Abstract
Hypertension is a leading cause of global disease, mortality, and disability. While individuals of African descent suffer a disproportionate burden of hypertension and its complications, they have been underrepresented in genetic studies. To identify novel susceptibility loci for blood pressure and hypertension in people of African ancestry, we performed both single and multiple-trait genome-wide association analyses. We analyzed 21 genome-wide association studies comprised of 31,968 individuals of African ancestry, and validated our results with additional 54,395 individuals from multi-ethnic studies. These analyses identified nine loci with eleven independent variants which reached genome-wide significance (P < 1.25×10−8) for either systolic and diastolic blood pressure, hypertension, or for combined traits. Single-trait analyses identified two loci (TARID/TCF21 and LLPH/TMBIM4) and multiple-trait analyses identified one novel locus (FRMD3) for blood pressure. At these three loci, as well as at GRP20/CDH17, associated variants had alleles common only in African-ancestry populations. Functional annotation showed enrichment for genes expressed in immune and kidney cells, as well as in heart and vascular cells/tissues. Experiments driven by these findings and using angiotensin-II induced hypertension in mice showed altered kidney mRNA expression of six genes, suggesting their potential role in hypertension. Our study provides new evidence for genes related to hypertension susceptibility, and the need to study African-ancestry populations in order to identify biologic factors contributing to hypertension.
Author summary
Hypertension is a global health problem which affects disproportionally people of African descent. We conducted a genome-wide association study of blood pressure in 31,968 Africans and African Americans to identify genes conferring susceptibility to increased blood pressure. This research identified three novel genomic regions associated with blood pressure which have not been previously reported in studies of other race/ethnicity. Using experimental models, we also showed an altered expression of these genes in kidney tissue in hypertension. These findings provide new evidence for genes influencing hypertension risk and supports the need to study diverse ancestry populations in order to identify biologic factors contributing to hypertension.
Introduction
Genetic studies hold the promise of providing tools to better understand and treat clinical conditions. To achieve the clinical and public health goals of reducing hypertension and its sequelae, and to understand ethnic disparities in the risk for hypertension, there is a need to study susceptible populations for genetic determinants of blood pressure (BP). BP traits are highly heritable across world populations (30 to 55%).[1–4] Over 200 genetic loci have been identified in genome-wide association studies [5–13] and admixture mapping studies.[14–17] These variants explain approximately 3.5% of inter-individual variation in BP.[5, 7] However, there is still a paucity of studies focused on individuals of African descent. Most of the loci identified in the literature have not been replicated in individuals of African ancestry.[18, 19]
African Americans have higher mean BP, an earlier onset of hypertension, and a greater likelihood to have treatment-resistant hypertension than other ethnic groups.[20–23] Emerging research on Africans shows increasing prevalence of hypertension in urban African communities [24, 25] which are more Westernized than rural African communities and, so, more closely resemble communities in which African Americans live in the U.S. Hypertension contributes to a greater risk of coronary heart disease, stroke, and chronic kidney disease.[26–30] African Americans experience increased risk of these hypertension-related outcomes [31–34] but the underlying mechanisms, whether environmental exposures or increased genetic susceptibility, are unknown.
We hypothesized that additional variants associated with BP can be identified in people of African ancestry; some variants may be African-specific, as has been observed for multiple traits, including kidney disease [35] and metabolic syndrome.[36, 37] Other variants may be identified in novel loci based on a higher frequency of risk alleles in this population. We used high density imputed genotypes from the 1000 Genomes Project (1000G) to expand the genome coverage of genetic variants so that we could examine the evidence for association with BP traits.
Here, we report three novel loci associated with BP which are driven by variants that are common in or unique to African-ancestry populations. Through bioinformatics and experimental evidence of kidney gene expression in mice submitted to angiotensin-II (Ang II) induced hypertension, we provide evidence for a key role of these genes in the pathogenesis of hypertension. In addition, our study extends the discovery of BP loci to genes related to kidney and the immune systems, and provides biological relevance for these loci to BP regulation.
Results
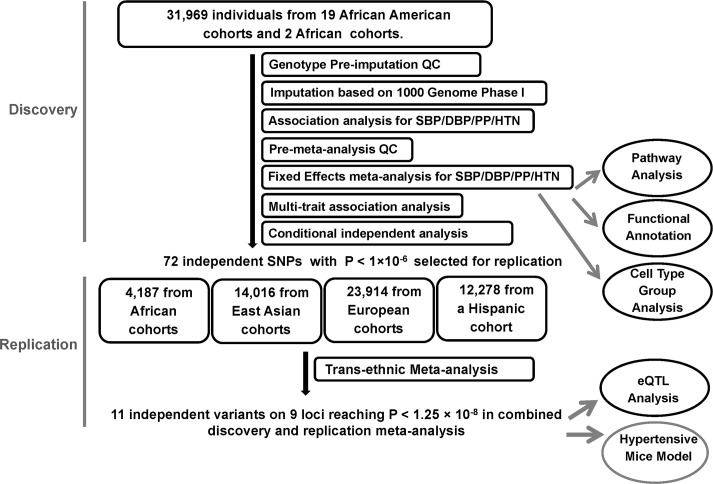
The study design and analysis process are shown in Fig 1. Study characteristics, genotyping, and quality control (QC) for discovery and replication samples are shown in S1 and S2 Tables. The discovery samples included 31,968 individuals of African ancestry from 19 studies. The replication samples included 4,184 individuals of African ancestry from three studies, 23,914 individuals of European ancestry from five studies, 14,016 individuals of Korean ancestry from three studies, and 12,278 individuals of Hispanic/Latino ancestry from one study.
Fig 1. Study design schematic for discovery and replication of loci.
QC, quality control; SBP, systolic blood pressure; DBP, diastolic blood pressure; PP, pulse pressure; HTN, hypertension; eQTL, expression quantitative loci.
Single-trait and multi-trait meta-analysis genome-wide association study (GWAS) results
Study-specific genomic-control inflation ranged from 0.98–1.06 (S3 Table, S1 Fig) and the linkage disequilibrium (LD) score regression intercepts of the single-trait BP meta-analyses calculated by the LD score regression approach ranged from 1.02–1.04. [38] These results suggest well-controlled population stratification.
The single-trait BP meta-analyses identified several genome-wide significant single nucleotide polymorphisms (SNP) at eight loci (P < 5.0×10−8, systolic BP (SBP): three loci, four SNPs; diastolic BP (DBP): three loci, three SNPs; pulse pressure (PP): three loci, four SNPs; and hypertension (HTN): one locus, one SNP), with the EVX1/HOXA locus identified for SBP, DBP and HTN (S2A–S2D Fig). When combining summary statistics for SBP, DBP, and HTN using the multi-trait approach CPASSOC,[39] we identified one locus by the multi-trait statistic SHom (EVX1/HOXA) and six loci by SHet (ULK4, TCF21, EVX1/HOXA, IGFBP3, CDH17, ZNF746) at P < 5×10−8 (S2E and S2F Fig). Note some loci overlap between single-trait and multi-trait findings.
We observed 264 variants with P < 1×10−6 for either single- or multi- trait GWAS and these variants were further analyzed by conditional association on the most associated SNPs at each locus (S4 Table). These analyses resulted in 72 independent associations, which included 58 SNPs with minor allele frequency (MAF) ≥ 0.05 and 14 with low frequency variants (0.01< MAF < 0.05) (S5 Table).
