50%, respectively, and a complication rate of 45.8%. The computed tomography protocol was not in accordance with guidelines in 52.8% of referrals. In 45 patients (54.2%) who underwent drainage in regional centers, additional drainage procedures were required after referral. Initial inadequate biliary drainage at a regional center was significantly associated with more procedures and a prolonged waiting time until surgery. A trend toward more drainage-related complications was observed among patients with inadequate initial drainage (54.7% vs. 39.0%, p = 0.061).
Conclusion
Despite available guidelines, suboptimal management of PHC persists in many regional centers and affects eventual treatment strategies.
Keywords: Perihilar cholangiocarcinoma, guidelines, compliance, biliary drainage, management
Introduction
Perihilar cholangiocarcinoma (PHC) is a rare tumor originating in the bile ducts at the liver hilum. It is recognized as one of the most complex gastrointestinal malignancies because of pitfalls in diagnosis and the extensive preoperative optimization that is required in patients with potentially resectable tumors.1 Most patients with malignant obstructive jaundice require biliary drainage to relieve symptoms, which is attempted endoscopically at the referring center in the majority of cases.2,3 However, endoscopic biliary drainage (EBD) in malignant hilar strictures is considered a difficult procedure that requires considerable skill and experience.4 Moreover, additional percutaneous transhepatic biliary drainage (PTBD) is often required to obtain adequate drainage or to treat drainage-induced cholangitis.3 Preferably, biliary drainage is performed after adequate radiologic staging and resectability assessment with contrast-enhanced computed tomography (CT) and/or magnetic resonance imaging (MRI), since plastic or metal biliary stents may cause artifacts and periductal inflammation, thereby affecting evaluation of both bile duct and vascular involvement.5,6
Importantly, success of biliary drainage has been associated with outcomes in PHC. Initial failure of adequate palliative drainage in patients with unresectable tumors has been shown to result in lower survival.7 Furthermore, preoperative drainage-induced cholangitis is a major concern as it increases postoperative mortality risk more than three-fold.8–10 To minimize the complication risk in potentially resectable tumors, drainage of only the future liver remnant (FLR) segments is advocated, whereas for selected patients with a large FLR volume the risk of drainage-induced cholangitis and related increase in mortality may even outweigh the benefits of biliary decompression.10,11
Given the complex treatment strategy in PHC and associated risks of inadequate work-up, recent guidelines, including the European Society of Gastrointestinal Endoscopy (ESGE) and American Society for Gastrointestinal Endoscopy (ASGE) clinical guideline, recommend that resectability assessment, biliary drainage and surgical resection should be performed in high-volume centers, specializing both in EBD and PTBD.12–15 In 2013, a national guideline was developed in the Netherlands that specifically recommends not to attempt biliary drainage at regional centers that have limited expertise with both drainage techniques.16 The aim of this study was to evaluate whether regional centers adhere to these recommendations following implementation in 2013.
Materials and methods
Study population
All consecutive patients with suspected PHC who were referred to the Academic Medical Center (AMC) in Amsterdam, the Netherlands, between June 2013 and December 2015 following introduction of the national guideline in May 2013, were selected from a prospectively maintained database. PHC was defined as a tumor mass or seemingly malignant stricture at or near the biliary confluence, arising between the origin of the cystic duct and the segmental bile ducts.17 Within the Netherlands, the AMC is considered the largest tertiary referral center for the management of PHC. A waiver was granted from the institutional review board for approval of this retrospective study.
All patients and radiological imaging were discussed in a multidisciplinary, hepato-pancreato-biliary team meeting (MDT), which included surgeons, gastroenterologists, radiologists, pathologists and medical oncologists. Additional imaging, usually with CT, was indicated when the initial CT scan did not include adequate contrast-enhanced series (according to guideline) enabling assessment of vascular tumor involvement. Patients with potentially resectable tumors underwent a further preoperative work-up in our center, whereas patients with unresectable disease either underwent (additional) palliative biliary drainage with stenting and chemotherapy in our center or in the referring hospital according to patients’ preferences. Additional biliary drainage in our center was indicated when prior endoscopic or percutaneous drainage at the regional hospital had failed to obtain adequate drainage with ongoing cholestasis in the FLR and/or elevated total bilirubin level. In unresectable patients, drainage of more than 50% liver volume was ensured.13 The optimal method for additional drainage procedures was decided at the MDT meeting, based on the cause of failure of drainage and individual biliary anatomy.
