Abstract
Rationale: Among patients with nontuberculous mycobacterial lung disease is a subset of previously healthy women with a slender body morphotype, often with scoliosis and/or pectus excavatum. We hypothesize that unidentified factors predispose these individuals to pulmonary nontuberculous mycobacterial disease.
Objectives: To compare body morphotype, serum adipokine levels, and whole-blood cytokine responses of patients with pulmonary nontuberculous mycobacteria (pNTM) with contemporary control subjects who are well matched demographically.
Methods: We enrolled 103 patients with pNTM and 101 uninfected control subjects of similar demographics. Body mass index and body fat were quantified. All patients with pNTM and a subset of control subjects were evaluated for scoliosis and pectus excavatum. Serum leptin and adiponectin were measured. Specific cytokines important to host-defense against mycobacteria were measured in whole blood before and after stimulation.
Measurements and Main Results: Patients with pNTM and control subjects were well matched for age, gender, and race. Patients with pNTM had significantly lower body mass index and body fat and were significantly taller than control subjects. Scoliosis and pectus excavatum were significantly more prevalent in patients with pNTM. The normal relationships between the adipokines and body fat were lost in the patients with pNTM, a novel finding. IFN-γ and IL-10 levels were significantly suppressed in stimulated whole blood of patients with pNTM.
Conclusions: This is the first study to comprehensively compare body morphotype, adipokines, and cytokine responses between patients with NTM lung disease and demographically matched controls. Our findings suggest a novel, predisposing immunophenotype that should be mechanistically defined.
Keywords: leptin, adiponectin, pectus excavatum, scoliosis, Marfan syndrome
At a Glance Commentary
Scientific Knowledge on the Subject
Most patients with nontuberculous mycobacterial lung disease have no obvious risk factors, although prior studies and clinical observation suggest that many possess a distinct body phenotype.
What This Study Adds to the Field
We found that a subset of patients with nontuberculous mycobacterial lung disease without clear risk factors possess a novel predisposing syndrome, characterized by a distinct body phenotype, lower levels of whole-blood IFN-γ after ex vivo stimulation, and altered serum adipokine levels normalized for body fat.
Chronic lung disease due to nontuberculous mycobacteria (NTM) is a growing public health concern (1–3). Recent studies estimate the incidence in the United States to be five to six cases per 100,000 and as high as 15.5 cases per 100,000 in persons over 50 years of age (3–5). Because the duration of symptomatic NTM lung disease is often years, the prevalence of disease is estimated to be 10 to 40 cases per 100,000 (1).
In the United States, the most common NTM species associated with lung disease are Mycobacterium avium complex (MAC), Mycobacterium kansasii, and Mycobacterium abscessus. Although NTM are widespread in water and soil (6, 7), relatively few persons develop disease. Thus, intact immunity is likely pivotal for protection against NTM.
Chronic lung disease is the most common form of NTM infection, manifested by two main radiographic patterns: (i) an upper lobe fibrocavitary pattern that occurs mostly in men with underlying lung disease such as chronic obstructive pulmonary disease (COPD) and (ii) a nodular-bronchiectasis pattern that often involves the right middle lobe and lingula and which appears to be more common in women with no clear risk factors (8). However, each type is not exclusively seen in one gender, and both types may be evident in a single patient (9). The proportion of patients with pulmonary NTM (pNTM) with nodular bronchiectasis has increased over the past few decades (9).
Prior reports indicate that slender body habitus predisposes individuals to mycobacterial infections. Large population studies showed that otherwise healthy subjects with low body mass index (BMI) are at increased risk for acquiring and dying from tuberculosis (TB) (10–12). These findings were corroborated by a Hong Kong study showing that obesity was associated with a lower risk of TB (13). It has been reported that a disproportionate number of patients with a nodular-bronchiectatic pattern of pNTM are elderly women with a distinct body morphotype, characterized by slender habitus, above average height, mitral valve prolapse, and thoracic abnormalities including scoliosis, pectus excavatum (PEX), and straight back syndrome (14–17).
We hypothesize that slender individuals have abnormal expression of leptin and adiponectin, adipokines that are important immune modulators (18, 19). Leptin typically increases proportionately with adiposity, whereas adiponectin decreases. The relative deficiency of leptin as seen in thin individuals accounts for reduced lymphopoiesis and impaired differentiation of T cells into the IFN-γ–producing TH1 phenotype (20). Leptin also activates macrophages and increases production of host-protective cytokines such as tumor necrosis factor-α (TNF-α) and IL-12 (21). Reduced leptin levels in slender individuals may contribute to the increased susceptibility to TB (22, 23). Tasaka and colleagues (24) found that patients with pNTM had 30% lower serum leptin as compared with healthy control subjects matched for age, gender, and BMI (24). Mouse studies show that leptin is a host-protective factor against M. tuberculosis and M. abscessus (25, 26). Conversely, serum adiponectin levels are increased in thin individuals (18, 19). Because adiponectin can induce immunosuppressive cytokines such as IL-10 and IL-1 receptor antagonist (18, 19), elevated levels in slender individuals may impair their resistance to NTM.