Trans-ethnic replication
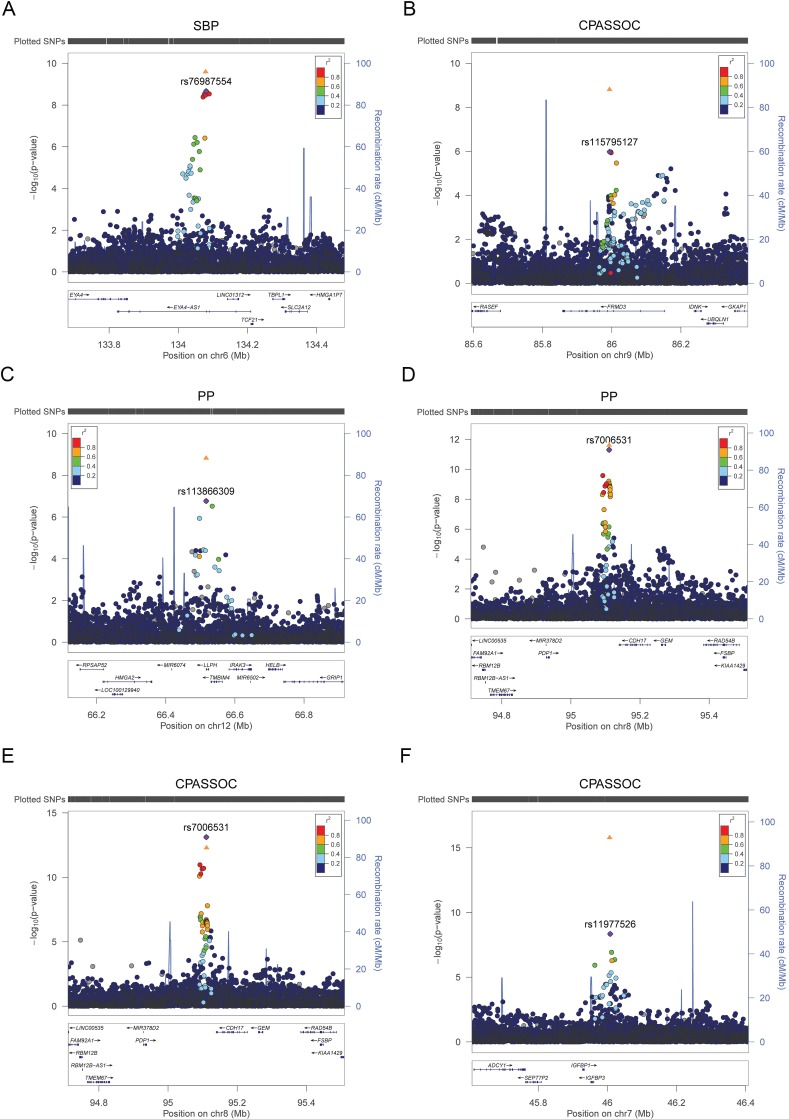
Among these 72 variants carried forward for trans-ethnic replication, nine variants, all low frequency variants (MAF<0.02), were not available in replication cohorts because they were either monomorphic in the replication population or had a low imputation quality, reducing our replication effort to 63 variants (S6 Table). Eleven independent variants at nine loci were significantly associated with BP traits at P < 1.25×10−8 in the combined discovery and replication analyses and are reported in Table 1. This significance level was determined by adjusting for two independent traits for SBP, DBP, PP and HTN, and two tests of multiple trait analysis. This includes six variants that reached significance level at discovery stage (P <5 x10-8). Two loci were identified only through multi-trait analyses (FRMD3, IGFBP3). Three of these nine loci are novel: TARID /TCF21, FRMD3, and LLPH/TMBIM4 (Fig 2A–2C). Four loci (ULK4, PLEKHG1, EVX1/HOXA cluster, and GPR20) have been reported in our previous BP GWAS of African ancestry (S3 Fig),[7, 18] and two loci (IGFBP3, CDH17) have been reported in multiple-trait analyses of African-ancestry studies (Fig 2D–2F).[39] A composite genetic-risk score using the eleven variants identified accounted for 1.89%, 2.92%, 1.03% and 1.08% of the variance for SBP, DBP, PP and HTN respectively.
Table 1. Loci identified in combined COGENT-BP African ancestry discovery samples and multi-ethnic replication samples.
| SNP |
Effect Allele/ Other Allele |
Chr | Nearby Gene |
COGENT-BP Allele Frequency |
1000G Phase 1 Allele Frequency |
Single or Multi-Trait (CPASSOC) Statistic | COGENT-BP Discovery (Up to N = 31,155) | Trans-Ethnic Replication (Up to N = 54,245) |
Combined Meta-analyses (Up to N = 85,397) |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AFR | AMR | ASN | EUR | Beta (SE) | P | P | P | ||||||
| SNPs in novel loci | |||||||||||||
| rs76987554 | C/T | 6 |
TARID/ TCF21 |
0.91 | 0.91 | 0.99 | 1 | 1 | SBP | 1.85 (0.31) | 2.2x10-9 | 2.0x10-2 | 2.3x10-10 |
| rs115795127 | T/C | 9 | FRMD3 | 0.89 | 0.86 | 1 | 1 | 1 | CPASSOC SHet | NA | 1.1x10-6 | 8.4x10-6 | 7.3x10-9 |
| rs113866309 | C/T | 12 |
LLPH/ TMBIM4 |
0.02 | 0.02 | 0.01 | 0.00 | 0.00 | PP | 3.28 (0.63) | 1.7x10-7 | 1.5x10-3 | 8.2x10-9 |
| SNPs in published BP loci | |||||||||||||
| rs7651190 | G/A | 3 | ULK4 | 0.65 |
0.72 | 0.17 | 0.15 | 0.19 | DBP | 0.45 (0.11) | 4.2x10-5 | 1.0x10-5 | 2.0x10-9 |
| CPASSOC SHet | NA | 6.9x10-9 | 2.0x10-4 | 9.8x10-11 | |||||||||
| rs7372217 | G/A | 3 | ULK4 | 0.66 | 0.71 | 0.20 | 0.16 | 0.20 | DBP | 0.55 (0.11) | 9.5x10-7 | 8.1x10-7 | 5.3x10-12 |
| CPASSOC SHet | NA | 8.2x10-6 | 6.5x10-8 | 1.4x10-11 | |||||||||
| rs62434120 | T/A | 6 | PLEKHG1 | 0.85 | 0.83 | 0.82 | 0.95 | 0.92 | SBP | 1.19 (0.24) | 1.1x10-6 | 2.7x10-3 | 5.7x10-9 |
| rs11563582 | A/G | 7 |
EVX1/ HOXA cluster |
SBP | 1.61 (0.28) | 7.1x10-9 | 4.2x10-4 | 4.5x10-10 | |||||
| 0.13 | 0.16 | 0.09 | 0.05 | 0.08 | DBP | 1.02 (0.17) | 8.4x10-10 | 1.4x10-4 | 1.7x10-11 | ||||
| CPASSOC SHom | NA | 1.5x10-10 | 8.0x10-4 | 1.9x10-11 | |||||||||
| CPASSOC SHet | NA | 1.1x10-9 | 9.4x10-3 | 1.8x10-9 | |||||||||
| rs6969780 | C/G | 7 | HOXA | SBP | 0.82 (0.19) | 1.7x10-5 | 6.5x10-5 | 6.2x10-9 | |||||
| 0.30 |
0.35 | 0.21 | 0.13 | 0.10 | DBP | 0.62 (0.12) | 7.0x10-8 | 2.1x10-4 | 3.3x10-10 | ||||
| CPASSOC SHom | NA | 4.1x10-7 | 4.0x10-4 | 9.9x10-9 | |||||||||
| rs11977526 | A/G | 7 | IGFBP3 | 0.34 | 0.34 | 0.31 | 0.78 | 0.41 | CPASSOC SHet | NA | 4.5x10-9 | 2.9x10-9 | 7.3x10-16 |
| rs7006531 | G/A | 8 | CDH17 | 0.15 | 0.19 | 0.02 | 0.00 | 0.00 | PP | 1.16 (0.17) | 5.0x10-12 | 9.7x10-2 | 5.9x10-12 |
| CPASSOC SHet | NA | 7.6x10-14 | 6.1x10-3 | 2.2x10-13 | |||||||||
| rs78192203 | T/A | 8 | GPR20 | 0.80 | 0.79 | 0.98 | 1 | 1 | DBP | 0.77 (0.14) | 1.3x10-8 | 2.7x10-4 | 4.1x10-11 |
Bold P-values represent either significance level at 5.0x10-8 in discovery sample or at 1.25x10-8 at combined discovery and replication samples. 1000G samples: AFR, African ancestry; AMR, American ancestry; ASN, Asian ancestry; EUR, European ancestry
Fig 2.
Regional plots of the significant loci A. TARID/TCF21 for SBP B. FRMD3 for SHet of CPASSOC C. LLPH locus for PP D. CDH17 for PP E. CDH17 for SHet of CPASSOC F. IGFBP3 for SHet of CPASSOC. The y axis shows the −log10 P values of SNP associations, and the x axis shows their chromosomal positions. The lowest P value SNP is plotted as a purple diamond and its correlation with other SNPs in the region is shown in color. The orange triangle is P value in the combined discovery and replication trans-ethnic meta-analysis of the lowest P value SNP.
Newly identified loci harbor variants common only in African-ancestry populations
Five of the eleven replicated variants are common in individuals of African ancestry but rare or monomorphic in individuals of non-African ancestry (rs76987554, rs115795127, rs113866309, rs7006531, and rs78192203)(Table 1). These five variants were 1) either low frequency or common variants in COGENT-BP African-ancestry samples; 2) low frequency in 1000G Phase I Integrated Release Ad Mixed-American ancestry (AMR); and 3) monomorphic in 1000G Asian ancestry (ASN) or European ancestry (EUR). One common variant was present in only 1000G samples of African ancestry (rs115795127 at FRMD3, Table 1). These variants were located at the three novel loci (TARID/TCF21, FRMD3, and LLPH/TMBIM4). Given the differences in allele frequency across continental-ancestry populations, we examined the evidence for selection at each of these loci using iHS, which measures the amount of extended haplotype homozygosity at a given SNP along the ancestral allele relative to the derived allele.[40] The iHS score for rs115795127 was 2.7 in African American samples from the Candidate-gene Association Resource (CARe) consortium (see Methods), suggesting selection at the FRMD3 locus (S7 Table).
