Abstract
Elucidating the genetic mechanisms of adaptation to the hydrothermal vent in organisms at genomic level is significant for understanding the adaptive evolution process in the extreme environment. We performed RNA-seq on four different tissues of a vent crab species, Austinograea alayseae, producing 725,461 unigenes and 134,489 annotated genes. Genes related to sensory, circadian rhythm, hormone, hypoxia stress, metal detoxification and immunity were identified. It was noted that in the degenerated eyestalk, transcription of phototransduction related genes which are important for retinal function was greatly reduced; three crucial neuropeptide hormones, one molt-inhibiting and two crustacean hyperglycemic hormone precursors were characterized with conserved domains; hypoxia-inducible factor 1 and two novel isoforms of metallothioneins in the vent crabs were discovered. An analysis of 6,932 orthologs among three crabs A. alayseae, Portunus trituberculutus and Eriocheir sinensis revealed 19 positive selected genes (PSGs). Most of the PSGs were involved in immune responses, such as crustins and anti-lipopolysaccharide factor, suggesting their function in the adaptation to environment. The characterization of the first vent crab transcriptome provides abundant resources for genetic and evolutionary studies of this species, and paves the way for further investigation of vent adaptation process in crabs.
Introduction
Deep-sea hydrothermal vents and their resident animals were first discovered in the 1970's after an extensive exploration along the Galápagos Rift [1–3]. Vents are well known for their challenging environment, such as low oxygen, lack of light, high hydrostatic pressure and thermal gradient, high levels of sulfide and heavy metals. One additional surprise of the vents is that they are unique ecosystems based on microbial chemoautotrophic production [4]. However, these seemingly toxic hydrothermal fluids directly support productive biological communities with a high proportion of endemic species [5,6]. Therefore, the organisms living in the hydrothermal vents have developed well adaptive mechanisms to survive in this extreme habitat.
Many invertebrates dominate the hydrothermal vents and decapod crustaceans occupy approximately 10% of all taxa identified from these sites [7,8]. Among them, six genera (Allograea, Austinograea, Bythograea, Cyanagraea, Gandalfus and Segonzacia) of brachyuran crabs have been found and all belong to a single family, Bythograeidae Williams, 1980. They are the most common and abundant species, and predators at the top of food chain in the vent ecosystem [9]. As living in the extreme environment, the vent crabs are characterized with specific morphological traits such as whole white body, and reduced eyestalks with vestigial cornea. Therefore, exploring the gene profile and determination of the selective force that drive molecular evolution of the vent crabs are fundamental to understand the genetic basis of the adaptation to the hydrothermal vent environment.
Most studies for the vent crabs are mainly focused on the complete mitochondrial genome sequences [10,11] and the molecular systematics of the vent species [9,12,13]. There is currently limited genomic resource for the vent crabs as well as other decapods, although it is significant to reveal the mechanism of adaptation to the vent. The cDNA sequences of metallothionein (MT) genes have been obtained and characterized in three species of the Bythograeidae, and in spite of the unique environmental conditions, no specific amino acid residue for the MT of the vent crabs has been found [14]. By the subtractive suppression hybridization experiments in G. yunohana and P. trituberculatus, 51 transcripts with annotations have been identified to be differentially expressed, and theoredoxin (Trx) and heat shock protein 90 (Hsp90) have been primarily analyzed [15]. However, the information at the transcriptome level of the vent crabs is far from sufficient.
The development of next generation sequencing (NGS) technology and bioinformatics provides a global perspective on taxonomic profiling of genes expressed under the influence of different environmental conditions where these organisms live [16], and the coordinated transcriptional changes in multiple genes that involve in various biological pathways [17]. For instance, recent high-throughput transcriptomic studies have been performed in the vent and cold seep mussels (Bathymodiolus azoricus and B. platifrons), respectively [18,19]. An extensive collection of transcripts have been obtained, and many genes related to innate immunity, heavy metal detoxification and sulfide metabolism have been identified. It can serve as basis for future molecular studies on extreme environment adaptation, including the host-symbiont interaction mechanism in mussels. To date, no such gene profile in a large scale has been explored in any crustaceans living in deep-sea hydrothermal vents.
The objective of this study is to generate a comprehensive transcriptome database (including eyestalk, gill, hepatopancreas and muscle) for the hydrothermal vent crab, A. alayseae Guinot, 1990 and clarify the intertwined gene regulatory mechanism for the vent adaptation preliminarily. Candidate genes that may facilitate inhabiting in the vent environment have been identified. By orthologous gene comparison with two shallow-water crab species (P. trituberculutus and E. sinensis), genes that might have experienced positive selection among the crabs have been explored. This is the possible inner driving force for the vent crab adaptive evolution.
Materials and methods
Ethics statement
The experiments were conducted in strict accordance with the guidelines of the Institutional Animal Care and Use Committee (IACUC) of the Chinese Academy of Sciences (No. 2011–2). This study was specifically approved by the Committee on the Ethics of Animal Experiments of the Institute of Oceanology at the Chinese Academy of Sciences. All efforts were made to minimize the suffering of the crabs.
Sample collection
Two deep-sea crabs A. alayseae (one male and one female) were collected from the South China Sea (151°52′50.084″E, 3°42′47.259″S) with a depth of 1995 m and temperature of 1.01°C on June 10, 2015. Four different tissues (eyestalk, gill, hepatopancreas and muscle) from each individual were sampled in order to obtain as many expressed genes as possible. After dissection, the samples were immediately frozen in liquid nitrogen for RNA extraction.
Illumina sequencing and de novo assembly
The total RNA from the eight samples was extracted using the TRIzol kit (Invitrogen, USA) according to the manufacturer’s protocol. For each sample, 1.5 μg RNA was prepared for RNA-Seq. Eight libraries for the tissues were constructed using NEBNext® Ultra™ RNA Library Prep Kit and sequenced on an Illumina HiSeq 2500 platform following the manufacturer’s instructions (Illumina, USA) and paired-end reads were generated. All raw sequences were deposited at NCBI Short Read Archive (SRA) database (http://www.ncbi.nlm.nih.gov/Traces/sra/). Clean reads were obtained by removing reads containing adaptor, reads containing ploy-N (the ratio of ‘N’ to be more than 10%) and low quality reads (the ratio of base pairs with quality score < 5 more than 50%). Transcriptome assembly was accomplished based on clean reads using Trinity [20] with min_kmer_cov set to 2 and all other default parameters. The longest copy of redundant transcripts was regarded as a unigene.
Gene functional annotation and expression analysis
All the assembled unigenes were searched against the NCBI Nr (non-redundant protein sequences), Nt (non-redundant nucleotide) and Swiss-Prot (http://www.ebi.ac.uk/uniprot/) databases for gene annotation with an E-value cutoff of 1E-5. Genes encoding protein domains were identified by searching against Protein Family (Pfam) database (http://pfam.janelia.org/; E-value < 0.01). After that, GO (Gene Ontology; http://www.geneontology.org/; E-value < 1E-6) and KOG (euKaryotic Ortholog Group; http://www.ncbi.nlm.nih.gov/COG/; E-value < 1E-3) annotations were performed to classify the potential functions of the unigenes based on known orthologous gene products. GO enrichment analysis was implemented by the GOseq R packages, in which gene length bias was adjusted [21]. Biochemical pathway information of the unigenes was collected from KEGG (Kyoto Encyclopedia of Genes and Genomes; http://www.genome.jp/kegg/; E-value < 1E-10), and KOBAS software was used to test the statistical enrichment of unigenes in KEGG pathway [22]. To estimate gene expression levels, the clean reads of each sample were mapped to the assembled transcriptome to obtain read counts for each gene using RSEM [23]. The read counts were normalized to fragments per kilobase of exon model per million mapped reads (FPKM).