Study endpoints
The primary endpoint of this study was the compliance of referring centers to guideline recommendations, specifically with regard to imaging and biliary drainage. Guidelines recommend a multiphase contrast-enhanced CT including a late arterial and portal-venous phase.12,14,16 An MRI (with magnetic resonance cholangiopancreatography (MRCP)) may be additionally performed. We analyzed whether biliary drainage was performed prior to referral and the outcome of biliary drainage was reported in terms of technical and therapeutic success. Technical success was defined as successful bile duct cannulation with stent/catheter placement providing internal biliary drainage.3 Therapeutic success of the initial procedure was defined as a more than 50% decrease in total bilirubin level within two weeks18,19 or, when laboratory values were missing, as a decrease in jaundice and cholestasis. A decrease in cholestasis was defined by diminished bile duct dilatation in the drained liver segments on subsequent imaging. The need for additional imaging and/or biliary drainage at our center was also assessed.
Secondary outcomes were the total number of drainage procedures required, the time interval between diagnosis or suspicion on PHC (on imaging or at endoscopic retrograde cholangiopancreatography (ERCP)) and surgery, and drainage-related complications in the referral center and in our center. Complications of biliary drainage in our center were scored until 30 days after the procedure in palliative patients and until surgery in potentially resectable patients. In particular, the incidences of cholangitis, pancreatitis, bleeding, perforation and stent dysfunction were assessed. Stent dysfunction was defined as rising bilirubin level after initial therapeutic success, without signs of cholangitis, requiring new bile duct cannulation. Lastly, the impact of inadequate initial biliary drainage (technical failure or therapeutic failure) on the incidence of complications, total number of required drainage procedures and interval until surgery was assessed.
Data regarding imaging, indications for biliary drainage, technical aspects of biliary drainage at the regional center, laboratory values and complications were retrieved from referral letters, reports of CT and MRI, and from ERCP or PTBD reports and images, which were all accessible from the electronic patient files.
Statistical analysis
Continuous variables are presented as mean ± standard deviation (SD) or median with interquartile range (IQR) for variables that did not follow a normal distribution. Categorical variables are expressed as counts with percentages. Univariable analysis of the impact of inadequate initial drainage on the incidence of complications and time interval until surgery was assessed using Pearson’s chi-squared for categorical variables and using Mann-Whitney U or unpaired t-test for continuous variables. Analyses were performed using IBM SPSS Statistics for Windows, version 23.0 (IBM Corp., Armonk, NY, USA). Two-tailed p values of < 0.05 were considered to indicate statistical significance.
Results
Study population
A total of 170 patients with suspected PHC were discussed at our MDT within the study period. Twelve patients were excluded from further analyses as seven patients were referred for a second opinion after evaluation in other tertiary centers and five patients were primarily seen in our center. Characteristics of the remaining 158 patients, all referred by clinicians from other centers, are listed in Table 1. Patients were referred from 44 regional centers (median four patients per center (IQR: 1–5)). Patients had a median age of 66 years, included 104 males (65.8%) and presented with jaundice in the majority of cases (75.9%). Median time interval between first suspicion of PHC at the regional center and referral to our MDT was 11 days (IQR: 6–20). In 39 of 158 cases (24.7%), the time to referral was 21 days or more.
Table 1.