Distinct associations at EVX1/HOXA, ULK4, and GPR20 in African-ancestry populations
We observed two independent genome-wide significant variants at the EVX1/HOXA locus (P < 1.25×10−8). The two variants, rs11563582 and rs6969780, are in weak LD (r2 = 0.21) (S3A–S3C Fig), and the LD pattern suggests that these SNPs are located in two blocks (S4 Fig). SNP rs11563582 is in strong LD with the previously reported SNP in the region (rs17428741).[18] SNP rs6969780 remained significant when conditioning on rs11563582 (S4 Table), thus demonstrating the presence of allelic heterogeneity at this locus. Two independent variants at ULK4 reached the significance threshold: rs7651190 and rs7372217 (LD r2 = 0.15) (S4E Fig). SNP rs7372217 is in strong LD with the previous reported SNP rs1717027.[18] The association evidence of rs1717027 can be explained by rs7372217 but not by rs7651190 in conditional analysis (S4 Table). Thus, rs7651190 is an independent association at this locus. At the GPR20 locus, our most significant SNP, rs78192203, is 8kb away and it is not in LD with the published SNP, rs34591516 (r2 = 0.008, D’ = 0.68 in African American CARe participants).
Pathway analyses suggest enrichment of immune pathways for BP traits
To gain insight into biologic mechanisms underlying genes associated with BP traits, we performed pathway analysis using publicly available databases. [41] The most relevant pathways identified were GSK3, Th1/Th2 differentiation, and Sonic Hedgehog (SHH) pathways (BIOCARTA): pyrimidine metabolism, apoptosis signaling pathway, and B cell activation (Panther); JAK Stat signaling, T cell receptor signaling, and B cell receptor signaling (Ingenuity); cytokine-cytokine receptor interaction and vascular smooth muscle contraction (KEGG); and neuronal activity, T cell mediated immunity, and tumor suppressor (Panther Biological Process) (Gene Set Enrichment Analysis [GSEA] P-value < 0.01, S8 Table). These analyses suggest enrichment of immune pathways for BP traits.
Tissue and cell type group enrichment analyses identify immune, kidney, and cardiovascular enriched systems
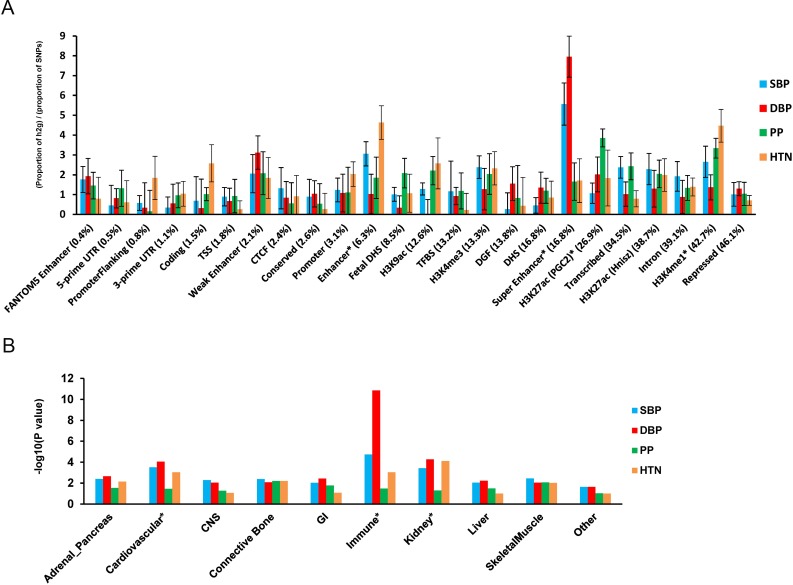
We performed functional annotation and cell type group enrichment analysis using the stratified LD score regression approach which uses data from ENCODE and the Roadmap Epigenetic Project, as well as GWAS results while accounting for the correlation among markers. [42] We estimated functional categories of enrichment using an enrichment score, which is the proportion of SNP-heritability in the category divided by the proportion of SNPs. We identified super enhancer (PEnrich = 5.4×10−5, Enrichment = 5.6 for DBP), enhancer (PEnrich = 4.8 ×10−4, Enrichment = 4.3 for HTN), and H3K27ac (PEnrich = 3.2×10−4, Enrichment = 3.6 for HTN) significant enrichment (Fig 3). These results support a role of identified noncoding regulatory regions in BP regulation. In addition, the following cell types showed significant enrichment (P ≤ 2.5 × 10−3): the immune (PEnrich = 1.4×10−9, Enrichment = 8.4 for DBP), kidney (PEnrich = 5.4×10−5, Enrichment = 4.8 for DBP), and cardiovascular (PEnrich = 8.9×10−5, Enrichment = 4.2 for SBP) systems (Fig 3).
Fig 3. Enrichment for functional annotations and cell-type groups using stratified LD score regression.
A. Enrichment estimates of 24 main annotations for each of four BP traits. Annotations are ordered by size. Error bars represent jackknife standard errors around the estimates of enrichment, and stars indicate significance at P < 0.05 after Bonferroni correction for 24 hypotheses tested and four BP traits. B. Significance of enrichment of 10 cell-type groups for four BP traits. Dotted line and stars indicate significance at P < 0.05 after Bonferroni correction for 10 hypotheses tested and four BP traits.
We next determined the enrichment of variants at the eleven genome-wide significant loci for DNase l hypersensitive (DHS) sites in 34 tissue categories from ENCODE. At each locus, we identified variants in r2>0.1 with the index variant and calculated causal evidence (Bayes Factors) for each variant. We then tested for enrichment in the causal evidence of variants in DHS sites using fGWAS.[43] We found enrichment of blood/immune DHS (Enrichment = 3.1) and cardiovascular DHS (blood vessel Enrichment = 28.7, heart Enrichment = 2.0), in addition to DHS in several fetal tissues (S5 Fig). Candidate causal variants at several loci overlapped enriched DHS sites. For example, at the LLPH/TMBIM4 locus, the most likely causal variant, rs12426813, overlaps a DHS site active in immune (CD14+, CD4+, CD34+), blood vessel (HMVEC), and heart (HCF) cells (S5 Fig).
Overlap with eQTL at specific tissues
To examine whether the eleven significant SNPs are eQTL, we searched the genotype-tissue expression (GTEx) pilot database, which includes non-disease human tissue.[42] Among the eleven SNPs, three SNPs have been identified as eQTL: rs6969780 (HOXA2), rs7651190 (ULK4), and rs62434120 (PLEKHG1) (S9 Table). SNP rs6969780 is an eQTL for expression of HOXA2, HOXA7, HOTAIRM1, and HOXA5 in multiple tissues, including esophagus, artery, lung, skin, nerve, adipose, skeletal muscle, and stomach tissues. SNP rs7651190 is an eQTL for ULK4 and RPL36P20 in artery, whole blood, thyroid, nerve, esophagus, skeletal muscle, skin, brain, and stomach cells/tissues. SNP rs62434120 is an eQTL for PLEKHG1 in testis tissue.
