Abstract
Background
Currently, there is inadequate evidence on which to base clinical management of neurotoxic snakebite envenoming, especially in the choice of initial antivenom dosage. This randomised controlled trial compared the effectiveness and safety of high versus low initial antivenom dosage in victims of neurotoxic envenoming.
Methodology/ Principal findings
This was a balanced, randomised, double-blind trial that was conducted in three health care centers located in the Terai plains of Nepal. Participants received either low (two vials) or high (10 vials) initial dosage of Indian polyvalent antivenom. The primary composite outcome consisted of death, the need for assisted ventilation and worsening/recurrence of neurotoxicity. Hourly evaluations followed antivenom treatment. Between April 2011 and October 2012, 157 snakebite victims were enrolled, of which 154 were analysed (76 in the low and 78 in the high initial dose group). Sixty-seven (43·5%) participants met the primary outcome definition. The proportions were similar in the low (37 or 48.7%) vs. high (30 or 38.5%) initial dose group (difference = 10·2%, 95%CI [-6·7 to 27·1], p = 0·264). The mean number of vials used was similar between treatment groups. Overall, patients bitten by kraits did worse than those bitten by cobras. The occurrence of treatment-related adverse events did not differ among treatment groups. A total of 19 serious adverse events occurred, including seven attributed to antivenom.
Conclusions
This first robust trial investigating antivenom dosage for neurotoxic snakebite envenoming shows that the antivenom currently used in Nepal performs poorly. Although the high initial dose regimen is not more effective than the low initial dose, it offers the practical advantage of being a single dose, while not incurring higher consumption or enhanced risk of adverse reaction. The development of new and more effective antivenoms that better target the species responsible for bites in the region will help improve future patients’ outcomes.
Trial registration
The study was registered on clinicaltrials.gov (NCT01284855) (GJ 5/1)
Author summary
Snakebite is an important medical problem in tropical regions, including in Nepal where tens of thousands of people are bitten every year. Snakebite can result in life-threatening envenoming and correct identification of the biting species is crucial for doctors to choose appropriate treatment and anticipate complications. This paper compares two different doses of antivenom for the treatment of neurotoxic snakebite envenoming. Out of 157 snakebite victims presenting to one of the study centers, 78 received a low initial dose and 79 received a high initial dose. The proportion of patients who either died, needed breathing support or additional doses of antivenom were the same in the two groups. Overall, patients bitten by kraits did worse than those bitten by cobras. The occurrence of adverse reactions was comparable among those received low or high initial dose respectively. This study is the first to use a rigorous and robust method for comparing doses of antivenom in snakebite victims in South Asia. Although the high initial dose was not more effective than the low initial dose, it offers the practical advantage of being simpler to administer and was as safe as the low initial dose. The development of new and more effective antivenoms that better target the species responsible for bites in the region will help improve future patients’ outcomes.
Introduction
Snakebite envenoming is a neglected disease par excellence that primarily affects poor communities in the tropics, but attracts little interest from pharmaceutical companies, health agencies and research funding bodies. This neglect has resulted in a paucity of scientific evidence on which to base therapeutic decisions and support robust guidelines. The antivenom development pipeline remains desperately dry [1,2]. Moreover, the absence of rigorous regulatory oversight has resulted in the marketing of antivenom of doubtful efficacy and variable quality and safety [1–3]. The optimal dosage of antivenom is highly debated. Antivenom potency varies widely, and the initial doses recommended by manufacturers range from 1 to over 30 vials [4]. Recommendations are usually based on median lethal (LD50) and effective dose (ED50) assays in which venom and antivenom are incubated in vitro before being injected into mice. However, rodent models are poor substitutes for clinical trials [5], and few randomised, dose ranging controlled trials (RCT) of snakebite envenoming have been conducted [6–16]. Moreover, a recent systematic review found that trials conducted in South Asia generated very low quality evidence [17].
In Nepal, snakebite is an important public health problem [18–20] with peak annual incidence and mortality rates of up to 1162/100 000 and 162/100 000, respectively, reported in eastern regions [19]. Snakebite is a disease of poverty. Farmers, plantation workers and herders are the main victims [21]. Elapid snakes, notably the Indian spectacled cobra (Naja naja) and the common krait (Bungarus caeruleus), cause most cases of snakebite envenoming in Nepal [22,23]. Elapid envenoming is characterized by a progressive, descending neuromuscular paralysis, leading to respiratory failure and death [24,25]. Since 1998, Indian polyvalent antivenom has been provided free of charge to all hospitals in Nepal by the Ministry of Health (MoH). The treatment of envenoming varies widely, with antivenom total doses ranging from 2 to 115 vials [18]. Case fatality rates (CFR) also vary widely, from 3% to 58% [18].
Indian polyvalent antivenom costs between 6·50 to 11·00 US$ per vial [26]. In order to minimise expense, the Nepalese MoH recommends a low initial dose (2 vials) of antivenom as an intravenous (IV) push, followed by an infusion of additional vials titrated to clinical response [27], consistent with some manufacturer guidelines. However, this dosing regimen contrasts with expert opinion which recommends a high loading dose of 10 vials (100 ml) administered as an IV push, arguing that this should neutralize neurotoxins more effectively before they become irreversibly bound to tissue receptors [28,29]. World Health Organization (WHO) guidelines also recommend an initial dose of 10 vials for envenoming after bites by South Asian cobras and kraits [30]. There are no published RCTs addressing the optimal dose of antivenom in neurotoxic envenoming and observational studies are unhelpful [31,32]. Given the lack of data and the need to optimise treatment of snakebite envenoming in Nepal, we conducted an RCT comparing high versus low initial antivenom dose in patients with neurotoxic envenoming.