Candidate gene discovery and sequence analysis
According to the annotation mainly from NR and Swiss-Prot databases, the genes with functions we concerned were manually checked by further searching UniProt database (http://www.uniprot.org/) as well as published literatures. The searched genes were related to sensory, circadian rhythm, neuropeptide hormone, immune system, chemical stress and metal transporter. SignalP 4.1 Server (http://www.cbs.dtu.dk/services/SignalP/) was used to predict the signal peptide. The multiple sequences alignment and the neighbor-joining (NJ) phylogenetic tree were conducted with MEGA 6.0 [24] based on amino acid sequences. Functional domain analysis was performed with SMART (http://smart.embl.de/).
Orthologous genes identification and phylogenic tree construction
According to Williams, vent crabs Bythograeidae exhibit certain characters of the family Portunidae, and resemblance to the freshwater Potamidae [25]. Therefore, we also sequenced the transcriptomes of four tissues (eyestalk, gill, hepatopancreas and muscle) in two shallow water crabs, E. sinensis and P. trituberculatus (unpublished). The comparison among species was expected to reveal the genetic basis of the vent adaptation in A. alayseae. The CDS of each putative unigene was extracted according to the BLASTX results, and ESTScan software [26] was used to determine the direction of sequences that did not have align results. The resultant CDS extracted from each unigene was translated into amino acid sequences with the standard codon table. Reciprocal BLASTP was conducted for all amino acid sequences with a cut-off E-value of 1E-5. Orthologous groups were constructed with OrthoMCL v2.0.3 using default settings [27]. The one-to-one orthologous genes alignment was performed by MUSCLE based on protein sequences [28]. The phylogeny tree was constructed using PhyML [29] and visualized by MEGA6.0 [24].
Positive selected genes identification
Ka/Ks ratio was estimated with PAML [30] package using default settings. Orthologous genes with a high Ka/Ks ratio were usually supposed to be evolving under positive selection, while genes with a Ka/Ks close to zero mainly had synonymous substitutions, indicating that these genes were under purified selection and conserved [17]. In this study, we defined genes with Ka/Ks > 1 as positively selected genes (divergent genes) and genes with Ka/Ks < 0.1 as conserved genes. In addition, GO and KEGG enrichment pathway analyses were also used to determine gene functional categories of divergent and conserved genes.
Results and discussion
De novo assembly, gene annotation and expression
A total of 591,265,622 raw reads for the vent crab transcriptomes were obtained. All raw data were deposited in the NCBI Sequence Read Archive repository (accession number: SRR4416314). After filtering, 75.13 Gbp clean data were remained. Then 864,469 transcripts and 725,461 unigenes were assembled with the N50 length of 423 bp for transcripts and 506 bp for unigenes. Detailed information of the sequence data was summarized in Table 1. Compared with other transcriptomic study for vent species, the average length of the transcripts here is shorter [18], which might be due to the slight RNA degradation of the sampled tissues. However, the large sequencing data should ensure the complete gene information obtained.
Table 1. Summary statistics of RNA sequencing data from Austinograea alayseae.
| Assembly and Annotation | |
|---|---|
| Number of transcripts | 864,469 |
| Mean length of transcripts | 414 |
| N50 of transcripts | 423 |
| Number of unigenes | 725,461 |
| Mean length of unigenes | 470 |
| N50 of unigenes | 506 |
| Annotated in NR | 32,056 (4.41%) |
| Annotated in NT | 16,559 (2.28%) |
| Annotated in KO | 36,810 (5.07%) |
| Annotated in Swiss-Prot | 59,339 (8.17%) |
| Annotated in Pfam | 107,290 (14.78%) |
| Annotated in GO | 109,411 (15.08%) |
| Annotated in KOG | 46,862 (6.45%) |
| Annotated in all databases | 2,999 (0.41%) |
| Annotated in at least one database | 134,489 (18.53%) |
Based on different databases, 134,489 (18.53%) unigenes were finally annotated in at least one database (Table 1; S1 Table). All these genes were divided into 56 subcategories in GO analysis, mainly including ‘cellular process’, ‘metabolic process’, ‘single-organism process’, ‘cell’, ‘cell part’, ‘binding’ and ‘catalytic activity’ (S1 Fig). In KOG analysis, the main function classifications were found to be ‘General function prediction only’, ‘Signal transduction mechanisms’, ‘Posttranslational modification, protein turnover, chaperones’ and ‘Translation, ribosomal structure and biogenesis’ (S2 Fig). In the KEGG analysis, the major categories were ‘Translation’, ‘Signal transduction’, ‘Transport and catabolism’, ‘Folding, sorting and degradation’, ‘Endocrine system’, ‘Carbohydrate metabolism’ and ‘Energy metabolism’ (S3 Fig). These annotation and classification will facilitate the following interpretation of the gene function.
Except ribosomal protein, the ten most abundant unigenes were different among tissue types (S2 Table), which reflected the distinct function of each tissue. In hepatopancreas, the typical immune organ of crustacean, hemocyanin and serine proteinase were abundant as expected, while in the muscle, genes related to muscle formation were highly expressed, such as skeletal muscle actin 1, troponin and myosin heavy chain. It was noted that in the eyestalk, immune related genes, ferritin, metallothionein (MT) and hemocyanin were highly expressed, indicating the eyestalk might be involved in the vent crab immune responses as revealed in other decapod species [31–33]. The gill also displayed the character of resistance to stress, with an anti-lipopolysaccharide factor (ALF) gene and a heat shock protein (HSP) gene over expressed. ALFs have been reported to have strong antibacterial effect on Gram-positive and Gram-negative bacteria [34,35], and many HSPs are normally up-regulated by heat stimulus, osmotic stress and toxic chemicals, particularly heavy metals [36–39]. These data are important to complete the transcriptomic resources of the vent fauna species, and we will characterize the gene families in detail as follows on different aspects of the crab adaptation to the vent environment.
Adaptation of visual photopigments and chemical sense
Different marine and fresh water environments require extensive adaptation, and the most affected areas are the acoustic, visual and chemical senses, due to the stimuli of chemical and physical properties of the medium [40]. Understanding the molecular underpinnings of the adaptation may provide broad insight into the crab evolution and behavior.