Kidney gene expression in experimental angiogensin II-induced hypertension
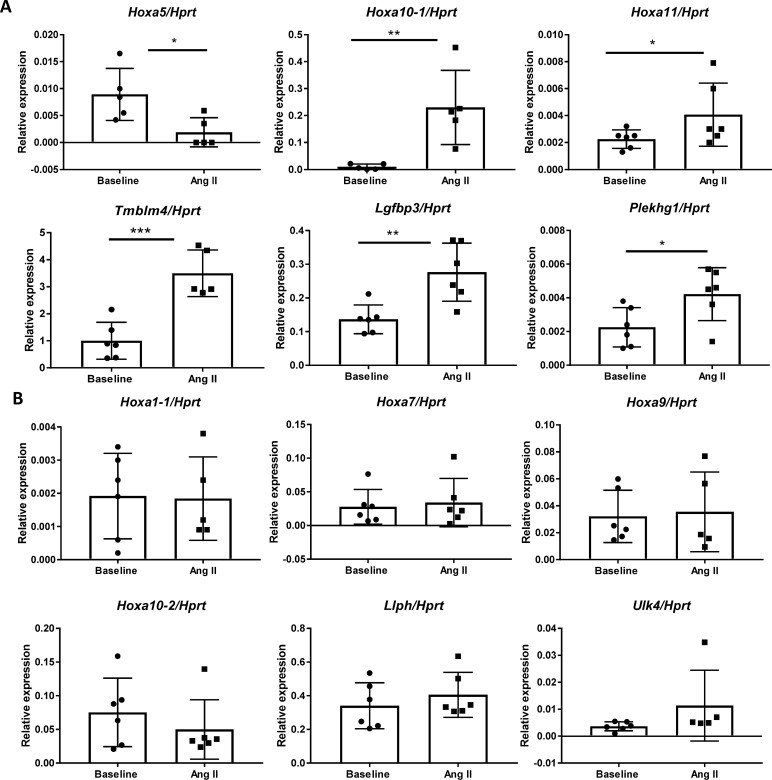
To determine if identified genes are functionally involved in BP regulation in the kidney during hypertension,[44] we quantified gene expression in mice kidneys at baseline and during the hypertensive state induced by Ang II. This hypertensive model was chosen for two reasons: 1) to mimic the low plasma renin state, albeit more exaggerated than the level observed, in African-ancestry individuals that has been suggested to reflect the elevated renin-angiotensin system activity at the tissue level in the kidney [45], and 2) maintenance of hypertension in the Ang II model requires activation of the immune system that is implicated in several identified loci.[46, 47] Kidney gene expressions of the identified genes were compared to age-matched untreated mice after two weeks of Ang II infusion, which increases SBP. For the HOXA locus, we examined the expression of genes that are known to be expressed in the mouse kidney: Hoxa1 (2 isoforms), 5, 7, 9, 10 (2 isoforms), and 11. Among all the genes examined, Tmbim4 was the most abundantly expressed gene in the kidney at baseline. Six genes—Hoxa5, Hoxa10-1 isoform, Hoxa11, Tmbim4, Igfbp3, and Plekhg1—were significantly differentially expressed in the kidney after Ang II treatment compared to baseline (Fig 4). Except for Hoxa5, which showed a significant decrease (Fig 4A), the expression of all these genes increased after the intervention. The expression of six genes—Hoxa1-1 isoform, Hoxa7, Hoxa9, Hoxa10-2 isoform, Llph, and Ulk4—were unchanged after Ang II infusion (Fig 4B). The following genes were not expressed in the adult mouse kidney at baseline or after Ang II intervention: Frmd3-1 isoform, Frmd3-2 isoform, Grp20, Tcf21, Cdh17, and Hoxa1-2 isoform.
Fig 4. Relative renal mRNA levels of genes identified at baseline and after 2 weeks of Ang II-induced hypertension.
HPRT gene was used for normalization. N ≥ 5 in each group. A. Genes that were differentially expressed between baseline and Ang II conditions. B. Genes that were not altered between the two conditions. * P < 0.05. ** P < 0.01. *** P < 0.001.
Discussion
To date, this is the largest genome-wide analysis of African-ancestry populations to study genetic variants underlying BP traits using dense-coverage imputed genotypes. Our main findings are eleven independent variants at nine loci, significantly associated with BP traits, including three newly identified loci (TARID/TCF21, FRMD3, LLPH/TMBIM4). We also found evidence for additional independent SNP associations in fine-mapping of three previously described loci, ULK4, EVX1/HOXA, and GRP20.[18, 39]
The most significant variants at TARID/TCF21, FRMD3, GPR20, and CDH17 are common variants in COGENT-BP African-ancestry participants, but monomorphic or low frequency in non-African-ancestry populations. For example, rs115795127 at FRMD3 is rare in European populations (MAF = 0.0007) and absent in East Asian and Hispanic/Latino populations. Therefore, they could not be identified in GWAS of non-African-ancestry populations even when increasing sample sizes. We also show evidence for selection for the variant at FRMD3, although additional studies should confirm these findings. The African-specific variants were not well tagged by HAPMAP2 data and therefore were not detected in our previous African-ancestry GWAS.[18] Overall, our results suggest additional gain in discovery when using dense imputed genotypes and support a role of population-specific alleles in African and African-admixed populations contributing to BP regulation and hypertension. Furthermore, they support the rationale and the need to study diverse populations in order to more effectively characterize the genetic architecture of BP in populations and the ethnic disparities in hypertension.
Functional annotation of our lead variants showed co-localization with annotated elements, including super enhancer, enhancer, and H3K27ac chromatic mapping in immune cells and kidney tissues, which has not been previously reported, in addition to cardiovascular tissues. There was also evidence for regulatory function in these relevant tissues through gene expression regulation (eQTL) and through overlaps with DHS in relevant tissues/cells. This evidence was additionally supported by experimental findings of differential expression of six genes (Hoxa5, Hoxa10-1 isoform, Hoxa11, Tmbim4, Igfbp3, and Plekhg1) in the mouse kidney after HTN induced by Ang II treatment. Overall, our results suggest the functional importance of identified genes in regulating BP in both normal and hypertension states.
At the newly identified loci, SNP rs76987554 is an intronic variant in TARID (TCF21 antisense RNA inducing promoter demethylation) which has not been previously reported to be associated with BP traits. A nearby gene, TCF21 (transcription factor 21), is a transcription factor of the basic helix-loop-helix family, which is mainly expressed in the liver, kidney, and heart. TCF21 is involved in epithelial differentiation and branching morphogenesis in kidney development,[48] and was associated with hypertension in a study of individuals of Japanese ancestry.[49] At the chromosome 7, rs115795127 is an intronic variant to FRMD3 (FERM domain containing 3) which encodes a protein involved in maintaining cell shape and integrity. FRMD3 has been associated with type 1 and type 2 diabetic kidney diseases in different ethnic populations, including those of European, African, and Asian ancestries.[50] The diabetes variant, rs10868025, is not in LD with rs115795127 in our African American samples or in 1000G EUR samples (r2 = 0.00028 and 0.0018, respectively), thus representing an independent association at this locus.
At chromosome 9, the functions of LLPH and TMBIM4 genes in BP regulation are currently unknown. LLPH belongs to the learning-associated protein family and is highly expressed in the immune system and the adrenal gland. TMBIM4 encodes the transmembrane BAX inhibitor motif-containing protein 4 and is highly expressed in whole blood, the immune system, and the adrenal gland.[51] The most significant variant at this locus, rs113866309, overlaps a DHS in immune, blood vessel, and heart cells. In our experimental model in mice, Tmbim4 gene expression was significantly increased after Ang II-induced HTN. This gene has been shown to inhibit apoptosis[52] and to decrease the efficacy of inositol 1,4,5-triphosphate (IP3)-dependent release of intracellular Ca2+. [53] This raises the possibility that the TMBIM4 protein may serve to dampen the effect of Ang II, which activates IP3 in vascular smooth muscle cells through the stimulation of the angiotensin type 1 receptor.[51, 53, 54] Therefore, it is possible that in conditions of activated renin-angiotensin system, genetic variants that lower the expression of TMBIM4 may augment BP, whereas genetic variants that increase its expression may attenuate BP.
Other genes, such as Hoxa5, Hoxa10-1, Hoxa11, Igfbp3, and Plekhg1, were significantly differentially expressed after Ang II-induced HTN in our mice experimental models. The HOXA-cluster has been identified in our previous GWAS of BP in African ancestry and in a recent GWAS of BP in European ancestry[5] though the underlying mechanisms related to BP control are unknown. We identified two independent variants at this locus; further studies are needed to delineate which of the HOXA genes are most likely involved in the association. In our experimental mice model, the Hoxa10-1 isoform had a greater than 20-fold increase in kidney expression during Ang II-induced HTN compared to baseline levels. However, it remains to be determined whether it is an effect of Ang II in hypertension, or a compensatory response to hypertension. Future studies using genetic manipulation in rodents are required to determine whether these changes are specific response related to BP and Ang II or simply a generic response to stress.
We identified several additional pathways involved in BP traits, including the GSK3 pathway, which has been reported to influence Wnt-mediated central BP regulation.[55] The Th1/Th2 pathway is involved in the regulation of immune responses[56] and has been linked to hypertension and atherosclerosis.[57, 58] The role of the immune system in the development of hypertension has been suggested in clinical studies and experimental animal models.[59–64] This includes reports of overlap of genetic variant associations between BP traits and immune-disorders [65] and evidence of enrichment of immune pathways from GWAS of BP.[66] Mutations of SH2B3, a gene identified in a GWAS of hypertension, have been recently shown to attenuate Dahl salt-sensitivity hypertension through inflammatory modulation.[67] In addition, the actions of Ang II in the pathophysiology and maintenance of hypertension are in part mediated through the activation of the immune system.[46]
Our assessment of the clinical implications of identified variants is limited by available data on African-ancestry populations. For example, there are currently no large publicly available GWAS of coronary heart disease or stroke outcomes in African-ancestry populations. It should also be noted that most of our replication cohorts were from populations other than those of African ancestry. Therefore, the power of replication analysis could still be low, which explains why only 11 of 63 variants were successfully replicated.