Methods
Ethics statement
Ethical approvals were obtained from the B.P. Koirala Institute of Health Sciences Ethics Committee, the Nepal Health Research Council (NHRC) and the Geneva University Hospitals Ethics Committee. The study was registered on clinicaltrials.gov (NCT01284855). Written informed consent was obtained from all adult participants prior to inclusion, and from guardians for minor participants. Whenever possible assent was also sought from children. For participants who were unable to read and/or write, an independent witness was present during the consent process and signed the consent form next to the participant’s thumbprint.
Study design
This was a balanced, randomised, double-blind, parallel trial comparing two dosing regimens of antivenom. The study was conducted between April 2011 and March 2013 at the Snake Bite Treatment Centre of Damak Red Cross Society, the Snake Bite Management Centre of Charali, both in Jhapa district, and the Bharatpur District Hospital, Bharatpur, in Chitwan district. All centres are located in the Terai plains of Nepal.
Participants
Snakebite victims were enrolled in the study if they gave written informed consent (assent if aged 12 to 18) and had ≥1 sign(s) of neurotoxic envenoming: bilateral ptosis; inability to frown, open the mouth, protrude the tongue, or clear secretions; broken neck sign; skeletal muscle weakness (power < 3 UK MRC scale); gag reflex loss; and paradoxical breathing.
Those presenting >24 hours post-bite, with a proven viper bite, who had already received antivenom, or were children below 5 years, pregnant or breast feeding women, individuals with a history of neuromuscular disease, known allergy to horse protein, and those with an immediate need for mechanical ventilation were excluded.
Randomisation and masking
Randomisation was stratified by centre, and within each stratum, patients were randomized in blocks of variable sizes, according to a computer generated list. Sealed sequentially numbered envelopes containing the antivenom regimen were prepared accordingly. For quality control, 10% of the envelopes were double-checked by an independent statistician.
The envelopes were kept in the site pharmacy, to which only the trial pharmacist had access. Upon inclusion of a trial participant, the pharmacist opened the envelope in sequence. Reconstitution of antivenom, dilution and preparation of push injections and perfusions took place in the pharmacy. To maintain blinding, the total volume and appearance of push injections and infusions were identical in the two study arms. The study clinicians and patients were unaware of treatment allocation. If neurotoxicity persisted or worsened, the clinician asked the pharmacist to prepare additional doses of antivenom, according to the indications found in the randomisation envelope for that patient. This was done in the same concealed manner. Compliance with randomisation and masking procedures was assessed as part of the GCP monitoring of the trial.
Intervention and trial procedures
We used two batches of lyophilised polyvalent antivenom raised against Indian Daboia russelii, Echis carinatus, Bungarus caeruleus and Naja naja venoms, manufactured by VINS Bioproducts Ltd, Hyderabad, India. The neutralizing potency of the antivenom (mg of Indian snake venom neutralized per mL of antivenom) as stated by the manufacturer in the Certificate of Analysis (CoA) was: 0.681 mg and 0.636 mg for N. naja, 0.541 mg for B. caeruleus, 0.704 mg for D. russelii, and 0.612 mg and 0.616 mg for E. carinatus. Trial participants received either the dose regimen recommended by the Nepalese national protocol (low initial dose group) or a high initial dose as recommended by WHO guidelines (high initial dose group). The low initial dose regimen consisted of an initial dose of 2 vials given by IV push, followed by the infusion of 4 vials over 4 hours. If signs of envenoming persisted after the initial 4 hours, the 4 vial infusion was repeated up to three times. If signs of envenoming persisted after 12 hours, an infusion of 2 vials of antivenom was given over 4 hours, every 4 hours, until recovery. In the case of neurotoxic deterioration, 2 vials were administered by IV push as recommended by national guidelines. To maintain the blinding, the high initial dose regimen was adapted to the administration method used in the low initial dose arm. It consisted of an initial dose of 2 vials given by IV push followed by an 8 vial infusion over one hour and an infusion of saline over 3 hours. If signs of envenoming persisted after these first 4 hours, the saline infusion was repeated, to mimic the infusion given in the low initial dose arm. In the case of deteriorating neurotoxic signs, 5 vials of antivenom were given by IV push. The two regimens are described in S2 Fig. The total number of vials of antivenom administered was restricted to 30, irrespective of treatment allocation.
Patients were hospitalised throughout the treatment period. After initial dosing, clinical evaluation was performed every hour until full recovery. Anaphylaxis was managed by stopping the antivenom immediately and administering intramuscular (IM) adrenaline, IV hydrocortisone and IV chlorphenamine. Oxygen, salbutamol inhalations, or the rapid administration of normal saline were given as indicated clinically. Following the publication of an RCT showing the benefits of subcutaneous adrenaline premedication [33], we adopted this strategy after April 2012.
Three follow-up visits were scheduled to assess short and medium-term patient outcome: 7 days, 21 days and 6 months after hospital discharge.
Outcomes
The primary effectiveness endpoint was a combination of (a) in-hospital death, (b) the need for assisted ventilation and (c) worsening or recurrence of neurotoxicity after the initial dose of antivenom.
The clinical indications for intubation and assisted ventilation were (1) absent gag reflex, (2) presence of paradoxical breathing, (3) respiratory distress or cyanosis, whichever was detected first, and/or (4) oxygen saturation <90% despite high flow oxygen supplementation.
The primary composite endpoint was evaluated at each clinical evaluation, i.e., every hour until full recovery from neurotoxic envenoming. If a patient presented at least one of the sub-criteria, the primary endpoint was deemed positive. If all the sub-criteria were indicated as being absent until full recovery, the primary composite endpoint was deemed negative. Patients with missing data in one of the sub-criteria always presented with at least one other sub-criterion, enabling us to define presence of the primary composite outcome for all patients.
Secondary endpoints included time to recovery and number of antivenom vials used. The safety endpoints were incidence of adverse events (AEs) and serious adverse events (SAEs).