Baseline characteristics of study cohort.
| Total N = 158 | |
|---|---|
| Male gender, n (%) | 104 (65.8) |
| Age, years, median (IQR) | 66 (62–75) |
| Jaundice at initial presentation, n (%) | 120 (75.9) |
| Total bilirubin at initial presentation, mg/dl, median (IQR) | 118 (35–223) |
| Total bilirubin at tertiary MDT, mg/dl, median (IQR) | 138 (20–303) |
| CA 19-9, kU/l, median (IQR) | 289 (43–1270) |
| IgG4, g/l, median (IQR) | 0.60 (0.32–1.19) |
| Diagnosis tertiary MDT, n (%) | |
| Perihilar cholangiocarcinoma (unchanged) | 139 (88.0) |
| Benign | 7 (4.4) |
| Gallbladder carcinoma | 8 (5.1) |
| Distal cholangiocarcinoma | 2 (1.3) |
| Choledochal cyst with malign degeneration | 1 (0.6) |
| Cystadenoma with malign degeneration | 1 (0.6) |
| Resectability assessment tertiary MDT, n (%) | |
| Unresectable | 55 (34.8) |
| Potentially resectable | 96 (60.8) |
| Benign | 7 (4.4) |
IQR: interquartile range; MDT: multidisciplinary team meeting; CA: carbohydrate antigen; IgG4: immunoglobulin G4.
Diagnosis and resectability assessment
The referral diagnosis was changed by the tertiary MDT for 19 patients (12.7%); 12 patients had other malignant tumors and seven patients were diagnosed with benign disease (bile duct stones or fibrosing/sclerosing inflammation). Judgment of resectability based on available imaging was made in 21 of 158 (13.3%) patients at the regional centers. Of 15 patients who were deemed unresectable before referral, five patients (33.3%) were considered to have potentially resectable disease after revision in our MDT. At the regional hospital, these patients were believed to have unresectable tumors because of bilateral segmental biliary involvement (Bismuth-Corlette type 4) or large tumor size. Two of these patients eventually underwent resection. Of six patients who were judged to have resectable PHC at the regional hospital, one was considered to have unresectable disease upon staging in our tertiary MDT and one patient was diagnosed with a benign inflammatory biliary stricture. Ultimately, according to the MDT, 55 patients (34.8%) had unresectable tumors, whereas 96 patients (60.8%) were considered to have potentially resectable PHC (Table 1).
Imaging
Table 2 provides an overview of imaging techniques and CT scanning protocols at the regional centers. A CT scan was performed in 144 patients (91.1%), but a multiphase contrast-enhanced CT protocol (according to guideline) was used in only 76 cases (52.8%). An MRI/MRCP was performed in 69 centers (43.7%), mostly in addition to CT. The CT and/or MRI reports included information on biliary tumor extension in only 42.4%, vascular involvement in only 29.7% and presence or absence of metastases in 78.5% of cases. Additional CT imaging to adequately assess tumor staging by the MDT radiologist in our center was required in 64 patients (40.5%) at our center.
Table 2.
Imaging at regional centers.
| Total N = 158 | |
|---|---|
| Imaging technique, n (%) | |
| US + CT | 89 (56.3) |
| US + MRI/MRCP | 14 (8.9) |
| CT + MRI/MRCP | 19 (12.0) |
| US + CT + MRI/MRCP | 36 (22.8) |
| CT scanning protocol (N = 144), n (%) | |
| Routine abdomen (non-enhanced or portal venous phase only) | 68 (47.2) |
| Pancreatic (portal venous and arterial phase) | 47 (32.6) |
| Four-phase (pre-contrast, portal venous and arterial, delayed phase) | 29 (20.1) |
| CT/MRI report information, n (%) | |
| Biliary extension (e.g. Bismuth-Corlette classification, segmental involvement) | 67 (42.4) |
| Vascular involvement | 47 (29.7) |
| Metastases | 124 (78.5) |
US: ultrasound; CT: computed tomography; MRI: magnetic resonance imaging; MRCP: magnetic resonance cholangiopancreatography.
Biliary drainage at regional centers
Of the 158 patients, 83 (52.5%) had undergone a drainage attempt at the regional center before referral. Indications for biliary drainage at the regional center are listed in Table 3. Most patients had suspicion of a malignant hilar obstruction (based on imaging) prior to the drainage procedure. ERCP was performed in all but two patients who underwent PTBD. Technical success after the initial procedure was achieved in 66 (79.5%) patients and eventually in 69 (83.1%) patients after additional procedures at the regional center. Therapeutic success was achieved in 37 of 74 (50%) jaundiced patients.
Table 3.