In summary, we report 11 independent variants at nine loci that are potential regulators of BP in our African-ancestry population study. Three loci are new. Identified BP variants are enriched in immune, kidney, heart, and vascular system pathways. Our experimental findings suggest that several of these genes may be involved in the renin-angiotensin pathways in the kidney during hypertension. Further population studies and experimental models are required for a comprehensive assessment of the identified genes across the immune, kidney, and cardiovascular systems. Our study demonstrates the need to further study individuals of African ancestry in order to identify loci and new biological pathways for BP.
Methods
Samples and BP phenotypes
Each study followed protocols for phenotype harmonization. For individuals taking anti-hypertensive medications, we added 15 and 10 mm Hg to measured SBP and DBP, respectively, a standard method used in other BP GWAS.[6, 68] PP was calculated as the difference between SBP and DBP after addition of the constant values. HTN was defined by a SBP ≥ 140 mm Hg, a DBP ≥ 90 mm Hg, or use of antihypertensive drugs.[69]
Genotyping and imputation
Each cohort was genotyped on either Affymetrix or Illumina genotyping platforms. Pre-imputation quality criteria were applied as described in S2 Table, and included exclusion of individuals with discordant self-reported gender and genetic gender. Imputation was performed using the software MACH-ADMIX, MACH-minimac or IMPUTE2 [70–72] using the Phase 1 integrated (March 2012 release) multi-ethnic reference panel from the 1000G Consortium (http://www.internationalgenome.org/).[73]
Association analysis
Autosomal chromosome SNP associations for SBP, DBP, and PP were assessed by linear regression for unrelated data or by the generalized linear mixed-effects model for family data, under the assumption of an additive genetic model. All models were adjusted for age, age2, sex, and body mass index. Up to ten principal components were included, as needed as covariates in the regression models, to control population stratification.[74, 75] We used standardized pre-meta-analysis QC criteria for all 21 discovery studies.[76] At the SNP level, we excluded variants with 1) imputation quality r2 < 0.3 in MACH or <0.4 in IMPUTE2; 2) the number of informative individuals (2×MAF×N×r2) ≤ 30; 3) an effect allele frequency (EAF) difference larger than 0.3 in comparison with the mixture of 80% YRI and 20% CEU of 1000G; and 4) the absolute regression coefficient ≥ 10. SNPs that passed the QC were carried forward for inverse variance weighted meta-analyses, implemented in METAL.[77]
Multi-trait statistical analyses using CPASSOC
We applied the CPASSOC software to combine association evidence of SBP, DBP, and HTN. CPASSOC provides two statistics, SHom and SHet, as previously described.[39] SHom is similar to the fixed effect meta-analysis method[77] but accounts for the correlation of summary statistics of the multi-traits and for overlapping or related samples among the cohorts. SHom uses the trait sample size as the weight, so that it is possible to combine traits with different measurement scales. SHet is an extension of SHom, and it can increase the statistical power over SHom when a variant affects only a subset of traits. The distribution of SHet under the null hypothesis was obtained through an estimated beta distribution. To calculate the statistics, SHom and SHet, and to account for the correlation among the traits, a correlation matrix is required. In this study, we used the correlation matrix calculated from the residuals of the three BP traits after adjustments for covariates and principal components.
Replication and meta-analyses
All independent SNPs identified with P < 10−6 (threshold chosen for suggestive association) in the discovery stage were carried forward for replication in African-ancestry individuals and in multi-ethnic samples of European Americans, East Asians, or Hispanics/Latinos (Fig 1). For single-trait analyses, we conducted fixed effect meta-analyses in the replication sets for each of four BP traits (SBP, DBP, PP and HTN), followed by a combined trans-ethnic meta-analysis of each trait. This was followed by a mega-meta-analyses, combining the results of discovery and replication for single traits using fixed-effects meta-analysis. We also performed a multi-trait CPASSOC analysis of SBP, DBP, and HTN in each replication study. Because CPASSOC only generated test statistics SHom/SHet and corresponding P values without effect sizes, we combined the association P values from all four replication populations using Fisher’s method (http://hal.case.edu/zhu-web/). Finally, we combined the CPASSOC meta-analysis results from the discovery and replication stages using Fisher’s method.
Multiple-testing thresholds
For a single trait GWAS discovery analysis, we used genome-wide significant level P = 5.0×10−8. We performed six different analyses, four single trait (SBP, DBP, PP and HTN) analyses and two CPASSOC (SHom and SHet) analyses for each SNP. For the four single correlated traits (SBP, DBP, PP and HTN), we calculated the number of independent traits using the eigenvalues of the correlation matrix, [78] which resulted two independent traits. Therefore, we counted four independent analyses, which were two independent single traits and two statistics of CPASSOC analyses, and applied an experimental significance level P = 1.25×10−8 for claiming a genome-wide significance when combining discovery and replication samples. We should point out that the two CPASSOC test statistics and a single trait statistic are not independent. Thus, the significance level P = 1.25×10−8 is conservative.
Conditional analysis
Since a locus may consist of multiple independent signals, we applied approximate conditional analysis implemented in GCTA-COJO[79, 80] using the summary statistics of SNPs with P < 1.0×10−6 from both of the individual trait meta-analyses (http://cnsgenomics.com/software/gcta/cojo.html). The LD among variants was estimated from the five African American cohorts from the CARe consortium.[79]
Pathway analysis
Pathway analysis was performed using the Meta-Analysis Gene-set Enrichment of variant Associations (MAGENTA) program (http://www.broadinstitute.org/mpg/magenta/).[41] Using the summary statistics from the four BP traits and two statistics from CPASSOC, from the discovery stage, we tested whether sets of functionally-related genes are enriched for associations. This method first converts the P values of SNPs into gene scores with correcting for confounders, such as gene site, number of variants in a gene, and their LD patterns, and then calculated a gene set enrichment P value for each biological pathway or gene set of interest using a non-parametric statistical test. The nominal GSEA P value refers to the nominal gene set enrichment P value for a gene set. The database of pathway/gene-sets to be tested include Ingenuity (June 2008), KEGG (2010), GO, and the Panther, signaling pathways downloaded from MSigDB and PANTHER (http://www.broad.mit.edu/gsea/msigdb/collections.jsp; http://www.pantherdb.org/).[81] We applied the parameters suggested by the authors, which includes the 75th percentile cut off of gene scores, the nominal GSEA P-value < 0.01 and the false discovery rate (FDR) < 0.3.
Functional annotation enrichment analysis
The enrichment of heritability of genomic regions to different functional categories, including cell type-specific elements, was evaluated using the method of LD score regression (https://github.com/bulik/ldsc).[42, 82] This method partitioned the heritability from the discovery GWAS summary statistics of four BP traits (SBP, DBP, PP, and HTN) while accounting for LD among markers.[42] We calculated enrichment, in functional regions and in expanded regions (+500bp) around each functional class, based on functional annotation, using a “full baseline model” previously created from 24 publicly available main annotations that are not specific to any cell type.[42] Enrichment was calculated based on the ratio of explained heritability and the proportion of SNPs in each annotation category. The standard error of enrichment was estimated with a block jackknife to calculate z scores and P values.[42] The multiple testing threshold was determined using the Bonferroni correction while accounting for two independent-trait analyses based on Ji and Li’s method[78] (P of 0.05/[25 classes × 2 traits]). We also performed cell-type-specific group enrichment analysis using cell-type-specific annotations from four histone marks (H3K4me1, H3K4me3, H3K9ac, and H3K27ac), which corresponded to 220 cell types. We divided the 220 cell-type-specific annotations into 10 groups: adrenal/pancreas, central nervous system (CNS), cardiovascular, connective/bone, gastrointestinal, immune/hematopoietic, kidney, liver, skeletal muscle and other. The analysis characterized cell-type-specific annotations within each group and calculated the enrichment of heritability for each group.[42]
Genomic annotation enrichment
We selected sets of variants in LD r2 > 0.1 from the eleven replicated variants, and calculated Bayes Factors and posterior causal probabilities for each variant from the effect sizes and standard errors, as previously described.[83] Each distinct variant associated with multiple traits was included in the analysis only once. The genomic annotations of DHS sites for 348 cell types from the ENCODE project were obtained and grouped into cell types associated with 34 tissues (http://genome.ucsc.edu/ENCODE/cellTypes.html). Four gene-based annotations—coding exon, 5-UTR, 3-UTR, and 1kb upstream of transcription start site (TSS)—from GENCODE transcripts were also obtained. Variants overlapping each of these annotations were then identified. Using the variant annotations and fGWAS (https://github.com/joepickrell/fgwas), we tested for enrichment of variants across all signals in 38 DHS categories, including in the four gene-based annotations in each model.[43]
Expression quantitative trait loci (eQTL) analysis
We used the GTEx pilot database [82] (http://www.gtexportal.org/home/) to identify eQTLs in the successfully replicated SNPs.