The evolution of neurotoxicity was assessed by a scoring method (S1 Fig). Worsening of neurotoxicity was defined as (1) appearance of ≥ 2 new signs, or (2) appearance of one severe sign (i.e., loss of gag reflex or paradoxical breathing). Persistence of neurotoxicity was defined as the persistence of ≥ 1 sign/s in the absence of criteria of neurotoxicity worsening. Patients were assessed hourly until signs of neurotoxicity disappeared (i.e., clinical score = 0). Complete neurological recovery was defined as reaching and sustaining a score of 0.
Dead snakes brought by victims were labelled and preserved in ethanol. Blinded identification was conducted by taxonomic experts. Morphological features of snakes and mitochondrial cytochrome b sequences of snakes generated using trace DNA swabbed from bite sites were analysed by comparison with reference specimens in museum collections and existing nucleotide sequence databases [23].
Sample size, and statistical analyses
We assumed a 60% rate of the composite primary outcome in the low dose group, and hypothesised that this would be reduced to 40% in the high dose group. Therefore, 99 patients would be needed in each arm (1-β = 80%, two-sided α = 0·05) and, assuming a dropout rate of 20%, the total estimated sample size was 250 patients.
Effectiveness and safety analyses were performed on a modified intention-to-treat (mITT) population, i.e., all patients who received antivenom and had at least one post-baseline effectiveness evaluation. Analyses were also performed on a per-protocol (PP) population to support conclusions made using the mITT population. The PP population was defined by comparing the total dose of antivenom that participants should have received based on the treatment allocation and their clinical evolution, to the actual total dose administered.
We described patients’ baseline characteristics overall and per treatment arm as frequencies for categorical data and median and inter-quartile ranges (IQR) or means and standard deviations (SD), as appropriate, for continuous data.
Categorical data were compared using Chi-squared or Fisher’s exact test, as appropriate. Continuous data were compared using the Student t or the Mann Whitney U tests.
Survival analyses were conducted (1) on the time free from primary endpoint; (2) on the time to reach a neurotoxicity score of 0. For the latter, fatal cases were included and considered as having never recovered. Survival estimates were obtained with the Kaplan-Meier estimator and the comparison between groups was performed using a log-rank test stratified by center. Adverse Events (AEs) were compared between treatment arms using Fisher’s exact test. All AEs were coded using the MedDRA dictionary version 17·0.
Except for the safety endpoints (AEs and SAEs) for which missing values were considered as no events, no missing data imputation was used.
A two-sided p value ≤ 0·05 was considered statistically significant for all analyses. All analyses were performed on R software (R foundation for Statistical Computing, Vienna, Austria, URL http://www.Rproject.org).
Results
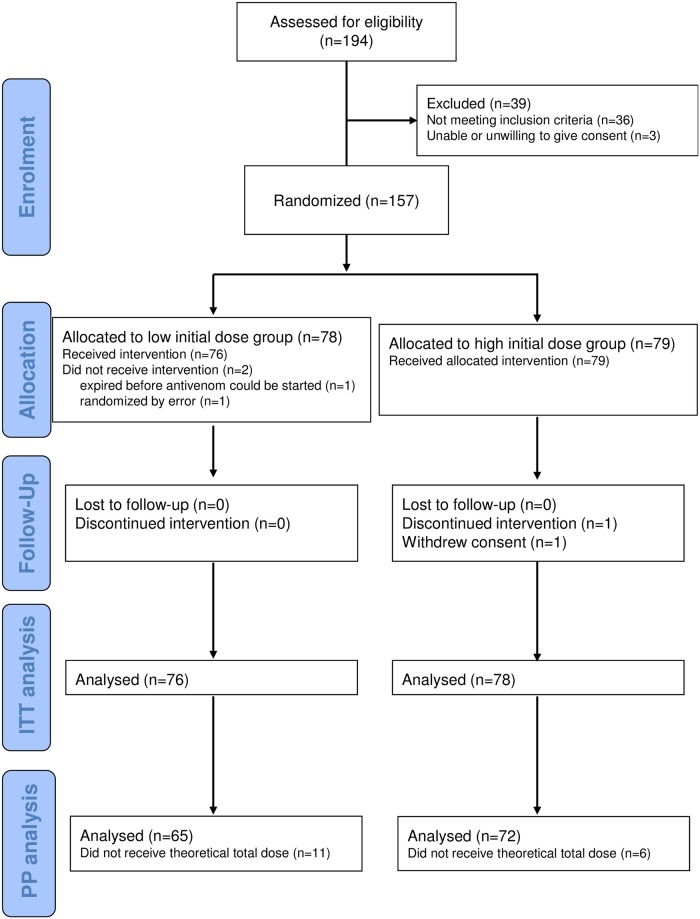
Between April 2011 and October 2012, we assessed 194 snakebite victims with signs of envenoming, of whom 157 were enrolled into the trial (patient flow shown in Fig 1). Of these, 78 and 79 were randomised to the low and high initial dose groups, respectively. Two participants did not receive antivenom because one died before antivenom could be started, and the other had been wrongly randomised (absence of neurotoxicity). One patient in the high dose group withdrew consent. Finally, 154 patients could be included in the mITT analysis while 137 patients were included in the PP analysis.
Fig 1. Flow diagram of the progress of participants through the parallel, randomized trial of high initial dose versus low initial dose of snake antivenom for the treatment of neurotoxic envenoming.
The two treatment groups were similar with respect to baseline characteristics and sex ratio (Table 1). Thirty-two patients out of 154 (20·8%) were aged < 15 years. The severity of envenoming on admission was similar in both groups. The snake species was identified in 53 (34·4%) of 154 trial participants: 29 had been bitten by spectacled cobras (Naja naja), 20 by common kraits (Bungarus caeruleus), two by other kraits (B. lividus and B. niger), and two by other cobras (Naja kaouthia and Naja sp.). The distribution of snake species was balanced between treatment groups. Kraits caused more bites in Bharatpur than Damak (see S1 Table).