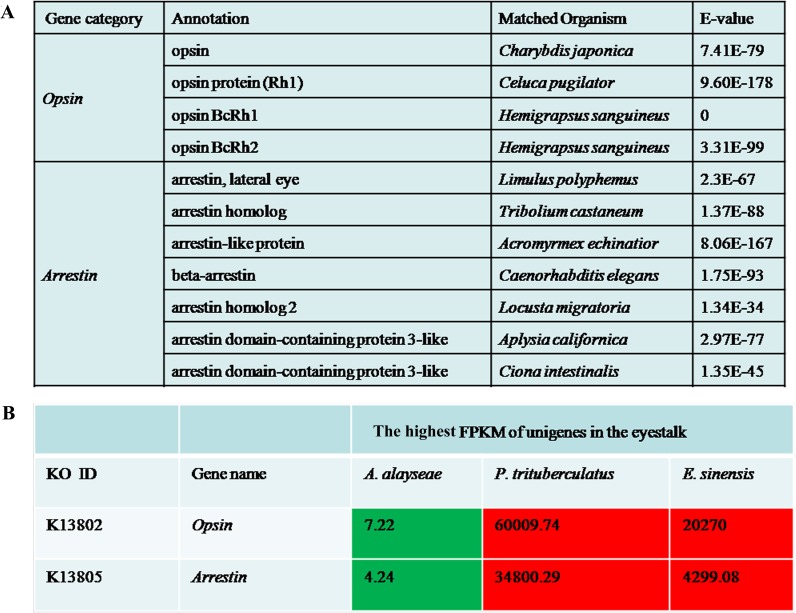
The vent crabs lose eyes and body pigmentation and evolve alternatives in adapting to constant darkness. In the eyestalk transcriptome, all genes involved in the phototransduction-fly pathway, KO04745 (http://www.genome.jp/kegg-bin/show_pathway?ko04745), were found as well as other visual related genes (S3 Table). In addition to a single class of Rhodopsin 1 (Rh1, with a λmax ~500nm) visual pigment, a middle-wavelength-sensitive Rh2 (with λmax 480-530nm) [41] was also identified in A. alayseae (Fig 1A). However, expression analysis showed that these photoreceptor genes were greatly down regulated in the vent species, as well as their regulators arrestins, when compared with that of two shallow water species, P. trituberculatus and E. sinensis (Fig 1B). Therefore, it indicates that reduced transcription of phototransduction related genes is consistent with the degenerated retinal function as revealed in the study for cavefish [42].
Fig 1. Key phototransduction regulation genes identified in Austinograea alayseae.
(A) Photoreceptor related genes in the eyestalk of A. alayseae. (B) Comparison of the highest FPKM values of the phototransduction related genes in the eyestalks of three crabs.
Since eyes of the vent crabs become vestigial, chemical communication should be the predominant mode of their behavior, such as predation and mating. In the degraded eyestalk, 10 olfactory receptor and three gustatory receptor genes were identified (S3 Table), including four ionotropic receptors (IRs) and two metabotropic glutamate receptors. Thereinto, IR4, IR7, IR8a and IR93a, the insect chemosensory variants of ionotropic glutamate receptors, were reported to be expressed in the eyestalk of crab for the first time. As suggested in the study for the spiny lobsters, Panulirus argus, IRs expression was detected in not only olfactory tissues, but other types of known chemosensory tissues [43]. The expression of IRs in eyestalk of the vent crabs supports the idea that IRs may play a more general role in arthropod chemosensation than just mediating detection of olfactory ligands. However, when the transcriptome of two shallow water crab species were searched for sensory related genes, our comparison results showed no difference in the genetic make-up connected to the variable habitats. Studying the important sensory organ, the first pair of antennae, housing their sense of smell, might bring more insights into their sensory function.
Circadian rhythm regulation and neuropeptide hormones
Many crustaceans are known to exhibit robust circadian rhythms in physiology and behavior, such as feeding, molting, reproduction, hatching and larval release [44,45]. As no light can penetrate through the deep sea, it is interesting to investigate the circadian rhythm of the vent crabs in the deep sea environment.
As a first step to study the molecular mechanisms of biological rhythms in the vent crabs, we mined its transcriptome for homologs of insect proteins known to play roles in the establishment of circadian clock systems (KO04711, http://www.genome.jp/kegg-bin/show_pathway?ko04711). As a result, 13 homologous genes in the insect circadian rhythm pathway were identified (S3 Table), including the core clock proteins, Bmal1, Clock, Period and Timeless, which constitute an interactive feedback loop [46]. Other related proteins were also found, such as casein kinase 1 epsilon, glycogen synthase kinase 3, aryl hydrocarbon receptor nuclear translocator-like protein 1, bZIP transcription factor, and hepatic leukemia factor. Moreover, an astakine gene and two prepro-beta-pigment dispersing hormone (PDH) genes were revealed to be expressed in the eyestalk of A. alayseae, which have been reported to function in circadian regulation of crustaceans [47–49]. Given the identification of an essentially full complement of circadian transcripts from A. alayseae, it seems highly likely that these molecules form the basis of a molecular clock in this species.
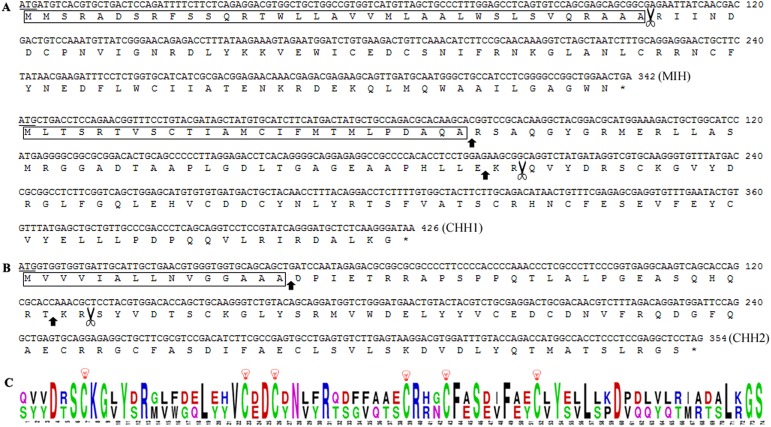
Furthermore, the eyestalk synthesizes and secretes several structurally-related peptides belonging to crustacean hyperglycemic hormone (CHH) family, which are considered as major physiological regulators during the crustacean life cycle. Although eyestalks of the hydrothermal vent crabs are degraded, we characterized three transcripts encoding important eyestalk prohormone and hormone of the CHH family in the ‘eyeless’ A. alayseae, which were one molt-inhibiting hormone (MIH) and two CHH precursors. The deduced hormones contained 141, 117 and 111 amino acids (aa), respectively (Fig 2A and 2B). The MIH included a signal peptide of 35aa and a mature peptide of 78aa (Fig 2A). The CHH1 precursor could be partitioned into a signal peptide of 25aa, followed by a 41aa CPRP (CHH precursor-related peptide), a dibasic cleavage site, and finally a mature peptide of 73aa. CHH2 started with a 15aa signal peptide, followed by a 27aa CPRP and a mature peptide of 73aa (Fig 2B). The mature peptides of the two CHHs were aligned and both have six cysteine residues (Fig 2C), which was a consensus amino acid pattern in the central part of the molecule and predicted to form three disulfide bridges [50]. Thus far, only one CHH has been reported in the hydrothermal vent crabs, Bythograeidae [51]. In this study, CHH2 is a novel neuropeptide found in the vent crabs, while CHH1 is more similar to the reported in B. thermydron. In B. thermydron, it starts with a 29aa signal peptide, followed by a 41aa CPRP and a mature peptide of 72aa. Other neuropeptides and related genes were also found in the eyestalk of the crab as shown in S3 Table, including neurophysin, allatostatin, buccalin, eclosion hormone and serotonin receptors. These genes lay foundation for the study of neuroendocrine regulation in the vent crabs.
Fig 2. Structure analysis of the three neuropeptides encoding genes in Austinograea alayseae.
(A) Nucleotide and deduced amino acid sequences of molt-inhibiting hormone (MIH). (B) Nucleotide and deduced amino acid sequences of two crustacean hyperglycemic hormone (CHH) precursors. (C) Web Logos of CHH1 precursor consensus regions compared to CHH2 precursor. The initiation codons are underlined; the signal peptides are marked by boxes; amino acids between vertical arrows indicate CHH precursor related peptide; the cleavage sites are shown by scissors. The six cysteines are marked by bulbs.