Integrated haplotype score (iHS) analysis
To evaluate population differentiation and natural selection, using Haplotter,[40] we calculated the integrated haplotype score (iHS) in five cohorts of CARe so that we could measure the amount of extended haplotype homozygosity (http://coruscant.itmat.upenn.edu/whamm/ihs.html). Hence, we tested the evidence of recent positive selection at five significant SNPs with differences in allele frequency across continental-ancestry populations. The measures were standardized (mean 0, variance 1) empirically to the distribution of observed iHS scores over a range of SNPs with similar derived allele frequencies. This method assesses the evidence for selection by comparing the extended homozygosity for haplotypes on a high frequency derived allele relative to the ancestry background.[40]
Experimental mouse models
Experiments were carried out in accordance with local and the National Institutes of Health guidelines. The animal protocol was approved by the University of Virginia Institutional Animal Care and Use Committee. Wild-type male mice on the 129S6 background at ~ 3 months of age were used for gene expression analyses. All mice were maintained on a 12-hour light-dark cycle with free access to standard chow and water in the animal facility of the University of Virginia.
The hypertension experimental model was induced using Ang II (Sigma-Aldrich, St. Luis, MO) delivered at 600 ng/kg/min for 2 weeks via Alzet mini-osmotic pumps (Durect Corporation, Cupertino, CA, model 2004), as previously described.[84] For gene expression analyses, RNA from kidney tissue was isolated by RNeasy Mini kit (Qiagen) and transcribed to cDNA by iScript TM cDNA synthesis kit (Bio-Rad). Real time PCR analyses were performed on iQTM5 Multicolor real time PCR Bio-Rad instruments using iQTM SYBER® Green Supermix. Hprt was used as a reference gene for normalization. Sequences of forward and reversed primers (FP and RP) for the gene expression studies are shown in S10 Table.
Ethic statement
All research involving human participants have been approved by the Institutional Review Board (IRB) # 04-95-72 and study-related Publication and Presentation committees. All participants have provided informed consent for DNA research and data are publicly available in dbGap.
Animal experiments were carried out following the guidelines established locally at the University of Virginia (UVA) and by the National Institutes of Health. The animal protocol was approved by the UVA Institutional Animal Care and Use Committee (Protocol # 3791, Protocol Title: Genes regulating Hypertension and Kidney Disease). Wild-type male mice on the 129S6 background at ~ 3 months of age were used for gene expression analyses. All mice were maintained on a 12-hour light-dark cycle with free access to standard chow and water in the animal facility UVA.
Supporting information
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(DOCX)
Acknowledgments
This is included in the Supplemental Note.
Data Availability
Study-specific phenotypes and genotypes are available from dbGaP (ARIC: phs000280.v1.p1, CHS: phs000287.v1.p1, WHI: phs000200.v1.p1, MESA: phs000283.v1.p1, Cleveland Family Study: phs000284.v1.p1, CARDIA: phs000285.v3.p). Discovery meta-analyses results for this study and readme file related to meta-analyses are available in GRASP and can be accessed from http://apps.nhlbi.nih.gov/GRASP/.
Funding Statement
The work was supported by the National Institutes of Health, the National Heart, Lung and Blood Institute R21HL123677 (NF) and the National Human Genome Research Institute grant HG003054 (XZ). JLi is supported by HL007567-31 (T32) from the National Heart, Lung and Blood Institute. MAN is supported by a consulting contract between Data Tecnica International and the National Institute on Aging, NIH, Bethesda, MD, USA. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Levy D, DeStefano AL, Larson MG, O'Donnell CJ, Lifton RP, Gavras H, et al. Evidence for a gene influencing blood pressure on chromosome 17. Genome scan linkage results for longitudinal blood pressure phenotypes in subjects from the framingham heart study. Hypertension. 2000;36(4):477–83. [DOI] [PubMed] [Google Scholar]
- 2.Hopkins PN, Hunt SC. Genetics of hypertension. Genet Med. 2003;5(6):413–29. doi: 10.109701.GIM.0000096375.88710.A6 [DOI] [PubMed] [Google Scholar]
- 3.Samani NJ. Genome scans for hypertension and blood pressure regulation. Am J Hypertens. 2003;16(2):167–71. [DOI] [PubMed] [Google Scholar]
- 4.Cooper RS, Guo X, Rotimi CN, Luke A, Ward R, Adeyemo A, et al. Heritability of angiotensin-converting enzyme and angiotensinogen: A comparison of US blacks and Nigerians. Hypertension. 2000;35(5):1141–7. [DOI] [PubMed] [Google Scholar]
- 5.Ehret GB, Ferreira T, Chasman DI, Jackson AU, Schmidt EM, Johnson T, et al. The genetics of blood pressure regulation and its target organs from association studies in 342,415 individuals. Nature genetics. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ehret GB, Munroe PB, Rice KM, Bochud M, Johnson AD, Chasman DI, et al. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature. 2011;478(7367):103–9. doi: 10.1038/nature10405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Surendran P, Drenos F, Young R, Warren H, Cook JP, Manning AK, et al. Trans-ancestry meta-analyses identify rare and common variants associated with blood pressure and hypertension. Nature genetics. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cho YS, Go MJ, Kim YJ, Heo JY, Oh JH, Ban HJ, et al. A large-scale genome-wide association study of Asian populations uncovers genetic factors influencing eight quantitative traits. Nature genetics. 2009;41(5):527–34. doi: 10.1038/ng.357 [DOI] [PubMed] [Google Scholar]
- 9.Kato N, Loh M, Takeuchi F, Verweij N, Wang X, Zhang W, et al. Trans-ancestry genome-wide association study identifies 12 genetic loci influencing blood pressure and implicates a role for DNA methylation. Nature genetics. 2015;47(11):1282–93. doi: 10.1038/ng.3405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kato N, Takeuchi F, Tabara Y, Kelly TN, Go MJ, Sim X, et al. Meta-analysis of genome-wide association studies identifies common variants associated with blood pressure variation in east Asians. Nature genetics. 2011;43(6):531–8. doi: 10.1038/ng.834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Surendran P, Drenos F, Young R, Warren H, Cook JP, Manning AK, et al. Trans-ancestry meta-analyses identify rare and common variants associated with blood pressure and hypertension. Nature genetics. 2016;48(10):1151–61. doi: 10.1038/ng.3654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu C, Kraja AT, Smith JA, Brody JA, Franceschini N, Bis JC, et al. Meta-analysis identifies common and rare variants influencing blood pressure and overlapping with metabolic trait loci. Nature genetics. 2016;48(10):1162–70. doi: 10.1038/ng.3660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Warren HR, Evangelou E, Cabrera CP, Gao H, Ren M, Mifsud B, et al. Genome-wide association analysis identifies novel blood pressure loci and offers biological insights into cardiovascular risk. Nature genetics. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang YJ, Tayo BO, Bandyopadhyay A, Wang H, Feng T, Franceschini N, et al. The association of the vanin-1 N131S variant with blood pressure is mediated by endoplasmic reticulum-associated degradation and loss of function. PLoS genetics. 2014;10(9):e1004641 doi: 10.1371/journal.pgen.1004641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu X, Young JH, Fox E, Keating BJ, Franceschini N, Kang S, et al. Combined admixture mapping and association analysis identifies a novel blood pressure genetic locus on 5p13: contributions from the CARe consortium. Hum Mol Genet. 2011;20(11):2285–95. doi: 10.1093/hmg/ddr113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu X, Cooper RS. Admixture mapping provides evidence of association of the VNN1 gene with hypertension. PloS one. 2007;2(11):e1244 doi: 10.1371/journal.pone.0001244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu X, Luke A, Cooper RS, Quertermous T, Hanis C, Mosley T, et al. Admixture mapping for hypertension loci with genome-scan markers. Nature genetics. 2005;37(2):177–81. doi: 10.1038/ng1510 [DOI] [PubMed] [Google Scholar]