Table 1. Baseline demographic and epidemiological characteristics of trial participants.
Figures are numbers of participants (percentage) unless stated otherwise.
| Parameter | Overall N = 154 | Low dose N = 76 | High dose N = 78 | |
|---|---|---|---|---|
| Study Center | Damak | 55 (35·7%) | 27 (35·5%) | 28 (35·9%) |
| Charali | 26 (16·9%) | 12 (15·8%) | 14 (18·0%) | |
| Bharatpur | 73 (47·4%) | 37 (48·7%) | 36 (46·2%) | |
| Sex | Female | 80 (51·9%) | 39 (51·3%) | 41 (52·6%) |
| Male | 74 (48·1%) | 37 (48·7%) | 37 (47·4%) | |
| Age (years) | Median (IQR) | 28 (16–46) | 26 (16–44) | 32 (17–49) |
| Time to reach center (min) | Median (IQR) | 75 (45–148) | 66 (41–134) | 80 (50–150) |
| Missing | 7 | 2 | 5 | |
| Neurotoxic score on admission | Mean ± sd | 2·14 ± 1·18 | 2·21 ± 1·33 | 2·08 ± 1·01 |
| Snake species | Unidentified | 101 (65·6%) | ||
| Identified | 53 (34·4%) | |||
| Cobra | 31 (58%) | 15 (58%) | 16 (59%) | |
| Krait | 22 (42%) | 11 (42%) | 11 (41%) |
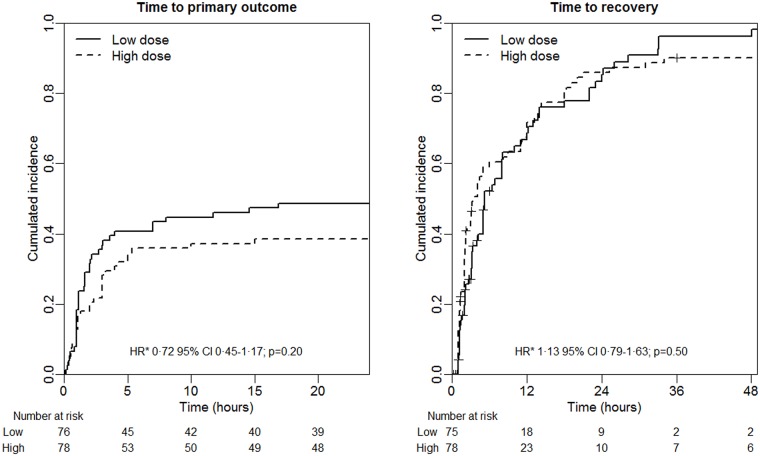
Of the 154 participants included in the mITT analysis, 67 (43·5%) participants met the primary composite outcome definition of death, need for ventilation or worsening of neurotoxicity (Table 2). The proportion was slightly higher in the low vs. high initial dose group but this difference was not statistically significant (risk difference = 10·2%, 95%CI [-6·7; 27·1], p = 0·264). The proportions of patients who either died, required assisted ventilation or experienced worsening neurotoxicity did not differ among treatment groups. In a similar way, neither the time to primary outcome (HR = 0·72 95%CI [0·45; 1·17], p = 0·20) nor the time to recovery (HR = 1·13 95%CI [0·79; 1·63], p = 0·50) was significantly different between the two groups (Fig 2A and 2B). Similar results were obtained in the PP analysis (HR = 0·62 95%CI [0·37; 1·05], p = 0·07 and HR = 1·38 95%CI [0·94; 2·02], p = 0·17, respectively) and in a per centrer analysis (see S2 Table and S3 Fig).
Table 2. Effectiveness endpoints in modified intention-to-treat population.
Figures are numbers of participants (percentage) unless stated otherwise.
| Low dose | High dose | Risk difference [95%CI] | p-value | HR* [95%CI] | p-value** | |
|---|---|---|---|---|---|---|
| n = 76 | n = 78 | |||||
| Primary composite outcome | 37 (48·7%) | 30 (38·5%) | 10·2% [-6·7; 27·1] | 0·264 | 0·72[0·45; 1·17] | 0·199 |
| Worsening toxicity1 | 31 (43·7%) | 27 (35·5%) | 8·1% [-9·0; 25·3] | 0·401 | ||
| Need for ventilation | 15 (19·7%) | 13 (16·7%) | 3·1% [-10·4; 16·6] | 0·776 | ||
| Death | 2 (2·6%) | 7 (9·0%) | -6·3% [-14·9; 2·2] | 0·167 | ||
| Number of vials, mean ± sd | 11·0 ± 7·9 | 12·5 ± 3·9 | 1·5 [-0·5; 3·5]*** | 0·142 | ||
| < 10 vials | 41 (53·9%) | 0 (0%) | ||||
| 10 to 15 vials | 17 (22·4%) | 70 (89·7%) | ||||
| > 15 vials | 18 (23·7%) | 8 (10·3%) |
1Seven patients had missing data for neurotoxicity score, 5 in the low dose group and 2 in the high dose group. All of these patients required ventilation so that a positive response to the primary composite outcome could be defined. It follows that no missing data remained for the primary composite outcome.
*adjusted for center;
**log-rank test stratified for center;
***mean difference [95%CI], p value from a Welch t test
Fig 2. Cumulative incidence by study arm for primary outcome1 (left panel) and recovery2 (right panel) obtained with Kaplan-Meier survival estimator in 154 patients (modified intent-to-treat population).
The observed average number of vials consumed was higher in the high initial dose group (mean = 12·5) than in the low initial dose group (mean = 11·0), but the mean difference was not statistically significant (mean difference = 1·5 95%CI [-0·5; 3·5], p = 0·14). However, the percentage of patients having 16 or more vials was higher in the low initial dose group (24% vs. 10%, p = 0·0446) (Table 2).