Response to the hypoxia and the reactive oxygen species
In the deep sea, there is devoid of oxygen (O2). However, it has been found that the O2 consumption rates of hydrothermal-vent animals at low O2 environment are similar to those of shallow water species at higher O2 environment [52,53], indicating specific adaptation for the O2 tension in the former group. Hemocyanins (Hcs) are blue extracellular proteins which are present in high concentrations in the blood of various mollusks and arthropods. Comparable to hemoglobin, Hc is an oxygen-carrier protein. The difference only lies in the nature of the metallic ions that coordinate oxygen, which is iron in case of hemoglobin, whereas copper for hemocyanins. The traditional role of hemocyanin is the transport and storage of molecular oxygen. In this study, six transcripts encoding different hemocyanin subunits were identified (S4 Table). All these transcripts showed high expression in hepatopancreas, which were similar with two shallow water crabs. This reveals that hemocyanin should be a multifunctional protein, and may be potentially involved in the immune responses of decapods with phenoloxidase activity, antimicrobial and antiviral properties as reported in other studies [54–56].
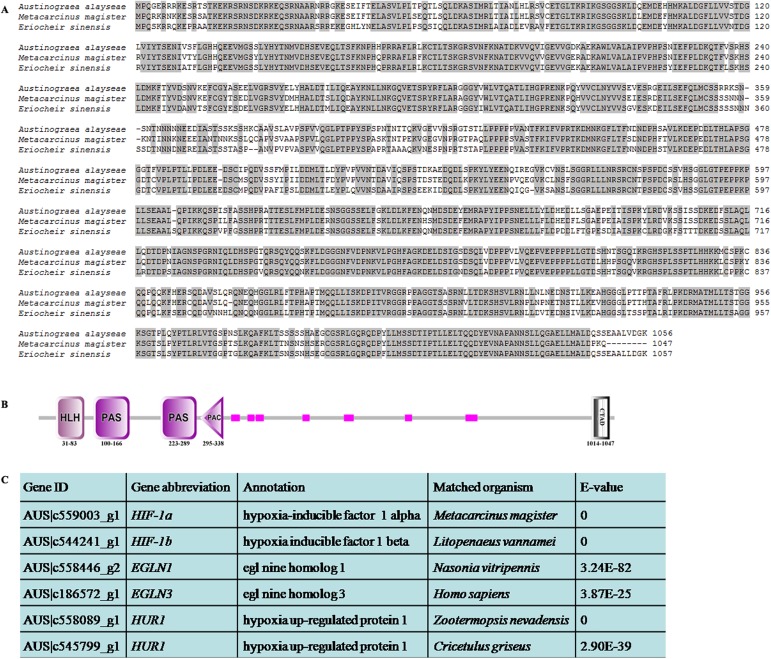
The transcription factor HIF-1 (hypoxia-inducible factor 1) has been shown to play an essential role in maintenance of homeostatic responses to hypoxia [57]. It is a dimeric protein consisting of a HIF-1α and a HIF-1ß subunits. HIF-1ß (also known as the aryl hydrocarbon receptor nuclear translocator, ARNT protein) is a common subunit for several transcription factors and constitutively expressed, while the HIF-1α subunit is unique to HIF-1 and O2-regulation [58]. From the vent crab transcriptomes, genes encoding HIF-1ɑ and HIF-1ß were both identified (Fig 3). The HIF-1α was composed of 1,056aa (Fig 3A), containing a typical HLH (helix loop helix) domain, two distinct PAS (PER-ARNT-SIM) domains, a PAC (PAS-associated C-terminal) domain and a transactivating domain (CTAD) (Fig 3B). Compared with those of other crabs, the HIF-1ɑ sequence of the vent crab showed high similarity with Metacarcinus magister and E. sinensis, with an identity of 88% and 83%, respectively (Fig 3A). Moreover, an oxygen sensor, Egl nine homolog 1 (EGLN1), also known as prolyl hydroxylase domain 2 (PHD2) protein, was found in the transcriptomes (Fig 3C), which have been reported to play pivotal roles in the HIF-1α pathway regulation [59]. Egl nine homolog 3 (EGLN3) was also detected, while its role in the regulation of HIF-1α has not been established. Moreover, two hypoxia up-regulated proteins were discovered in the transcriptomes (Fig 3C), which might be involved in the hypoxia response of the crab.
Fig 3. The hypoxia-inducible factor 1 (HIF1) identified in Austinograea alayseae.
(A) The alignment of HIF-1α from three different crabs. Conserved amino acid residues are in grey. The HIF-1α accession numbers are ABF83561 for Metacarcinus magister and AHH85804 for Eriocheir sinensis. (B) Specific domains in HIF-1α. HLH: helix loop helix; PAS: PER-ARNT-SIM; PAC: PAS-associated C-terminal; CTAD: transactivating domain. The pink squares represent low complexity regions. (C) HIF1 regulated genes in the transcriptomes.
The excessive production of reactive oxygen species (ROS) is another impact factor for the vent species, which is resulted from increased oxidative stress and detrimental to the cell [60]. Metals are known to enhance the production of ROS, and consequently increase the oxyradical stress for the species [61]. Many oxidase related genes existed in the vent crab transcriptomes, including catalase, dual oxidase, glutathione peroxidase (GPx), selenoprotein W and superoxide dismutase (SOD) (S4 Table). Three families of SODs were found and copper/zinc superoxide dismutase and manganese superoxide dismutase predominated.
The thioredoxin (Trx) is the main ubiquitously expressed thiol-reducing antioxidant systems [62]. In the transcriptomes, many thioredoxin-like proteins, Trx domain-containing proteins and Trx reductases, were found (S4 Table). Thereinto, two forms of Trx were identified, Trx1 and Trx2, both with conserved catalytic center -Cys-Gly-Pro-Cys- (-CGPC-). Trx1 exists primarily in cytosol and is also found in the nucleus and blood plasma [63], whereas Trx2 is a mitochondrial form with an N-terminal mitochondrial translocation and signal peptide, and lacks the other cysteine residues that are found in Trx1 [64]. It is noted that Trx2 of the A. alayseae showed high identity with that of crab P. trituberculatus, while Trx1 in the vent crabs was more similar with that of a sponge Amphimedon queenslandica. Therefore, Trx1 in A. alayseae might be a new form of Trx in the crabs.
Ion transport and metal detoxification
Extremely high hydrostatic pressure is one of the major characteristics of the hydrothermal vent. Gill is one of the most important organs involved in osmoregulation in crabs. Two crucial enzymes responsible for ion transport in crustacean gills, Na+/K+-ATPase, V-type H+ -ATPase, were both identified with high expression level in the gill transcriptome (S5 Table). Other regulators were also found, including solute carrier (SLC) family members, sodium/hydrogen exchanger (SLC9) and urea transporter 2 (SLC14). Notably, many SLC members related to metal ion transport were expressed in the gill, including SLC11 (3 unigenes), SLC30 (6 unigenes), SLC31 (2 unigenes), SLC39 (11 unigenes). Among them, SLC11 members are proton-coupled transporters involved in Fe2+, Cd2+, Co2+, Cu1+, Mn2+, Ni2+, Pb2+, Zn2+ transport. SLC30 and SLC39 are zinc transporters, while SLC31 are responsible for the copper transport. These gene encoding proteins are expected to facilitate the heavy metal transport of the crabs in the vent environment.