- 18.Franceschini N, Fox E, Zhang Z, Edwards TL, Nalls MA, Sung YJ, et al. Genome-wide association analysis of blood-pressure traits in African-ancestry individuals reveals common associated genes in African and non-African populations. American journal of human genetics. 2013;93(3):545–54. doi: 10.1016/j.ajhg.2013.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adeyemo A, Gerry N, Chen G, Herbert A, Doumatey A, Huang H, et al. A genome-wide association study of hypertension and blood pressure in African Americans. PLoS genetics. 2009;5(7):e1000564 doi: 10.1371/journal.pgen.1000564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Calhoun DA, Jones D, Textor S, Goff DC, Murphy TP, Toto RD, et al. Resistant hypertension: diagnosis, evaluation, and treatment. A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension. 2008;51(6):1403–19. doi: 10.1161/HYPERTENSIONAHA.108.189141 [DOI] [PubMed] [Google Scholar]
- 21.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, et al. Executive summary: heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation. 2012;125(1):188–97. doi: 10.1161/CIR.0b013e3182456d46 [DOI] [PubMed] [Google Scholar]
- 22.Hertz RP, Unger AN, Cornell JA, Saunders E. Racial disparities in hypertension prevalence, awareness, and management. Archives of internal medicine. 2005;165(18):2098–104. doi: 10.1001/archinte.165.18.2098 [DOI] [PubMed] [Google Scholar]
- 23.Stevens J, Truesdale KP, Katz EG, Cai J. Impact of body mass index on incident hypertension and diabetes in Chinese Asians, American Whites, and American Blacks: the People's Republic of China Study and the Atherosclerosis Risk in Communities Study. American journal of epidemiology. 2008;167(11):1365–74. doi: 10.1093/aje/kwn060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mufunda J, Chatora R, Ndambakuwa Y, Nyarango P, Kosia A, Chifamba J, et al. Emerging non-communicable disease epidemic in Africa: preventive measures from the WHO Regional Office for Africa. Ethnicity & disease. 2006;16(2):521–6. [PubMed] [Google Scholar]
- 25.Addo J, Smeeth L, Leon DA. Hypertension in sub-saharan Africa: a systematic review. Hypertension. 2007;50(6):1012–8. doi: 10.1161/HYPERTENSIONAHA.107.093336 [DOI] [PubMed] [Google Scholar]
- 26.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360(9349):1903–13. [DOI] [PubMed] [Google Scholar]
- 27.Hsu CY, McCulloch CE, Darbinian J, Go AS, Iribarren C. Elevated blood pressure and risk of end-stage renal disease in subjects without baseline kidney disease. Archives of internal medicine. 2005;165(8):923–8. doi: 10.1001/archinte.165.8.923 [DOI] [PubMed] [Google Scholar]
- 28.Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2224–60. doi: 10.1016/S0140-6736(12)61766-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr., et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. Jama. 2003;289(19):2560–72. doi: 10.1001/jama.289.19.2560 [DOI] [PubMed] [Google Scholar]
- 30.Berry JD, Dyer A, Cai X, Garside DB, Ning H, Thomas A, et al. Lifetime risks of cardiovascular disease. The New England journal of medicine. 2012;366(4):321–9. doi: 10.1056/NEJMoa1012848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Howard G, Lackland DT, Kleindorfer DO, Kissela BM, Moy CS, Judd SE, et al. Racial differences in the impact of elevated systolic blood pressure on stroke risk. JAMA Intern Med. 2013;173(1):46–51. doi: 10.1001/2013.jamainternmed.857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Safford MM, Brown TM, Muntner PM, Durant RW, Glasser S, Halanych JH, et al. Association of race and sex with risk of incident acute coronary heart disease events. Jama. 2012;308(17):1768–74. doi: 10.1001/jama.2012.14306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharma A, Colvin-Adams M, Yancy CW. Heart failure in African Americans: disparities can be overcome. Cleve Clin J Med. 2014;81(5):301–11. doi: 10.3949/ccjm.81a.13045 [DOI] [PubMed] [Google Scholar]
- 34.Zakai NA, McClure LA, Judd SE, Safford MM, Folsom AR, Lutsey PL, et al. Racial and regional differences in venous thromboembolism in the United States in 3 cohorts. Circulation. 2014;129(14):1502–9. doi: 10.1161/CIRCULATIONAHA.113.006472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Genovese G, Friedman DJ, Ross MD, Lecordier L, Uzureau P, Freedman BI, et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science. 2010;329(5993):841–5. doi: 10.1126/science.1193032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tekola-Ayele F, Doumatey AP, Shriner D, Bentley AR, Chen G, Zhou J, et al. Genome-wide association study identifies African-ancestry specific variants for metabolic syndrome. Mol Genet Metab. 2015;116(4):305–13. doi: 10.1016/j.ymgme.2015.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cooper RS, Tayo B, Zhu X. Genome-wide association studies: implications for multiethnic samples. Hum Mol Genet. 2008;17(R2):R151–5. doi: 10.1093/hmg/ddn263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bulik-Sullivan BK, Loh PR, Finucane HK, Ripke S, Yang J, Schizophrenia Working Group of the Psychiatric Genomics C, et al. LD Score regression distinguishes confounding from polygenicity in genome-wide association studies. Nature genetics. 2015;47(3):291–5. doi: 10.1038/ng.3211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu X, Feng T, Tayo BO, Liang J, Young JH, Franceschini N, et al. Meta-analysis of correlated traits via summary statistics from GWASs with an application in hypertension. American journal of human genetics. 2015;96(1):21–36. doi: 10.1016/j.ajhg.2014.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Voight BF, Kudaravalli S, Wen X, Pritchard JK. A map of recent positive selection in the human genome. PLoS Biol. 2006;4(3):e72 doi: 10.1371/journal.pbio.0040072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Segre AV, Consortium D, investigators M, Groop L, Mootha VK, Daly MJ, et al. Common inherited variation in mitochondrial genes is not enriched for associations with type 2 diabetes or related glycemic traits. PLoS genetics. 2010;6(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Finucane HK, Bulik-Sullivan B, Gusev A, Trynka G, Reshef Y, Loh PR, et al. Partitioning heritability by functional annotation using genome-wide association summary statistics. Nature genetics. 2015;47(11):1228–35. doi: 10.1038/ng.3404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pickrell JK. Joint analysis of functional genomic data and genome-wide association studies of 18 human traits. American journal of human genetics. 2014;94(4):559–73. doi: 10.1016/j.ajhg.2014.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Franceschini N, Le TH. Genetics of hypertension: discoveries from the bench to human populations. Am J Physiol Renal Physiol. 2014;306(1):F1–F11. doi: 10.1152/ajprenal.00334.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Price DA, Fisher ND. The renin-angiotensin system in blacks: active, passive, or what? Curr Hypertens Rep. 2003;5(3):225–30. [DOI] [PubMed] [Google Scholar]
- 46.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, et al. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. The Journal of experimental medicine. 2007;204(10):2449–60. doi: 10.1084/jem.20070657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Madhur MS, Lob HE, McCann LA, Iwakura Y, Blinder Y, Guzik TJ, et al. Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction. Hypertension. 2010;55(2):500–7. doi: 10.1161/HYPERTENSIONAHA.109.145094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Quaggin SE, Schwartz L, Cui S, Igarashi P, Deimling J, Post M, et al. The basic-helix-loop-helix protein pod1 is critically important for kidney and lung organogenesis. Development. 1999;126(24):5771–83. [DOI] [PubMed] [Google Scholar]
- 49.Fujimaki T, Oguri M, Horibe H, Kato K, Matsuoka R, Abe S, et al. Association of a transcription factor 21 gene polymorphism with hypertension. Biomedical reports. 2015;3(1):118–22. doi: 10.3892/br.2014.371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Buffon MP, Sortica DA, Gerchman F, Crispim D, Canani LH. FRMD3 gene: its role in diabetic kidney disease. A narrative review. Diabetology & metabolic syndrome. 2015;7:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lisak DA, Schacht T, Enders V, Habicht J, Kiviluoto S, Schneider J, et al. The transmembrane Bax inhibitor motif (TMBIM) containing protein family: Tissue expression, intracellular localization and effects on the ER CA(2)(+)-filling state. Biochimica et biophysica acta. 2015;1853(9):2104–14. doi: 10.1016/j.bbamcr.2015.03.002 [DOI] [PubMed] [Google Scholar]
- 52.Saraiva N, Prole DL, Carrara G, Johnson BF, Taylor CW, Parsons M, et al. hGAAP promotes cell adhesion and migration via the stimulation of store-operated Ca2+ entry and calpain 2. The Journal of cell biology. 2013;202(4):699–713. doi: 10.1083/jcb.201301016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guo DF, Sun YL, Hamet P, Inagami T. The angiotensin II type 1 receptor and receptor-associated proteins. Cell Res. 2001;11(3):165–80. doi: 10.1038/sj.cr.7290083 [DOI] [PubMed] [Google Scholar]