Technical aspects of biliary drainage in regional centers.
| Total N = 83 | |
|---|---|
| Jaundiced patient | 72 (86.7) |
| CT/MRI/MRCP prior to biliary drainage, n (%) | 70 (84.3) |
| Indications for biliary drainage, n (%) | |
| Malignant hilar obstruction | 52 (62.7) |
| Jaundice of unknown cause | 13 (15.7) |
| Cholangitis | 6 (7.2) |
| Increasing bilirubin | 5 (6.0) |
| Suspicion of bile duct stones | 4 (4.8) |
| Brush cytology | 3 (3.6) |
| Technical success, n (%) | |
| After initial procedure | 66 (79.5) |
| After additional procedures (at regional center) | 69 (83.1) |
| Therapeutic success if jaundiced (N = 74), n (%) | 37 (50.0) |
| Number of drainage procedures, median (IQR) | 1 (2) |
| >1 procedure, n (%) | 38 (45.8) |
| >2 procedures, n (%) | 10 (12.0) |
| Drainage method, n (%) | |
| ERCP only | 67 (80.7) |
| PTBD only | 2 (2.4) |
| Both | 14 (16.9) |
| Stent type (if technical success, N = 69), n (%) | |
| Plastic | 64 (92.8) |
| Metal | 5 (7.2) |
| Stent position, n (%) | |
| Left liver segments | 23 (27.7) |
| Right liver segments | 29 (34.9) |
| Left + right liver segments | 9 (10.8) |
| Common bile duct only | 7 (8.4) |
| Brush performed, n (%) | |
| No | 34 (41.0) |
| Yes (benign/inconclusive/malign) | 49 (59.0) (7/22/20) |
CT: computed tomography; MRI: magnetic resonance imaging; MRCP: magnetic resonance cholangiopancreatography; ERCP: endoscopic retrograde cholangiopancreatography; PTBD: percutaneous transhepatic biliary drainage.
More than one biliary drainage procedure was required in 38 (45.8%) patients. Additional PTBD was performed in 14 (16.9%) patients at regional centers. Drainage-related complications occurred in 38 (45.8%) patients and more than one complication was observed in 10 (12.0%) patients. Cholangitis (18.1%) and stent dysfunction (16.9%) were the most frequently observed complications (Table 4). Six patients had such a deteriorated condition due to complications that they were considered no candidates for surgery upon presentation at our center.
Table 4.
Outcomes of biliary drainage at regional and tertiary center.
| Regional center (N = 83) | Tertiary center (N = 96)a | |
|---|---|---|
| Patients with any complication, n (%) | 38 (45.8) | 39 (40.6) |
| Cholangitis | 15 (18.1) | 18 (18.8) |
| Cholecystitis | 2 (2.4) | 1 (1.0) |
| Pancreatitis | 7 (8.4) | 4 (4.2) |
| Bleeding | 4 (4.8) | 2 (2.1) |
| Perforationb | 3 (3.6) | 1 (1.0) |
| Stent dysfunction | 14 (16.9) | 13 (13.5) |
| PTBD dislocation | 0 | 4 (4.2) |
| Other | 6 (7.2) | 3 (3.1) |
| Patients with >1 complication, n (%) | 10 (12.0) | 8 (8.3) |
| Patients with >2 complications, n (%) | 1 (1.2) | 6 (6.3) |
| Additional drainage required by tertiary center, n (%) | 45 (54.2) | n/a |
| Inadequate FLR drainage | 16 (19.3) | |
| Stent dysfunction | 15 (18.1) | |
| Cholangitis | 3 (3.6) | |
| Palliative stenting | 11 (13.3) |
PTBD: percutaneous transhepatic biliary drain; FLR: future liver remnant; n/a: not applicable.
This group also included patients who had undergone previous drainage at the regional center.
Perforation of duodenum (3) in regional center and perforation of bile duct (1) at tertiary center.
In 13 (15.7%) of the patients who underwent drainage, no CT or MRI was performed prior to the procedure. One of these patients presented with cholangitis, and biliary stones were suspected in four patients. Eventually, these patients were all diagnosed with PHC.