We investigated the impact of the biting species on the effectiveness outcomes in the 53 patients for whom the species could be identified (Table 3). Patients bitten by kraits met the primary outcome more frequently, received more vials, recovered less often and when they did, the time for recovery was longer. The small number of victims for which the snake species could be identified precluded an effectiveness analysis by treatment group.
Table 3. Effectiveness endpoints by biting species.
| Snake species | Cobras N = 31 |
Kraits (3 species) N = 22 |
Difference [95%CI] | p-value |
|---|---|---|---|---|
| Primary composite outcome, N (%) | 8 (26%) | 15 (68%) | 0·004 | |
| Patients reaching full neurotoxic recovery, N (%) | 29 (94%) | 9 (41%) | <0·001 | |
| Time (h) to recovery, mean ± sd | 5·0 ± 6·0 | 18·3 ± 12·0 | 0·0102 | |
| Number of vials, mean ± sd | 8·9 ± 3·3 | 18·3 ± 6·8 | 9·4 [6·2; 12·6] | <0·0001 |
We also investigated the impact of the study centre on the effectiveness outcomes and found that the time to primary outcome was significantly shorter in Bharatpur than Damak, after adjustment for treatment arm (HR = 2·00 95%CI [1·2; 3·6], p = 0·014). The average number of vials consumed per patient was also higher in Bharatpur compared to the other two centers (mean difference = 3·7, 95%CI [1·8; 5·6], p = 0·0003).
A total of 137 patients (89%) reported ≥ 1 AE (Table 4) with no significant difference between high 73/78 (94%) and low dose 64/76 (84%) groups (risk difference = 9·4% 95%CI [-1·8; 20·5], p = 0·075). In 82 patients (53·2%) the AE was deemed related to antivenom treatment with no difference between high 42/78 (54%) vs. low dose 40/76 (53%) groups (p = 1). Seven out of 154 patients (5%) reported symptoms consistent with serum sickness (i.e., arthralgia occurring more than 7 days after antivenom treatment). In the PP analysis the proportion of patients experiencing at least one AE was higher in the high dose group (risk difference = 12·9% 95%CI [-0·6; 25·2], p = 0·0373). This difference in proportion was not specific to one type of AE. Among the 154 patients included in the mITT analysis, 18 experienced at least one SAE (Table 5), seven (9%) in the low dose group and 11 (14%) in the high dose group (p = 0·45). Among a total of 19 SAEs, seven were deemed definitely or probably related to the antivenom, two in the low dose group and five in the high dose group.
Table 4. Safety endpoints.
Figures are numbers of participants (percentage) unless stated otherwise.
| All (n = 154) | Low dose (n = 76) | High dose (n = 78) | p-value | |
|---|---|---|---|---|
| Patients reporting Adverse Events | 137 (89%) | 64 (84%) | 73 (94%) | 0·075 |
| Patients reporting Serious Adverse Events | 18 (12%) | 7 (9%) | 11 (14%) | 0·45 |
| Type of events reported | ||||
| Skin and subcutaneous tissue disorders | 101 (65·6%) | 49 (64·5%) | 52 (66·7%) | 0·866 |
| Infected bite | 62 (61·4%) | 34 (44·7%) | 28 (35·9%) | 0·324 |
| Pruritus, rash or angioedema | 59 (58·4%) | 29 (38·2%) | 30 (38·5%) | 1 |
| General disorders | 75 (48·7%) | 35 (46·1%) | 40 (51·3%) | 0·524 |
| Fever and chills | 73 (47·4%) | 35 (46·1%) | 38 (48·7%) | 0·750 |
| Gastrointestinal disorders | 51 (33·1%) | 28 (36·8%) | 23 (29·5%) | 0·393 |
| Epigastric discomfort | 28 (18·2%) | 16 (21·1%) | 12 (15·4%) | 0·408 |
| Vomiting | 23 (14·9%) | 11 (14·5%) | 12 (15·4%) | 1 |
| Abdominal pain | 6 (3·9%) | 4 (5·3%) | 2 (2·6%) | 0·439 |
| Respiratory, thoracic and mediastinal disorders | 39 (25·3%) | 18 (23·7%) | 21 (26·9%) | 0·712 |
| Tachypnoea | 24 (15·6%) | 10 (13·2%) | 14 (17·9%) | 0·507 |
| Wheezing/laryngeal edema | 13 (8·4%) | 7 (9·2%) | 6 (7·7%) | 0·779 |
| Respiratory failure | 6 (3·9%) | 0 (0%) | 6 (7·7%) | 0·028 |
| Nervous system disorders | 22 (14·3%) | 8 (10·5%) | 14 (17·9%) | 0·250 |
| Paraesthesia | 11 (7·1%) | 6 (7·9%) | 5 (6·4%) | 0·764 |
| Headache | 7 (4·5%) | 1 (1·3%) | 6 (7·7%) | 0·117 |
| Musculoskeletal and connective tissue disorders | 22 (14·3%) | 9 (11·8%) | 13 (16·7%) | 0·491 |
| Myalgia | 9 (5·8%) | 3 (3·9%) | 4 (5·1%) | 1 |
| Arthralgia1 | 7 (4·5%) | 3 (3·9%) | 4 (5·1%) | 1 |
1 Late arthralgia: defined as occurring later than 7 days after treatment initiation
Table 5. List of serious adverse events (SAE) occurring in snakebite victims with neurotoxic signs randomized to either a low or a high initial dose of antivenom.