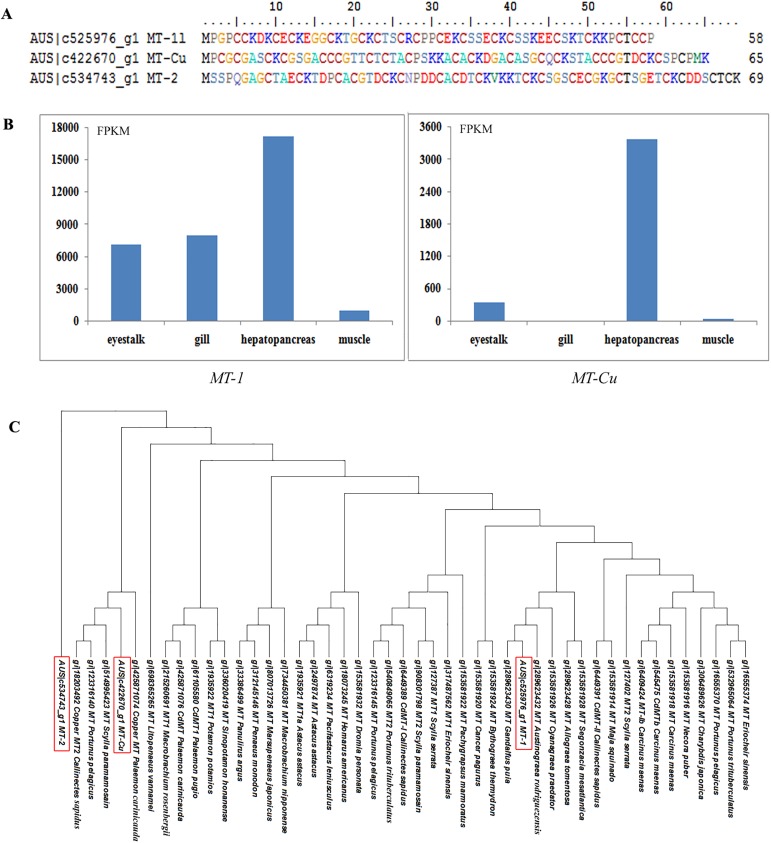
High levels of hydrogen sulfide and heavy metals are the typical feature of the hydrothermal vent, which requires specific adaptation for this condition. Metallothioneins (MTs) are unusual low molecular weight intracellular proteins found in a wide variety of vertebrates and invertebrates, displaying heat stability, high cysteine content and nonenzymatic nature. MTs can act as a metal reservoir to maintain the homeostasis of essential metals, as well as a main effector in the detoxification of excessive metals [65]. Three different MT cDNA forms were isolated from A. alayseae transcriptome, named MT-1, MT-Cu, MT-2. The open reading frames were 58aa, 65aa and 69aa in length, respectively (Fig 4A), of which 18aa, 21aa and 18aa were cysteine residues. By protein BLAST, MT-1 showed high similarity (98%) with another vent crab, G. puia. MT-Cu was annotated as copper-specific metallothionein-2 in Callinectes sapidus with identity 78%, while MT-2 was 97% identical to an abalone, Haliotis discus hannai. As shown in the phylogenetic tree for MTs of decapods, MT-1 of A. alayseae was clustered with the hydrothermal vent crab family group, Bythograeidae, while the other two distributed in separated clusters (Fig 4C). Therefore, MT-Cu and MT-2 might be novel isoforms identified in hydrothermal vent crabs. Among them, MT-1 showed high expression in all four tissues tested, while MT-2 was lowly expressed in four tissues (Fig 4B). Notably, the expression of MT-Cu was extremely high in hepatopancreas, a vital immune organ. It has been reported that hydrothermal vent organisms are exposed to copper concentrations up to a thousand times higher than those in the oceanic water [66], which might result in the high expression of copper specific MT.
Fig 4. The metallothioneins (MTs) identified in Austinograea alayseae.
(A) The alignment of the three MTs of A. alayseae. (B) Comparison of the FPKM values of two MT genes in four tissues. (C) Phylogenetic tree of the MTs from decapods. The MTs in A. alayseae are marked by red boxes.
Positively selected genes and immune adaptation
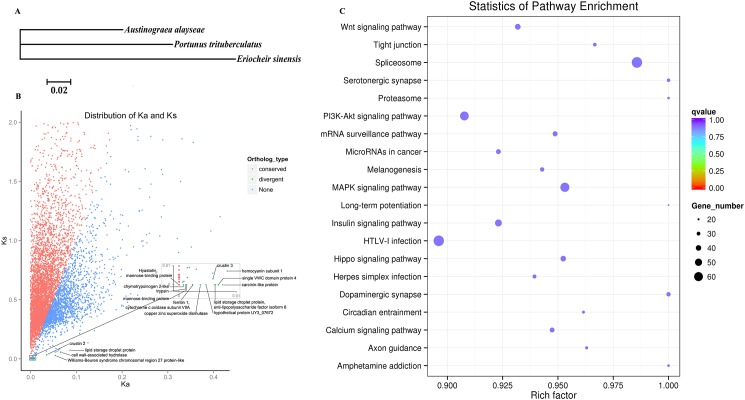
Among three crabs, A. alayseae, P. trituberculatus and E. sinensis, 6,932 orthologous genes were identified, while among five arthropods including three crabs, Daphnia pulex and Drosophilla virilis, 1,145 orthologous genes were identified and the phylogenic tree was constructed correspondingly (Fig 5A). Among them, A. alayseae was closest to P. trituberculatus, which is the representation of sea crab. The sea crab was then clustered with E. sinensis, which is a catadromous species with most of its life spending in freshwater and larvae in salt water. This phylogenetic relationship is consistent with the previous study [67].
Fig 5. Orthologous genes analysis results.
(A) Phylogenetic tree of five arthropods based on the orthologous genes. (B) Distribution of Ka/Ks for the orthologous genes among three crabs. We define genes with Ka/Ks >1 as divergent genes, and genes with Ka/Ks < 0.01 as conserved genes. (C) KEGG analysis for the conserved genes.
Ka, Ks and Ka/Ks ratios were calculated for the 6,932 orthologous genes of three crabs, resulting in 19 genes with Ka/Ks >1 and 5,040 genes with Ka/Ks < 0.1 (Fig 5B). Among the conserved genes, Wnt, MAPK, PI3K-Akt, insulin signaling pathway and hippo signaling pathway were the dominant categories based on KEGG enrichment analysis (Fig 5C). These are all related to development, indicating a conserved way of growth and development in crabs. In the 19 divergent genes (Fig 5B and Table 2), most of them were involved in immune responses. For instance, three crustin genes were identified as crustin2, crustin3 and carcinin-like protein genes. Crustin is an antibacterial peptide family composed of three members custin1-3, with a characteristic four-disulphide core-containing whey acidic protein (WAP) domain and a broad antibacterial spectrum [68]. Two other antibacterial peptides, hyastatin and anti-lipopolysaccharide factor isoform 6 were also discovered. Moreover, two mannose-binding proteins (MBLs) were positively selected, which are members of mannose pathway of complement system and involved in crab adaptive immunity. Two genes involved in the antioxidant system were also found, including ferritin 1 and copper zinc superoxide dismutase (SOD1), while the identified single VWC domain protein 4 (SVC4) can respond to environmental challenges, such as bacterial infection and nutritional status [69]. The discovered Dscam gene plays an essential function in neuronal wiring and appears to be involved in innate immune responses in many arthropods [70]. In addition, two serine-type endopeptidase chymotrypsinogen 2-like (Ctrbl) and trypsin, associated with proteolysis and metabolism, were also revealed to be under selection. These genes provide nice candidates to study their function in the adaptive evolution of crab species.