Biliary drainage at tertiary center
In 45 of the 83 patients (54.2%) who had undergone previous drainage at the regional center, additional biliary drainage was deemed necessary by the MDT at our center. Main indications were stent dysfunction or inadequate drainage of the FLR in patients with potentially resectable tumors (Table 4). In total, 96 patients, including those without a previous drainage attempt, underwent biliary drainage at our center. ERCP was chosen as the preferred method in 63 patients (65.6%) and PTBD in 33 (34.4%). Additional PTBD after ERCP was required in 17 patients (27%) to achieve complete drainage and, overall, more than one biliary drainage procedure at our center was required in 44 patients (45.8%).
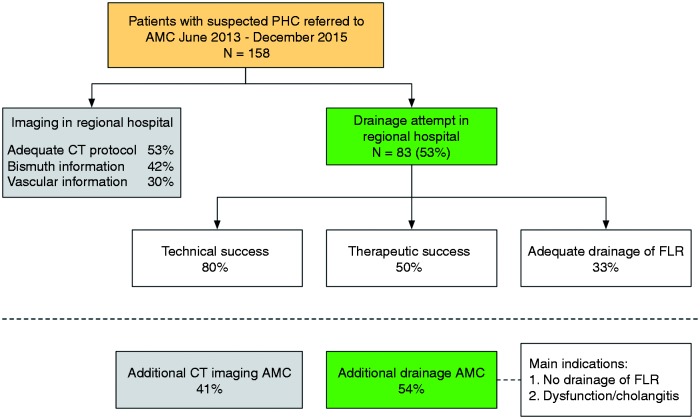
The risk of drainage-related complications was comparable at our center as 39 events (40.6%) were observed (Table 4). More than one complication occurred in eight patients (8.3%). A summarizing overview of imaging and biliary drainage at regional centers and implications for management at our center is provided in Figure 1.
Figure 1.
Overview of imaging and biliary drainage at regional centers and implications for management at a tertiary center.
PHC: perihilar cholangiocarcinoma; AMC: Academic Medical Center; CT: computed tomography; FLR: future liver remnant.
Impact of inadequate initial biliary drainage
Inadequate initial biliary drainage was observed in 53 patients (33.5%) and was associated with a higher number of total drainage procedures (p < 0.001) and nearly four-week longer interval until surgery (p = 0.006) (Table 5). There was a trend toward more drainage-related complications among patients who had undergone inadequate initial drainage (54.7% vs. 39.0%, p = 0.061).
Table 5.
Impact of inadequate initial biliary drainage.
| Adequate or no drainage (N = 105) | Inadequate drainage (N = 53) | p value | |
|---|---|---|---|
| Any drainage complication, n (%) | 41 (39.0) | 29 (54.7) | 0.061 |
| Total drainage procedures, median (IQR) | 1 (1–2) | 3 (1–2) | <0.001 |
| Interval suspicion-referral, days, median (IQR) | 9 (5–16) | 14 (8–28) | 0.002 |
| Interval suspicion-laparotomy, days, mean (sd) (N = 60)a | 81 (29) | 108 (42) | 0.006 |
IQR: interquartile range.
The need for portal vein embolization or staging laparoscopy was similar between groups.
Surgery outcomes
Eventually, 60 patients underwent laparotomy and resection was performed in 31 patients (51.7%). In three of 31 cases (9.7%), final histopathological examination revealed benign disease. Postoperative 90-day mortality was 9.7% (3/31). There were no differences in the incidence of post-resection complications between patients who had undergone initial inadequate or adequate biliary drainage.
Discussion
The present study shows that many patients with suspected PHC do not undergo the recommended work-up in regional centers prior to referral and further treatment in a tertiary center. Despite the availability of a national PHC guideline and clinical guidelines from the European and American societies for endoscopic biliary stenting, half of patients still undergo a biliary drainage attempt at non-tertiary referral centers with low success rates.