| Nature of the SAE | Seriousness criteria | Relationship to treatment | Outcome | |
|---|---|---|---|---|
| 1 | Anaphylactic reaction | Life-threatening | Definitely related | Resolved |
| 2 | Anaphylactic reaction | Results in death | Probably related | Death |
| 3 | Anaphylactic reaction | Life-threatening | Definitely related | Resolved |
| 4 | Delayed anaphylactic reaction | Results in death | Probably related | Death |
| 5 | Anaphylactic reaction | Life-threatening | Definitely related | Resolved |
| 6 | Anaphylactic reaction | Life-threatening | Definitely related | Resolved |
| 7 | Gangrene of bitten finger | Prolonged hospitalization and permanent disability | Unlikely to be related | Resolved with sequelae |
| 8 | Respiratory failure | Results in death | Not related | Death |
| 9 | Cardiac arrest | Life-threatening | Definitely related | Resolved |
| 10 | Tracheostomy1 | Results in death | Unlikely to be related | Not resolved |
| 11 | Sudden death after discharge (unexplained) | Results in death | Unlikely to be related | Not resolved |
| 12 | Anaphylactoid reaction | Life-threatening | Probably related | Resolved |
| 13 | Overdose2 | Overdose | NA | NA |
| 14 | Death (unexplained reason) | Results in death | Unlikely to be related | Death |
| 15 | Death (overwhelming envenoming) | Results in death | Unlikely to be related | Death |
| 16 | Post-anoxic myoclonus | Prolonged hospitalization | Unlikely to be related | Recovered |
| 17 | Anaphylactoid reaction | Results in death | Probably related | Death |
| 18 | Respiratory failure | Results in death | Unlikely to be related | Death |
| 19 | Death (overwhelming envenomation) | Results in death | Unlikely to be related | Death |
1Death consecutive to tracheostomy occurred in the same patient as the cardiac arrest.
2As per trial protocol, overdose of antivenom were to be considered as SAE and reported on expedited basis to the sponsor.
The incidence of anaphylactic reactions did not differ significantly before and after the implementation of the low-dose adrenaline pre-treatment in April 2012 (pruritus, rash or angiodema: 30/85 (35·3%) vs. 29/69 (42·0%) p = 0·491, wheezing/laryngeal oedema 10/85 (11·8%) vs. 3/69 (4·3%) p = 0·175, anaphylaxis 0/85 (0%) vs. 1/69 (1·5%) p = 0·448, cardiovascular shock 4/85 (4·7%) vs. 0/69 (0%) p = 0·128).
Discussion
This study failed to demonstrate that a high initial dose of antivenom was more effective than a low initial dose in treating neurotoxic envenoming among Nepali patients. None of the components of the composite primary end point were significantly different in the higher dose group, but the data suggested that the rate of progression was slower in this group. The occurrence of AEs appeared slightly higher in the high dose group (statistical significance was not achieved in mITT but in PP analyses), however this difference was not clinically relevant.
Our study took place in two dedicated snakebite clinics, one in a rural area and the other in a small town, and in a referral hospital of a larger town. Thus, our study mirrored the routine management of snakebite victims in Nepal and, probably, most of South Asia. This and the low number of losses-to-follow-up increased our study’s external validity. Another significant strength was the ability to ascertain the biting species in a third of patients. Identifying the snake species is extremely challenging in South Asia because of the lack of robust methods [23], while patients’ descriptions are unreliable. The response to antivenom is highly dependent on the toxins of the biting snake. Thus, determining the biting species is key to giving the correct antivenom and anticipating the clinical course and potential complications. Other studies have achieved higher rates of species identification [12,13], thanks to ELISA-based methods against circulating venom components.
To our knowledge, this is the first robust RCT to compare different dose regimens of antivenom in the treatment of neurotoxic envenoming. Most published studies lacked a proper power calculation [7,10,34], were un-blinded [7,9,10] and/or used inappropriate or incomplete randomization [10,12,34]. Moreover, several studies mixed neurotoxic and haematotoxic envenoming or included patients with nonspecific manifestations like confusion or bradycardia [7–9]. A systematic review in 2015 deemed these studies to be of very low quality evidence [17]. There is currently no validated and internationally recommended protocol to monitor the clinical progression of neurotoxic envenoming. We developed a scoring system, based on objective, readily-assessable, clinical signs that may be used by staff in small health posts or clinics. Although it has yet to be formally validated, the intra- and inter- observer reliability of the scoring method was tested during the planning phase of the trial and found to be high. The calculated scores also showed consistency across different observers and over different time periods in retrospective analysis, adding confidence to our endpoint measurements.
Almost half of the trial participants either died, developed respiratory paralysis, or experienced a worsening of neurotoxicity despite the administration of antivenom. Antivenom effectiveness depends on its ability to neutralise the venom of the local snakes. Several medically-important snake species in Nepal are not covered covered by the Indian antivenom, while E. carinatus is not present in the country [23]. Although most species responsible for envenoming bites in the present study were the same as those whose venom is used to raise Indian antivenom, venom composition is known to vary within a species from region to region. The pre-clinical efficacy of Indian antivenoms against the venoms of Nepali neurotoxic species is unknown. Both the greater species diversity and geographical variation in venom composition could have contributed to the overall poor performance of the antivenom. Moreover, the utility of antivenom in the management of krait envenoming has long been questioned [35,36]. The most lethal neurotoxins of krait venoms, β-bungarotoxins, are presynaptic in their mode of action, irreversibly destroying motor nerve terminals. Thus, clinical recovery is slow because it depends on the regeneration of the neuromuscular junction [36]. Results of our subgroup analyses confirmed that, compared to the cobra victims, patients bitten by kraits deteriorated more frequently, recovered more slowly and required more vials of antivenom. These findings are consistent with the observation that, in Bharatpur hospital where krait bite envenoming predominates [23], patients had a worse prognosis than at the other study sites. We support calls for the establishment of regional venom banks of geographically representative snake populations [37] for the development of new, better-targeted antivenoms. It is also an essential pre-requisite of national regulatory authorities to test independently the effectiveness of marketed antivenoms, in line with WHO recommendations [5].