Table 2. Function categories of positively selected genes in three crabs, Austinograea alayseaee, Portunus trituberculutus and Eriocheir sinensis.
| Orthology ID | Gene name | Annotation | Function |
|---|---|---|---|
| OG06005 | COX7A | cytochrome c oxidase subunit VIIA putative | Energy metabolism |
| OG09209 | WHL | cell wall-associated hydrolase | Metabolism |
| OG12001 | Hyastatin | antimicrobial peptide hyastatin | Immunity |
| OG12033 | Ctrb1 | chymotrypsinogen 2-like | Proteolysis |
| OG12037 | MBL | mannose-binding protein | Immunity |
| OG12105 | ferritin 1 | ferritin 1 | Immunity |
| OG12337 | hypothetical protein UY3_07672 [Chelonia mydas] | Unknown | |
| OG12436 | crustin3 | crustin 3 | Immunity |
| OG12521 | trypsin | trypsin | Proteolysis |
| OG12637 | crustin2 | crustin 2 | Immunity |
| OG13252 | ALF6 | anti-lipopolysaccharide factor isoform 6 | Immunity |
| OG13368 | carcinin-like | carcinin-like protein | Immunity |
| OG13448 | SOD1 | copper zinc superoxide dismutase | Immunity |
| OG13939 | Hc1 | hemocyanin subunit 1 | Energy metabolism |
| OG14016 | WBSCR27 | Williams-Beuren syndrome chromosomal region 27 protein-like | Unknown |
| OG14400 | MBL | mannose-binding protein | Immunity |
| OG14461 | SVC | single VWC domain protein 4 | Immunity |
| OG14472 | Dscam2 | DSCAM | Immunity |
| OG14474 | LSDP | lipid storage droplet protein | Metabolism |
Considering the significance of immune genes in the evolution of the crabs, we characterized 33 additional immune genes by searching the transcriptome of hepatopancreas, especially focus on genes related to pathogenic microorganism stress of the vent (S4 Table). There were 12 antimicrobial peptides, including five ALFs, seven crustins, and 21 genes involved in prophenoloxidase-activating system (serine protease, serine proteinase inhibitor, pacifastin, prophenoloxidase and serpin). Exploring these genes will contribute to understand the mechanisms of pathogens elimination and the symbiont phenomenon in the hydrothermal vent crabs.
Conclusions
The transcriptome analysis of A. alayseae revealed a comprehensive set of genes expressed in four different tissues, resulting in 725,461 unigenes and 134,489 annotated genes. Candidate genes related to harsh condition adaptation were identified, such as genes related to hypoxia, metal detoxification, high osmotic pressure and pathogens. Sequences and structures of many candidate genes were characterized, including MIH, CHHs, HIF1 and MTs. Orthologs among three crabs revealed 19 PSGs and most of them were involved in immune responses, indicating their important roles in the environment adaptation in crabs. This first transcriptome of the hydrothermal crabs sets the stage for expanding the genetic resources available for vent species, and provides candidate genes that are likely tied with physiological adaptation to the extreme environment.
Supporting information
(RAR)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(TIF)
(TIF)
A: Cellular Processes; B: Environmental Information Processing; C: Genetic Information Processing; D: Metabolism; E: Organismal Systems.
(TIF)
Acknowledgments
The samples were collected by RV KEXUE. We greatly appreciated members working in the RV KEXUE for the sampling and Dr. Jie Cheng in the Ocean University of China for revising the manuscript.
Data Availability
All reads are deposited in the NCBI Sequence Read Archive repository (accession number: SRR4416314).
Funding Statement
This work was supported by the Scientific and Technological Innovation Project of Qingdao National Laboratory for Marine Science and Technology (No.2015ASKJ02, http://www.qnlm.ac/index) to ZC, and the National Natural Science Foundation of China (No.31302187, http://www.nsfc.gov.cn/) to MH. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Williams DL, Herzen RPV, Sclater JG, Anderson RN (1974) The Galapagos Spreading Centre: lithospheric cooling and hydrothermal circulation. Geophysical Journal of the Royal Astronomical Society 38: 587–608. [Google Scholar]
- 2.Weiss RF, Lonsdale P, Lupton JE, Bainbridge AE, Craig H (1977) Hydrothermal plumes in the Galapagos Rift. Nature 267: 600–603. [Google Scholar]
- 3.Corliss JB, Dymond J, Gordon LI, Edmond JM, von Herzen RP, Ballard RD, et al. (1979) Submarine thermal sprirngs on the Galápagos Rift. Science 203: 1073–1083. doi: 10.1126/science.203.4385.1073 [DOI] [PubMed] [Google Scholar]
- 4.Brown C, Hodgson A (2001) The ecology of deep-sea hydrothermal vents. African Zoology 27: 119–120. [Google Scholar]
- 5.Little CT, Vrijenhoek RC (2003) Are hydrothermal vent animals living fossils? Trends in Ecology & Evolution 18: 582–588. [Google Scholar]
- 6.Bachraty C, Legendre P, Desbruyères D (2009) Biogeographic relationships among deep-sea hydrothermal vent faunas at global scale. Deep Sea Research Part I Oceanographic Research Papers 56: 1371–1378. [Google Scholar]
- 7.Martin JW, Haney TA (2005) Decapod crustaceans from hydrothermal vents and cold seeps: a review through 2005. Zoological Journal of the Linnean Society 145: 445–522. [Google Scholar]
- 8.Desbruyères D, Segonzac M, Bright M (2006) Handbook of deep-sea hydrothermal vent fauna. Marine Ecology 27: 119–120. [Google Scholar]
- 9.Kim SJ, Lee KY, Ju SJ (2013) Nuclear mitochondrial pseudogenes in Austinograea alayseae hydrothermal vent crabs (Crustacea: Bythograeidae): effects on DNA barcoding. Moecular Ecology Resources 13: 781–787. [DOI] [PubMed] [Google Scholar]
- 10.Yang JS, Nagasawa H, Fujiwara Y, Tsuchida S, Yang WJ (2008) The complete mitochondrial genome sequence of the hydrothermal vent galatheid crab Shinkaia crosnieri (Crustacea: Decapoda: Anomura): A novel arrangement and incomplete tRNA suite. BMC Genomics 9: 257 doi: 10.1186/1471-2164-9-257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang JS, Nagasawa H, Fujiwara Y, Tsuchida S, Yang WJ (2010) The complete mitogenome of the hydrothermal vent crab Gandalfus yunohana (Crustacea: Decapoda: Brachyura): a link between the Bythograeoidea and Xanthoidea. Zooogica Scripta 39: 621–630. [Google Scholar]
- 12.Mateos M, Hurtado LA, Santamaria CA, Leignel V, Guinot D (2012) Molecular systematics of the deep-sea hydrothermal vent endemic Brachyuran family Bythograeidae: a comparison of three Bayesian species tree methods. Plos One 7: e32066 doi: 10.1371/journal.pone.0032066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang JS, Lu B, Chen DF, Yu YQ, Yang F, Nagasawa H, et al. (2013) When did decapods invade hydrothermal vents? Clues from the Western Pacific and Indian Oceans. Molecular Biology & Evolution 30: 305–309. [DOI] [PubMed] [Google Scholar]
- 14.Leignel V, Marchand J, Moreau B, Chenais B (2008) Metallothionein genes from hydrothermal crabs (Bythograeidae, Decapoda): characterization, sequence analysis, gene expression and comparison with coastal crabs. Comparative Biochemistry and Physiology-Part C: Toxicology & Pharmacology 148: 6–13. [DOI] [PubMed] [Google Scholar]
- 15.Ma Y (2011) Specific genes screening and gene function analysis in the hydrothermal crab, Gandalfus yunohana. Master thesis (In Chinese), Zhejiang University.