An important finding of the present audit, which was conducted following implementation of the Dutch national guideline in 2013, was that therapeutic success of drainage at regional centers was achieved in only half of patients. Although the rate of endoscopic attempts prior to referral was somewhat lower than previously reported (53% vs. 72%), the need for additional drainage at our center had not decreased (54% vs. 41%).3 The most frequent indications for additional procedures were inadequate drainage of the FLR in patients with potentially resectable tumors and stent dysfunction. Furthermore, some patients with unresectable PHC also required adequate palliative stenting after initial drainage. One of the reasons for referring physicians to attempt drainage in their own center may be a lack of awareness of the adverse outcomes after failed or complicated biliary drainage. In the present study, patients who had undergone inadequate initial biliary drainage in the regional hospital required more procedures and had a longer time interval until surgery. Furthermore, a trend toward more drainage-related complications was observed in these patients. Previous studies have also shown a clear correlation between drainage-induced cholangitis and mortality after resection and between drainage failure and survival in the palliative setting.7,10,20 The overall relatively high therapeutic failure rate and inadequate drainage of the FLR in the study period resulted in exposure of patients to multiple drainage procedures and, consequently, to unnecessary risks of complications. The observation that the complication risks of biliary drainage were comparably as high in the regional hospitals as in experienced hands in our center, underscores the complex nature of drainage in hilar obstructions. Considering these high failure rates and complication risks, this demanding procedure should be performed in specialized centers with extensive expertise both in endoscopic and percutaneous biliary drainage techniques, especially as PTBD is often needed to salvage segmental cholangitis.
Tumor staging is of the utmost importance in order to decide which liver segments should be drained in suspected PHC.1,13 The surgical plan, which depends on bile duct and vascular involvement on imaging, often determines the desired drainage strategy. However, radiological staging of PHC is complex, often requires both CT and MR imaging and asks for assessment by specialized abdominal radiologists and hepatobiliary surgeons at tertiary centers. Remarkably, we observed that many referring physicians relied on incomplete imaging reports when deciding to attempt endoscopic stenting. These reports lacked sufficient information on tumor characteristics such as proximal bile duct involvement (Bismuth-Corlette classification). Inadequate staging in PHC is likely to lead to an inadequate biliary drainage strategy. Patients with unresectable tumors may benefit from direct placement of self-expandable metal stents instead of having to undergo prior drainage with plastic stents, as metal stents are associated with higher success rates and longer survival in palliative drainage.21,22
CT scans without multiphase contrast series enabling both assessment of the arterial and portal venous vasculature (as recommended by guidelines) were performed in only half of the cases. Additional imaging at our center was often required, increasing costs as well as work-up time. In addition to this, resectability assessment often is hampered by the presence of any biliary stents that have been introduced before performing adequate imaging. Because of the many pitfalls encountered in assessing resectability, this judgment should be made by a dedicated MDT consisting of specialists who frequently see and treat these patients. Given the rarity of the disease, this recommendation is widely accepted in our country. Judgment of resectability before referral was made in only a few patients in the present study.
Among all ERCP procedures, biliary stenting in malignant hilar strictures is recognized as an advanced procedure (Grade 2 of the Schutz classification) that is associated with increased risk of technical failure.4,23 In the present study, we observed a 20% failure rate of the initial endoscopic attempt at regional centers. These patients require additional endoscopic attempts or even PTBD, but interventional radiologists with expertise in this field may not be available in every hospital. It can be difficult to selectively cannulate the biliary tree through the endoscopic approach, especially in Bismuth type 3 and 4 tumors or when drainage of the left liver segments is preferred.3 The sharper angle of the left hepatic duct makes selective cannulation using the endoscopic guidewire technically demanding. We observed that the majority of stents were positioned in the right liver segments.
The usual route of referral for patients with suspicion of biliary cancer in most countries starts at the general practitioner (primary care). Patients often present with silent jaundice and are usually referred to a regional hospital (secondary care) for diagnostic studies and consultation with a gastroenterologist. When PHC is suspected, patients are referred for assessment of resectability at an hepato-pancreato-biliary unit within a tertiary hospital (tertiary care). The time from clinical symptoms until eventual treatment may thus take long. However, in a previous study, no correlation was found between treatment time and resectability, occurrence of metastases, tumor stage, or survival after resection of PHC.24 Although delay in referral and work-up for surgery may not affect oncological outcomes, it is likely that drainage-related complications, especially cholangitis, increase the operative risk.8–10 These complications should thus be prevented by careful evaluation of the drainage strategy within a dedicated MDT.