The absence of a significant difference between the high and the low-dose groups in the response to antivenom should not be interpreted as evidence of no benefit of a high initial dose. This absence of statistical significance is potentially due to a lack of power owing to a lower-than-targeted sample size, an optimistic hypothesized difference in the sample size calculation (the observed difference was only ~10% whereas we powered the study to detect a 20% difference), and the substantial proportion of krait bite envenoming in our study population. Convincing evidence of the benefit or superiority of the higher initial dose regimen would require a large study with a mortality endpoint and higher proportion of identified snakes, a very unlikely scenario for such a neglected disease. In view of the complexity of the dosing regimen recommended in the Nepal national guidelines, and since a high initial dose regimen does not result in a higher consumption of antivenom, the dose regimen recommended by WHO guidelines seems a reasonable preference for treating neurotoxic envenoming in Nepal and the rest of South Asia. Clinicians will have to balance the simplicity of administration of this regimen with a slight increase in the occurrence of adverse events.
Supporting information
Each sign scored one.
(DOCX)
(PDF)
(DOCX)
(DOCX)
Figures are numbers of participants (percentage) unless stated otherwise.
(DOCX)
Acknowledgments
We would like to thank Khaled Mostaguir for his support with the data management, as well as Varalakshmi Elango, Ninon Horie and Morgane Amman for performing the trial monitoring visits. We are also thankful to the clinicians who clinically managed snakebite victims in Nepal.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The study was funded by the Swiss National Science Foundation (grant number IZ70Z0_131223). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Alirol E, Lechevalier P, Zamatto F, Chappuis F, Alcoba G, Potet G. Antivenoms for snakebite envenoming: what is in the research pipeline? PLoS Negl Trop Dis 2015; 9: e0003896 10.1371/journal.pntd.0003896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown NI. Consequences of neglect: analysis of the sub-Saharan African snake antivenom market and the global context. PLoS Negl Trop Dis 2012; 6: e1670 10.1371/journal.pntd.0001670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Visser L E, Kyei-Faried S, Belcher D W, Geelhoed D W, van Leeuwen J S, van Roosmalen J. Failure of a new antivenom to treat Echis ocellatus snake bite in rural Ghana: the importance of quality surveillance. Trans R Soc Trop Med Hyg 2008; 102: 445–50 10.1016/j.trstmh.2007.11.006 [DOI] [PubMed] [Google Scholar]
- 4.Simpson ID, Norris RL. Snake antivenom product guidelines in India: ‘the devil is in the details’. Wilderness Environ Med 2007; 18:163–8. 10.1580/07-WEME-ED-099R.1 [DOI] [PubMed] [Google Scholar]
- 5.Guidelines for the production, control and regulation of snake antivenom immunoglobulins. World Health Organisation; Geneva: 2010. [DOI] [PubMed] [Google Scholar]
- 6.Jorge M T, Cardoso J L C, Castro S C B, et al. A randomized ‘blinded’ comparison of two doses of antivenom in the treatment of Bothrops envenoming in Sao Paulo, Brazil. Trans R Soc Trop Med Hyg 1995; 89: 111–4. [DOI] [PubMed] [Google Scholar]
- 7.Paul V, Pratibha S, Prahlad KA, Earali J, Francis S, Lewis F. High-dose anti-snake venom versus low-dose anti-snake venom in the treatment of poisonous snake bites—a critical study. J Assoc Physicians India 2004; 52: 14–7. [PubMed] [Google Scholar]
- 8.Tariang D D, Philip P J, Alexander G, Macaden S, Jeyaseelan L, Peter J V, Cherian A M. Randomized controlled trial on the effective dose of anti-snake venom in cases of snake bite with systemic envenomation. J Assoc Physicians India 1999; 47: 369–71. [PubMed] [Google Scholar]
- 9.Thomas P P, Jacob J. Randomised trial of antivenom in snake envenomation with prolonged clotting time. Br Med J 1985; 291: 177–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Srimanarayana J, Dutta T K, Sahai A, Badrinath S. Rational use of anti-snake venom (ASVS): trial of various regimens in hemotoxic snake envenomation. J Assoc Physicians India 2004; 52: 788–93. [PubMed] [Google Scholar]
- 11.Abubakar S B, Abubakar I S, Habib A G, et al. Pre-clinical and preliminary dose-finding and safety studies to identify candidate antivenoms for treatment of envenoming by saw-scaled or carpet vipers (Echis ocellatus) in northern Nigeria. Toxicon 2010; 55: 719–23. 10.1016/j.toxicon.2009.10.024 [DOI] [PubMed] [Google Scholar]
- 12.Ariaratnam C A, Meyer W P, Perera G, et al. A new monospecific ovine Fab fragment antivenom for treatment of envenoming by the Sri Lankan Russell’s viper (Daboia russelii russelii): A preliminary dose-finding and pharmacokinetic study. Am J Trop Med Hyg 1999; 61: 259–65. [DOI] [PubMed] [Google Scholar]
- 13.Allen G E, Brown S G A, Buckley N A, et al. Clinical effects and antivenom dosing in brown snake (Pseudonaja spp.) envenoming—Australian snakebite project (ASP-14). PLoS One 2012; 7: e53188 10.1371/journal.pone.0053188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rahmani A H, Jalali A, Alemzadeh-Ansari M H, Tafazoli M, Rahim F. Dosage comparison of snake anti-venom on coagulopathy. Iran J Pharm Res 2014; 13: 283–9. [PMC free article] [PubMed] [Google Scholar]
- 15.Yeung JM, Little M, Murray LM, Jelinek G A, Daly F S. Antivenom dosing in 35 patients with severe brown snake (Pseudonaja) envenoming in Western Australia over 10 years. Med J Aust; 181: 703–5. [PubMed] [Google Scholar]