- 54.de Mattia F, Gubser C, van Dommelen MM, Visch HJ, Distelmaier F, Postigo A, et al. Human Golgi antiapoptotic protein modulates intracellular calcium fluxes. Mol Biol Cell. 2009;20(16):3638–45. doi: 10.1091/mbc.E09-05-0385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cheng PW, Chen YY, Cheng WH, Lu PJ, Chen HH, Chen BR, et al. Wnt Signaling Regulates Blood Pressure by Downregulating a GSK-3beta-Mediated Pathway to Enhance Insulin Signaling in the Central Nervous System. Diabetes. 2015;64(10):3413–24. doi: 10.2337/db14-1439 [DOI] [PubMed] [Google Scholar]
- 56.Lederman S, Yellin MJ, Krichevsky A, Belko J, Lee JJ, Chess L. Identification of a novel surface protein on activated CD4+ T cells that induces contact-dependent B cell differentiation (help). The Journal of experimental medicine. 1992;175(4):1091–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lozovoy MA, Simao AN, Morimoto HK, Iryioda TM, Panis C, Reiche EM, et al. Hypertension is associated with serologically active disease in patients with systemic lupus erythematosus: role of increased Th1/Th2 ratio and oxidative stress. Scandinavian journal of rheumatology. 2014;43(1):59–62. doi: 10.3109/03009742.2013.834963 [DOI] [PubMed] [Google Scholar]
- 58.Tracy RP, Doyle MF, Olson NC, Huber SA, Jenny NS, Sallam R, et al. T-helper type 1 bias in healthy people is associated with cytomegalovirus serology and atherosclerosis: the Multi-Ethnic Study of Atherosclerosis. Journal of the American Heart Association. 2013;2(3):e000117 doi: 10.1161/JAHA.113.000117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mattson DL. Infiltrating immune cells in the kidney in salt-sensitive hypertension and renal injury. American journal of physiology Renal physiology. 2014;307(5):F499–508. doi: 10.1152/ajprenal.00258.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dell'Omo G, Penno G, Pucci L, Lucchesi D, Del Prato S, Pedrinelli R. Lack of association between TGF-beta-1 genotypes and microalbuminuria in essential hypertensive men. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association—European Renal Association. 2009;24(6):1864–9. [DOI] [PubMed] [Google Scholar]
- 61.Blasi ER, Rocha R, Rudolph AE, Blomme EA, Polly ML, McMahon EG. Aldosterone/salt induces renal inflammation and fibrosis in hypertensive rats. Kidney international. 2003;63(5):1791–800. doi: 10.1046/j.1523-1755.2003.00929.x [DOI] [PubMed] [Google Scholar]
- 62.Elmarakby AA, Quigley JE, Imig JD, Pollock JS, Pollock DM. TNF-alpha inhibition reduces renal injury in DOCA-salt hypertensive rats. American journal of physiology Regulatory, integrative and comparative physiology. 2008;294(1):R76–83. doi: 10.1152/ajpregu.00466.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nonaka-Sarukawa M, Okada T, Ito T, Yamamoto K, Yoshioka T, Nomoto T, et al. Adeno-associated virus vector-mediated systemic interleukin-10 expression ameliorates hypertensive organ damage in Dahl salt-sensitive rats. The journal of gene medicine. 2008;10(4):368–74. doi: 10.1002/jgm.1166 [DOI] [PubMed] [Google Scholar]
- 64.Trott DW, Harrison DG. The immune system in hypertension. Adv Physiol Educ. 2014;38(1):20–4. doi: 10.1152/advan.00063.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Andreassen OA, McEvoy LK, Thompson WK, Wang Y, Reppe S, Schork AJ, et al. Identifying common genetic variants in blood pressure due to polygenic pleiotropy with associated phenotypes. Hypertension. 2014;63(4):819–26. doi: 10.1161/HYPERTENSIONAHA.113.02077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Torkamani A, Topol EJ, Schork NJ. Pathway analysis of seven common diseases assessed by genome-wide association. Genomics. 2008;92(5):265–72. doi: 10.1016/j.ygeno.2008.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rudemiller NP, Lund H, Priestley JR, Endres BT, Prokop JW, Jacob HJ, et al. Mutation of SH2B3 (LNK), a genome-wide association study candidate for hypertension, attenuates Dahl salt-sensitive hypertension via inflammatory modulation. Hypertension. 2015;65(5):1111–7. doi: 10.1161/HYPERTENSIONAHA.114.04736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Levy D, Ehret GB, Rice K, Verwoert GC, Launer LJ, Dehghan A, et al. Genome-wide association study of blood pressure and hypertension. Nature genetics. 2009;41(6):677–87. doi: 10.1038/ng.384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr., et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA: the journal of the American Medical Association. 2003;289(19):2560–72. doi: 10.1001/jama.289.19.2560 [DOI] [PubMed] [Google Scholar]
- 70.Collins AJ, Foley RN, Herzog C, Chavers B, Gilbertson D, Ishani A, et al. US Renal Data System 2012 Annual Data Report. Am J Kidney Dis. 2013;61(1 Suppl 1):A7, e1-476. [DOI] [PubMed] [Google Scholar]
- 71.Howie B, Fuchsberger C, Stephens M, Marchini J, Abecasis GR. Fast and accurate genotype imputation in genome-wide association studies through pre-phasing. Nature genetics. 2012;44(8):955–9. doi: 10.1038/ng.2354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Howie BN, Donnelly P, Marchini J. A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS genetics. 2009;5(6):e1000529 doi: 10.1371/journal.pgen.1000529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Genomes Project C, Abecasis GR, Auton A, Brooks LD, DePristo MA, Durbin RM, et al. An integrated map of genetic variation from 1,092 human genomes. Nature. 2012;491(7422):56–65. doi: 10.1038/nature11632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nature genetics. 2006;38(8):904–9. doi: 10.1038/ng1847 [DOI] [PubMed] [Google Scholar]
- 75.Zhu X, Zhang S, Zhao H, Cooper RS. Association mapping, using a mixture model for complex traits. Genet Epidemiol. 2002;23(2):181–96. doi: 10.1002/gepi.210 [DOI] [PubMed] [Google Scholar]
- 76.Winkler TW, Day FR, Croteau-Chonka DC, Wood AR, Locke AE, Magi R, et al. Quality control and conduct of genome-wide association meta-analyses. Nature protocols. 2014;9(5):1192–212. doi: 10.1038/nprot.2014.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Willer CJ, Li Y, Abecasis GR. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics. 2010;26(17):2190–1. doi: 10.1093/bioinformatics/btq340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li J, Ji L. Adjusting multiple testing in multilocus analyses using the eigenvalues of a correlation matrix. Heredity. 2005;95(3):221–7. doi: 10.1038/sj.hdy.6800717 [DOI] [PubMed] [Google Scholar]
- 79.Yang J, Ferreira T, Morris AP, Medland SE, Genetic Investigation of ATC, Replication DIG, et al. Conditional and joint multiple-SNP analysis of GWAS summary statistics identifies additional variants influencing complex traits. Nature genetics. 2012;44(4):369–75, S1-3. doi: 10.1038/ng.2213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yang J, Lee SH, Goddard ME, Visscher PM. GCTA: a tool for genome-wide complex trait analysis. American journal of human genetics. 2011;88(1):76–82. doi: 10.1016/j.ajhg.2010.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Thomas PD, Campbell MJ, Kejariwal A, Mi H, Karlak B, Daverman R, et al. PANTHER: a library of protein families and subfamilies indexed by function. Genome research. 2003;13(9):2129–41. doi: 10.1101/gr.772403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bulik-Sullivan B, Finucane HK, Anttila V, Gusev A, Day FR, Loh PR, et al. An atlas of genetic correlations across human diseases and traits. Nature genetics. 2015;47(11):1236–41. doi: 10.1038/ng.3406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wakefield J. A Bayesian measure of the probability of false discovery in genetic epidemiology studies. American journal of human genetics. 2007;81(2):208–27. doi: 10.1086/519024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chu PL, Gigliotti JC, Cechova S, Bodonyi-Kovacs G, Chan F, Ralph DL, et al. Renal Collectrin Protects against Salt-Sensitive Hypertension and Is Downregulated by Angiotensin II. J Am Soc Nephrol. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(DOCX)
Data Availability Statement
Study-specific phenotypes and genotypes are available from dbGaP (ARIC: phs000280.v1.p1, CHS: phs000287.v1.p1, WHI: phs000200.v1.p1, MESA: phs000283.v1.p1, Cleveland Family Study: phs000284.v1.p1, CARDIA: phs000285.v3.p). Discovery meta-analyses results for this study and readme file related to meta-analyses are available in GRASP and can be accessed from http://apps.nhlbi.nih.gov/GRASP/.