To improve the observed discrepancy between the diagnostic work-up in regional centers and eventual treatment strategies in tertiary centers, a national multidisciplinary clinical pathway for patients with suspected PHC was recently developed in our country in collaboration with several tertiary hospitals and the Dutch Association of Comprehensive Cancer Centers. This clinical pathway further elaborates on existing guidelines and provides recommendations for early communication between physicians (prior to biliary drainage) and referral of patients to a specialized center at an early stage. Implementation will start at the end of 2016.
There are several limitations to our study. Firstly, although we included all consecutive patients with suspected PHC who were referred to our MDT within a period of two and a half years, the collected study cohort was relatively small. However, the described cohort includes patients both with unresectable and resectable tumors, and these groups are often analyzed separately in literature. Secondly, as some patients may have been managed at their local hospital without consulting a tertiary center, we are unaware of the adherence to current guidelines in those cases. In the present study, selection of difficult cases may have occurred as physicians may be more inclined to refer patients in the event of technical or therapeutic failure of drainage. Another suggestion for the selection of complex cases in this study may be the high rate of drainage-related complications observed both in the regional hospitals and tertiary centers. Furthermore, as this study was performed in only one academic center, our conclusions on the adequacy of imaging and biliary drainage in regional centers may not apply to every hospital in our country. A national audit may therefore be desirable. Thirdly, the retrospective design may have introduced reporting bias, as some outcome measures relied on the information available from reports by the referring physicians. Lastly, the recent study period did not permit sufficient follow-up time and subsequent survival analysis, although this was not the aim of the study.
In conclusion, despite available guidelines, suboptimal management of PHC persists in many regional centers and affects eventual treatment strategies. Implementation of a national multidisciplinary clinical pathway is therefore of great importance as it can potentially lead to further optimization of care in these patients.
Declaration of conflicting interests
None declared.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Notes
This study was previously presented at the 12th World Congress of the International Hepato-Pancreato-Biliary Association, April 20–23, 2016, São Paulo, Brazil
References
- 1.Mansour JC, Aloia TA, Crane CH, et al. Hilar cholangiocarcinoma: Expert consensus statement. HPB (Oxford) 2015; 17: 691–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kloek JJ, van der Gaag NA, Aziz Y, et al. Endoscopic and percutaneous preoperative biliary drainage in patients with suspected hilar cholangiocarcinoma. J Gastrointest Surg 2010; 14: 119–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wiggers JK, Groot Koerkamp B, Coelen RJ, et al. Preoperative biliary drainage in perihilar cholangiocarcinoma: Identifying patients who require percutaneous drainage after failed endoscopic drainage. Endoscopy 2015; 47: 1124–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schutz SM, Abbott RM. Grading ERCPs by degree of difficulty: A new concept to produce more meaningful outcome data. Gastrointest Endosc 2000; 51: 535–539. [DOI] [PubMed] [Google Scholar]
- 5.Masselli G, Manfredi R, Vecchioli A, et al. MR imaging and MR cholangiopancreatography in the preoperative evaluation of hilar cholangiocarcinoma: Correlation with surgical and pathologic findings. Eur Radiol 2008; 18: 2213–2221. [DOI] [PubMed] [Google Scholar]
- 6.Choi JY, Kim MJ, Lee JM, et al. Hilar cholangiocarcinoma: Role of preoperative imaging with sonography, MDCT, MRI, and direct cholangiography. AJR Am J Roentgenol 2008; 191: 1448–1457. [DOI] [PubMed] [Google Scholar]