- 16.Theakston RD, Fan HW, Warrell DA, Da Silva W D, Ward S A, Higashi H G. Use of enzyme immunoassays to compare the effect and assess the dosage regimens of three Brazilian Bothrops antivenoms. The Butantan Institute Antivenom Study Group (BIASG). Am J Trop Med Hyg 1992; 47: 593–604. [DOI] [PubMed] [Google Scholar]
- 17.Das RR, Sankar J, Dev N. High-dose versus low-dose antivenom in the treatment of poisonous snake bites: a systematic review. Indian J Crit Care Med 2015;19: 340–9. 10.4103/0972-5229.158275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharma SK, Khanal B, Pokhrel P, Khan A, Koirala S. Snakebite—reappraisal of the situation in eastern Nepal. Toxicon 2003; 41: 285–9. [DOI] [PubMed] [Google Scholar]
- 19.Sharma SK, Chappuis F, Jha N, Bovier P A, Loutan L, Koirala S. Impact of snake bites and determinants of fatal outcomes in southeastern Nepal. Am J Trop Med Hyg 2004; 71: 234–8. [PubMed] [Google Scholar]
- 20.Pyakurel R, Sharma N, Paudel D, et al. Cause of death in women of reproductive age in rural Nepal obtained through community-based surveillance: Is reducing maternal mortality the right priority for women’s health programs? Health Care Women Int 2014; 36: 1–8. [DOI] [PubMed] [Google Scholar]
- 21.Harrison RA, Hargreaves A, Wagstaff SC, Faragher B, Lalloo DG. Snake envenoming: a disease of poverty. PLoS Negl Trop Dis 2009; 3: e569 10.1371/journal.pntd.0000569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hansdak SG, Lallar KS, Pokharel P, Shyangwa P, Karki P, Koirala S. A clinico-epidemiological study of snake bite in Nepal. Trop Doct 1998; 28: 223–6. 10.1177/004947559802800412 [DOI] [PubMed] [Google Scholar]
- 23.Sharma SK, Kuch U, Höde P, et al. Use of molecular diagnostic tools for the identification of species responsible for snakebite in Nepal: a pilot study. PLoS Negl Trop Dis 2016; 10: e0004620 10.1371/journal.pntd.0004620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ariaratnam CA, Sheriff MHR, Theakston RDG, Warrell DA. Distinctive epidemiologic and clinical features of common krait (Bungarus caeruleus) bites in Sri Lanka. Am J Trop Med Hyg 2008; 79: 458–62. [PubMed] [Google Scholar]
- 25.Kularatne SAM, Budagoda BDSS, Gawarammana IB, Kularatne WKS. Epidemiology, clinical profile and management issues of cobra (Naja naja) bites in Sri Lanka: first authenticated case series. Trans R Soc Trop Med Hyg 2009; 103: 924–30. 10.1016/j.trstmh.2009.04.002 [DOI] [PubMed] [Google Scholar]
- 26.Whitaker R, Whitaker S. Venom, antivenom production and the medically important snakes of India. Curr Sci; 2012; 103: 635–43. [Google Scholar]
- 27.Shah K B, Shrestha J M, Thapa. Snake bite management guideline. Nepal Ministry of Health, Department of Health Services, Epidemiology and Disease Control Division; Kathmandu: 2003. [Google Scholar]
- 28.Theakston RD, Warrell DA. Antivenoms: a list of hyperimmune sera currently available for the treatment of envenoming by bites and stings. Toxicon 1991; 29: 1419–70. [DOI] [PubMed] [Google Scholar]
- 29.Isbister GK, Maduwage K, Saiao A, et al. Population pharmacokinetics of an Indian F(ab’)2 snake antivenom in patients with Russell's viper (Daboia russelii) bites. PLoS Negl Trop Dis 2015; 9: e0003873 10.1371/journal.pntd.0003873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.World Health Organization regional Office for South-East Asia. Guidelines for the management of snakebites. (2nd Edition WHO, New Delhi, 2016) http://apps.searo.who.int/PDS_DOCS/B5255.pdf?ua=1. [Google Scholar]
- 31.Agarwal R, Aggarwal AN, Gupta D, Behera D, Jindal SK. Low dose of snake antivenom is as effective as high dose in patients with severe neurotoxic snake envenoming. Emerg Med J 2005; 22:397–9. 10.1136/emj.2004.020727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vijeth SR, Dutta TK, Shahapurkar J, Sahai A. Dose and frequency of anti-snake venom injection in treatment of Echis carinatus (saw-scaled viper) bite. J Assoc Physicians India 2000; 48: 187–91. [PubMed] [Google Scholar]
- 33.De Silva HA, Pathmeswaran A, Ranasinha CD, et al. Low-dose adrenaline, promethazine, and hydrocortisone in the prevention of acute adverse reactions to antivenom following snakebite: a randomised, double-blind, placebo-controlled trial. PLoS Med 2011; 8: e1000435 10.1371/journal.pmed.1000435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pochanugool C, Limthongkul S, Wilde H. Management of Thai cobra bites with a single bolus of antivenin. Wilderness Environ Med 1997; 8: 20–3. [DOI] [PubMed] [Google Scholar]
- 35.Theakston RD, Phillips RE, Warrell DA, et al. Envenoming by the common krait (Bungarus caeruleus) and Sri Lankan cobra (Naja naja naja): efficacy and complications of therapy with Haffkine antivenom. Trans R Soc Trop Med Hyg 1990; 84: 301–8. [DOI] [PubMed] [Google Scholar]
- 36.Ranawaka UK, Lalloo DG, de Silva HJ. Neurotoxicity in snakebite—the limits of our knowledge. PLoS Negl Trop Dis 2013; 7: e2302 10.1371/journal.pntd.0002302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gutiérrez JM, Burnouf T, Harrison RA, et al. A multicomponent strategy to improve the availability of antivenom for treating snakebite envenoming. Bull World Health Organ 2014; 92: 526–32. 10.2471/BLT.13.132431 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Each sign scored one.
(DOCX)
(PDF)
(DOCX)
(DOCX)
Figures are numbers of participants (percentage) unless stated otherwise.
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.