- 16.Stefanni S, Bettencourt R, Pinheiro M, Moro GD, Bongiorni L, Pallavicini A (2014) Transcriptome of the deep-sea black scabbardfish, Aphanopus carbo (Perciformes: Trichiuridae): tissue-specific expression patterns and candidate genes associated to depth adaptation. International Journal of Genomics (ID 267482): 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang W, Qi Y, Fu J (2013) Exploring the genetic basis of adaptation to high elevations in reptiles: a comparative transcriptome analysis of two toad-headed agamas (genus Phrynocephalus). Plos One 9: e112218–e112218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bettencourt R, Pinheiro M, Egas C, Gomes P, Afonso M, Shank T, et al. (2010) High-throughput sequencing and analysis of the gill tissue transcriptome from the deep-sea hydrothermal vent mussel Bathymodiolus azoricus. BMC Genomics 11: 559 doi: 10.1186/1471-2164-11-559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wong YH. Sun J, He LS, Chen LG, Qiu J, Qiang PY, et al. (2015) High-throughput transcriptome sequencing of the cold seep mussel Bathymodiolus platifrons. Scientific Reports 5: 16597 doi: 10.1038/srep16597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grabherr MG, Haas BJ, Yassour M, Levin JZ, Thompson DA, Amit I, et al. (2011) Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nature Biotechnology 29: 644–652. doi: 10.1038/nbt.1883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Young MD, Wakefield MJ, Smyth GK, Oshlack A (2010) Method Gene ontology analysis for RNA-seq: accounting for selection bias. Genome Biology 11: R14 doi: 10.1186/gb-2010-11-2-r14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanehisa M, Araki M, Goto S, Hattori M, Hirakawa M, Itoh M, et al. (2008) KEGG for linking genomes to life and the environment. Nucleic Acids Research 36: D480–D484. doi: 10.1093/nar/gkm882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li B, Dewey CN (2011) RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics 12: 323 doi: 10.1186/1471-2105-12-323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Molecular Biology & Evolution 30: 2725–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williams AB (1980) A new crab family from the vicinity of submarine thermal vents on the Galapagos Rift (Crustacea: Decapoda: Brachyura). Proceedings of the Biological Society of Washington 93: 443–472. [Google Scholar]
- 26.Iseli C, Jongeneel CV, Bucher P (1999) ESTScan: a program for detecting, evaluating, and reconstructing potential coding regions in EST sequences In: Proceedings of intelligent systems for molecular biology. AAAI Press, Menlo Park. [PubMed] [Google Scholar]
- 27.Li L, Stoeckert CJ, Roos DS (2003) OrthoMCL: identification of ortholog groups for eukaryotic genomes. Genome Research 13: 2178–2189. doi: 10.1101/gr.1224503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research 32: 1792–1797. doi: 10.1093/nar/gkh340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guindon S, Dufayard J, Hordijk W, Lefort V, Gascuel O (2010) New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Systematic Biology 59: 307–321. doi: 10.1093/sysbio/syq010 [DOI] [PubMed] [Google Scholar]
- 30.Yang Z (2007) PAML 4: phylogenetic analysis by maximum likelihood. Molecular Bioogy & Evoution 24: 1586–1591. [DOI] [PubMed] [Google Scholar]
- 31.Liu Y, Cui Z, Song C, Wang S, Li Q (2011) Multiple isoforms of immune-related genes from hemocytes and eyestalk cDNA libraries of swimming crab Portunus trituberculatus. Fish & Shellfish Immunology 31: 29–42. [DOI] [PubMed] [Google Scholar]
- 32.Sainz-Hernández JC, Racotta IS, Dumas S (2008) Effect of unilateral and bilateral eyestalk ablation in Litopenaeus vannamei male and female on several metabolic and immunologic variables. Aquaculture 283: 188–193. [Google Scholar]
- 33.Asusena ACJ, Carlos SHJ, Arturo FCJ, Genaro DP (2012) The effects of eyestalk ablation on the reproductive and immune function of female Macrobrachium americanum. Journal of Aquaculture Research and Development 3: 156. [Google Scholar]
- 34.Yedery RD, Reddy KVR (2009) Identification, cloning, characterization and recombinant expression of an anti-lipopolysaccharide factor from the hemocytes of Indian mud crab, Scylla serrata. Fish & Shellfish Immunology 27: 275–284. [DOI] [PubMed] [Google Scholar]
- 35.Liu Y, Cui Z, Luan W, Song C, Nie Q, Wang S, et al. (2011) Three isoforms of anti-lipopolysaccharide factor identified from eyestalk cDNA library of swimming crab Portunus trituberculatus. Fish & Shellfish Immunology 30: 583–591. [DOI] [PubMed] [Google Scholar]
- 36.Ivanina AV, Taylor C, Sokolova IM (2009) Effects of elevated temperature and cadmium exposure on stress protein response in eastern oysters Crassostrea virginica (Gmelin). Aquatic Toxicology 91: 245–254. doi: 10.1016/j.aquatox.2008.11.016 [DOI] [PubMed] [Google Scholar]
- 37.Qian Z, Liu X, Wang L, Wang X, Li Y, Xiang J, et al. (2012) Gene expression profiles of four heat shock proteins in response to different acute stresses in shrimp, Litopenaeus vannamei. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology 156: 211–220. [DOI] [PubMed] [Google Scholar]
- 38.Yang Y, Ye H, Huang H, Li S, Liu X, Zeng X, et al. (2013) Expression of Hsp70 in the mud crab, Scylla paramamosain in response to bacterial, osmotic, and thermal stress. Cell Stress Chaperon 18: 475–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hui M, Liu Y, Song C, Li Y, Shi G, Cui Z (2014) Transcriptome changes in Eriocheir Sinensis megalopae after desalination provide insights into osmoregulation and stress adaption in larvae. Plos One 9: e114187 doi: 10.1371/journal.pone.0114187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Groh KC, Vogel H, Stensmyr MC, Grossewilde E, Hansson BS (2013) The hermit crab's nose-antennal transcriptomics. Frontiers in Neuroscience 7: 266 doi: 10.3389/fnins.2013.00266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Davies WIL, Collin SP, Hunt DM (2012) Molecular ecology and adaptation of visual photopigments in craniates. Molecular Ecology 21: 3121–3158. doi: 10.1111/j.1365-294X.2012.05617.x [DOI] [PubMed] [Google Scholar]
- 42.Meng F, Braasch I, Phillips JB, Lin X, Titus T, Zhang C, et al. (2013) Evolution of the eye transcriptome under constant darkness in Sinocyclocheilus cavefish. Molecular Biology and Evolution 30: 1527–1543. doi: 10.1093/molbev/mst079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Corey EA, Bobkov Y, Ukhanov K, Ache BW (2013) Ionotropic crustacean olfactory receptors. Plos One 8: e60551 doi: 10.1371/journal.pone.0060551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Farca Luna AJ, Heinrich R, Reischig T (2010) The circadian biology of the marbled crayfish. Frontiers in Bioscience 2: 1414–1431. [DOI] [PubMed] [Google Scholar]