- 7.Paik WH, Park YS, Hwang JH, et al. Palliative treatment with self-expandable metallic stents in patients with advanced type III or IV hilar cholangiocarcinoma: A percutaneous versus endoscopic approach. Gastrointest Endosc 2009; 69: 55–62. [DOI] [PubMed] [Google Scholar]
- 8.Sakata J, Shirai Y, Tsuchiya Y, et al. Preoperative cholangitis independently increases in-hospital mortality after combined major hepatic and bile duct resection for hilar cholangiocarcinoma. Langenbecks Arch Surg 2009; 394: 1065–1072. [DOI] [PubMed] [Google Scholar]
- 9.Nagino M, Ebata T, Yokoyama Y, et al. Evolution of surgical treatment for perihilar cholangiocarcinoma: A single-center 34-year review of 574 consecutive resections. Ann Surg 2013; 258: 129–140. [DOI] [PubMed] [Google Scholar]
- 10.Wiggers JK, Groot Koerkamp B, Cieslak KP, et al. Postoperative mortality after liver resection for perihilar cholangiocarcinoma: Development of a risk score and importance of biliary drainage of the future liver remnant. J Am Coll Surg 2016; 223: 321–331.e321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farges O, Regimbeau JM, Fuks D, et al. Multicentre European study of preoperative biliary drainage for hilar cholangiocarcinoma. Br J Surg 2013; 100: 274–283. [DOI] [PubMed] [Google Scholar]
- 12.Khan SA, Davidson BR, Goldin RD, et al. Guidelines for the diagnosis and treatment of cholangiocarcinoma: An update. Gut 2012; 61: 1657–1669. [DOI] [PubMed] [Google Scholar]
- 13.Dumonceau JM, Tringali A, Blero D, et al. Biliary stenting: Indications, choice of stents and results: European Society of Gastrointestinal Endoscopy (ESGE) clinical guideline. Endoscopy 2012; 44: 277–298. [DOI] [PubMed] [Google Scholar]
- 14.Rerknimitr R, Angsuwatcharakon P, Ratanachu-ek T, et al. Asia-Pacific consensus recommendations for endoscopic and interventional management of hilar cholangiocarcinoma. J Gastroenterol Hepatol 2013; 28: 593–607. [DOI] [PubMed] [Google Scholar]
- 15.Anderson MA, Appalaneni V, Ben-Menachem T, et al. The role of endoscopy in the evaluation and treatment of patients with biliary neoplasia. Gastrointest Endosc 2013; 77: 167–174. [DOI] [PubMed] [Google Scholar]
- 16.Richtlijn Galweg- en Galblaascarcinoom. IKNL. http://www.oncoline.nl/galweg-en-galblaascarcinoom (2013, accessed 1 January 2016).
- 17.Edge SB, Byrd DR, Compton CC, et al. (eds) AJCC cancer staging manual. 7th ed. New York, NY: Springer, 2010.
- 18.van der Gaag NA, Rauws EA, van Eijck CH, et al. Preoperative biliary drainage for cancer of the head of the pancreas. N Engl J Med 2010; 362: 129–137. [DOI] [PubMed] [Google Scholar]
- 19.Khashab MA, Van der Merwe S, Kunda R, et al. Prospective international multicenter study on endoscopic ultrasound-guided biliary drainage for patients with malignant distal biliary obstruction after failed endoscopic retrograde cholangiopancreatography. Endosc Int Open 2016; 4: E487–E496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ribero D, Zimmitti G, Aloia TA, et al. Preoperative cholangitis and future liver remnant volume determine the risk of liver failure in patients undergoing resection for hilar cholangiocarcinoma. J Am Coll Surg 2016; 223: 87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sangchan A, Kongkasame W, Pugkhem A, et al. Efficacy of metal and plastic stents in unresectable complex hilar cholangiocarcinoma: A randomized controlled trial. Gastrointest Endosc 2012; 76: 93–99. [DOI] [PubMed] [Google Scholar]
- 22.Sawas T, Al Halabi S, Parsi MA, et al. Self-expandable metal stents versus plastic stents for malignant biliary obstruction: A meta-analysis. Gastrointest Endosc 2015; 82: 256–267.e257. [DOI] [PubMed] [Google Scholar]
- 23.Ekkelenkamp VE, de Man RA, Ter Borg F, et al. Prospective evaluation of ERCP performance: Results of a nationwide quality registry. Endoscopy 2015; 47: 503–507. [DOI] [PubMed] [Google Scholar]
- 24.Ruys AT, Heuts SG, Rauws EA, et al. Delay in surgical treatment of patients with hilar cholangiocarcinoma: Does time impact outcomes? HPB (Oxford) 2014; 16: 469–474. [DOI] [PMC free article] [PubMed] [Google Scholar]