- 45.Strauss J, Dircksen H (2010) Circadian clocks in crustaceans: identified neuronal and cellular systems. Frontiers in Bioscience 15: 1040–1074. [DOI] [PubMed] [Google Scholar]
- 46.Allada R, Chung BY (2010) Circadian organization of behavior and physiology in Drosophila. Annual Review of Physiology 72: 605–624. doi: 10.1146/annurev-physiol-021909-135815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Watthanasurorot A, Söderhäll K, Jiravanichpaisal P, Söderhäll I (2011) An ancient cytokine, astakine, mediates circadian regulation of invertebrate hematopoiesis. Cellular & Molecular Life Sciences Cmls 68: 315–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang X, Ye H, Huang H, Yu K, Huang Y (2014) Two beta-pigment-dispersing hormone (β-PDH) isoforms in the mud crab, Scylla paramamosain: Implication for regulation of ovarian maturation and a photoperiod-related daily rhythmicity. Animal Reproduction Science 150: 139–147. doi: 10.1016/j.anireprosci.2014.09.004 [DOI] [PubMed] [Google Scholar]
- 49.Strauß J, Zhang Q, Verleyen P, Huybrechts J, Neupert S, Predel R, et al. (2011) Pigment-dispersing hormone in Daphnia interneurons, one type homologous to insect clock neurons displaying circadian rhythmicity. Cellular and Molecular Life Sciences Cmls 68: 3403–3423. doi: 10.1007/s00018-011-0636-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chan SM, Gu PL, Chu KH, Tobe SS (2003) Crustacean neuropeptide genes of the CHH/MIH/GIH family: implications from molecular studies. General and Comparative Endocrinology 134: 214–219. [DOI] [PubMed] [Google Scholar]
- 51.Toullec JY, Vinh J, Caer JPL, Shillito B, Soyez D (2002) Structure and phylogeny of the crustacean hyperglycemic hormone and its precursor from a hydrothermal vent crustacean: the crab Bythograea thermydron. Peptides 23: 31–42. [DOI] [PubMed] [Google Scholar]
- 52.Hourdez S, Weber RE, Green BN, Kenney JM, Fisher CR (2002) Respiratory adaptations in a deep-sea orbiniid polychaete from Gulf of Mexico brine pool NR-1: metabolic rates and hemoglobin structure/function relationships. Journal of Experimental Biology 205: 1669–1681. [DOI] [PubMed] [Google Scholar]
- 53.Hourdez S, Weber RE (2005) Molecular and functional adaptations in deep-sea hemoglobins. Journal of Inorganic Biochemistry 99: 130–141. doi: 10.1016/j.jinorgbio.2004.09.017 [DOI] [PubMed] [Google Scholar]
- 54.Zhang Y, Yan F, Hu Z, Zhao X, Min S, Du Z, et al. (2009) Hemocyanin from shrimp Litopenaeus vannamei shows hemolytic activity. Fish & Shellfish Immunology 27: 330–335. [DOI] [PubMed] [Google Scholar]
- 55.Nagai T, Osaki T, Kawabata S (2001) Functional conversion of hemocyanin to phenoloxidase by horseshoe crab antimicrobial peptides. Journal of Biological Chemistry 276: 27166–27170. doi: 10.1074/jbc.M102596200 [DOI] [PubMed] [Google Scholar]
- 56.Sun S, Chen L, Qin J, Ye J, Qin C, Jiang H, et al. (2012) Molecular cloning, characterization and mRNA expression of copper-binding protein hemocyanin subunit in Chinese mitten crab, Eriocheir sinensis. Fish & Shellfish Immunology 33: 1222–1228. [DOI] [PubMed] [Google Scholar]
- 57.Semenza GL (2004) Hydroxylation of HIF-1: oxygen sensing at the molecular level. Physiology 19: 176–182. doi: 10.1152/physiol.00001.2004 [DOI] [PubMed] [Google Scholar]
- 58.Iyer NV, Leung SW, Semenza GL (1998) The human hypoxia-inducible factor 1α gene: Hif1α structure and evolutionary conservation. Genomics 52: 159–165. doi: 10.1006/geno.1998.5416 [DOI] [PubMed] [Google Scholar]
- 59.Semenza GL (2007) Hypoxia-inducible factor 1 (HIF-1) pathway. Sciences Stke Signal Transduction Knowledge Environment 407: cm8. [DOI] [PubMed] [Google Scholar]
- 60.Kanzok SM, Fechner A, Bauer H, Ulschmid JK, Müller HM, Botella-Munoz J, et al. (2001) Substitution of the thioredoxin system for glutathione reductase in Drosophila melanogaster. Science 291: 643–646. doi: 10.1126/science.291.5504.643 [DOI] [PubMed] [Google Scholar]
- 61.Francesco R, Nigro M, Benedetti M, Gorbi S, Pretti C (2005) Interactions between metabolism of trace metals and xenobiotic agonists of the aryl hydrocarbon receptor in the antarctic fish Trematomus bernacchii: environmental perspectives. Environmental Toxicology and Chemistry 24: 1475–1482. [DOI] [PubMed] [Google Scholar]
- 62.Nordberg J, Arnér ESJ (2001) Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radical Biology and Medicine 31: 1287–1312. [DOI] [PubMed] [Google Scholar]
- 63.Watson WH, Yang X, Choi YE, Jones DP, Kehrer JP (2004) Thioredoxin and its role in toxicology. Toxicological. Sciences 78: 3–14. doi: 10.1093/toxsci/kfh050 [DOI] [PubMed] [Google Scholar]
- 64.Spyrou G, Enmark E, Miranda-Vizuete A, Gustafsson J (1997) Cloning and expression of a novel mammalian thioredoxin. Journal of Biological Chemistry 272: 2936–2941. [DOI] [PubMed] [Google Scholar]
- 65.Carpenè E, Andreani G, Isani GM (2007) Metallothionein functions and structural characteristics. Journal of Trace Elements in Medicine & Biology 21: 35–39. [DOI] [PubMed] [Google Scholar]
- 66.Hardivillier Y, Leignel V, Denis F, Uguen G, Cosson R, Laulier M (2004) Do organisms living around hydrothermal vent sites contain specific metallothioneins? The case of the genus Bathymodiolus (Bivalvia, Mytilidae). Comparative Biochemistry and Physiology-Part C: Toxicology & Pharmacology 139: 111–118. [DOI] [PubMed] [Google Scholar]
- 67.Yang JS, Lu B, Chen DF, Yu YQ, Yang F, Nagasawa H, et al. (2012) When did decapods invade hydrothermal vents? Clues from the Western Pacific and Indian Oceans. Molecular Biology & Evolution 30: 305–309. doi: 10.1093/molbev/mss224 [DOI] [PubMed] [Google Scholar]
- 68.Smith VJ, Fernandes JMO, Kemp GD, Hauton C (2008) Crustins: Enigmatic WAP domain-containing antibacterial proteins from crustaceans. Developmental & Comparative Immunology 32: 758–772. [DOI] [PubMed] [Google Scholar]
- 69.Sheldon TJ, Miguel-Aliaga I, Gould AP, Taylor WR, Conklin DA (2007) A novel family of single VWC-domain proteins in invertebrates. FEBS Letters 581: 5268–5274. doi: 10.1016/j.febslet.2007.10.016 [DOI] [PubMed] [Google Scholar]
- 70.Watthanasurorot A, Jiravanichpaisal P, Liu H, Söderhäll I, Söderhäll K (2011) Bacteria-induced dscam isoforms of the crustacean, Pacifastacus leniusculus. Plos Pathogens 7: 265–275. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(RAR)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(TIF)
(TIF)
A: Cellular Processes; B: Environmental Information Processing; C: Genetic Information Processing; D: Metabolism; E: Organismal Systems.
(TIF)
Data Availability Statement
All reads are deposited in the NCBI Sequence Read Archive repository (accession number: SRR4416314).