It remains controversial whether patients with pNTM with no known predisposing disorder are immunologically impaired (16, 27–31). Lim and colleagues found more IFNγ+CD4+ T cells in the blood of patients with pNTM stimulated with staphylococcal enterotoxin B and MAC-derived purified protein derivative (32). Kim and colleagues (16) found no difference in stimulated levels of proinflammatory cytokines in peripheral blood mononuclear cells (PBMCs) of control subjects and patients with pNTM. In contrast, other researchers found that stimulated PBMCs and whole blood from patients with pulmonary MAC produced less proinflammatory cytokines but more IL-10 than blood cells from healthy subjects or subjects with TB (27–31, 33).
We performed a prospective, cross-sectional study to determine whether a subset of patients with pNTM have a unique predisposing phenotype. To do this, we compared patients with pNTM and uninfected control subjects for body morphotype, serum leptin and adiponectin levels, and whole-blood cytokine levels after ex vivo stimulation. Some of the results of this study have been presented as abstracts (34, 35).
Methods
Materials
A detailed list of materials used can be found in the online supplement.
Subjects
After Institutional Review Board approval and informed consent, 103 patients with pNTM who fulfilled American Thoracic Society criteria for isolated NTM lung disease (7) were recruited consecutively from the Adult Day Unit of National Jewish Health (NJH) between December 1, 2009 and October 1, 2010. Patients were enrolled regardless of their disease stage, antibiotic therapy, or prior history of lung resection surgery. Control subjects (n = 101) were recruited consecutively from the University of Colorado Denver Anschutz Medical Campus Metabolic Bone Clinic. We selected this control group because the patients seen at this clinic possess similar demographics as the patients with pNTM at NJH (mostly elderly, white women). Demographic factors can influence body habitus and body fat content, which can affect a subject’s immunophenotype. Control subjects were excluded if they had a history of NTM infection. For both groups, individuals were excluded if they were pregnant, had active cancer, or were currently prescribed immunosuppressive or immunomodulatory agents.
All enrolled subjects were self-reported to be HIV negative by testing or were deemed to be of such low risk that testing was not clinically indicated. A detailed medical history was obtained and reviewed for all patients. Tobacco use was defined as a history of greater than 5 pack-years of smoking or current cigarette smoking.
Body Morphotype Measurements
Body morphometric measurements were completed on all enrolled subjects. All measurements were performed in triplicate on the right side of the body by a single investigator (M.K.). The mean of three measurements was used for final comparisons between groups. From these measurements, the BMI (kg/m2) and percent body fat (Durnin/Womersley Caliper Method) were calculated. The total body fat in kg was calculated by multiplying weight times the percent body fat. Height and arm span:height ratio were also measured.
Assessment for Scoliosis and PEX
The presence of scoliosis was based on the interpretation of posteroanterior and lateral chest X-rays by clinical radiologists and validated by a single investigator (M.K.). PEX was determined using the Haller index to analyze the axial chest CT image, available for all 103 patients with pNTM and 20 control subjects. The Haller index is calculated by dividing the transverse diameter inside the rib cage by the shortest anteroposterior diameter between the anterior vertebral column and the posterior sternum (36). Mild to moderate PEX is defined as Haller index of 2.5 to 3.5 and severe PEX as > 3.5.
Adipokine Measurements
Serum leptin and adiponectin concentrations were measured in duplicate using ELISA kits from R&D Systems (Minneapolis, MN).
Whole-Blood Cytokine Levels
A random subset of subjects (47 patients with pNTM and 53 control subjects) underwent whole-blood collection for cytokine measurements. Whole blood was diluted 5-fold with RPMI alone or with RPMI containing a final concentration of 20 ng/ml lipopolysaccharide (LPS), 6.2 μg protein/ml of heat-killed Staphylococcus epidermidis, or 5.5 × 106 live Mycobacterium intracellulare/ml. Additional details on preparation of the whole blood and cytokine analysis can be found in the online supplement.
Statistical Analyses
Statistical analyses assessed five primary outcomes: height, presence of scoliosis, leptin and adiponectin concentration per kg of body fat, and cytokine production in whole-blood samples. The two groups in this study (patients with pNTM and control subjects) were compared for each outcome. Details of the statistical analyses can be found in the online supplement.
Results
Demographics and Past Medical History
There was no significant difference in age, gender, or race between the patients with pNTM and control subjects (Table 1). More patients with pNTM were current or past smokers, but in both groups the vast majority with tobacco use were former smokers (Table 1). Although there was a trend toward greater prevalence of COPD in the pNTM cohort (Table 2), this was self-reported and may reflect airway dysfunction related to bronchiectasis and not classic COPD. The pNTM cohort was also more likely to have a history of childhood respiratory disease, sinusitis, and gastroesophageal reflux, consistent with previously published studies (16). There were no significant differences in vitamin D levels or use of nonsteroidal antiinflammatory drugs between groups (Table 2). Osteoporosis and osteopenia were more common among control subjects, although the prevalence was high in both groups (Table 2). Fifty-eight (56%) patients with pNTM were on antibiotics at the time of enrollment, with 33 receiving azithromycin. Of the 45 patients with pNTM not on antibiotics, 33 were therapy naive, and 12 had received antimicrobial therapy before enrollment.
TABLE 1.
DEMOGRAPHICS
| Control Subjects (n = 101) | Patients with pNTM (n = 103) | P Value | |
| Age, yr* | 60.8 (15.7) | 63.8 (11.2) | 0.11 |
| Female gender, N (%) | 90 (89) | 85 (83) | 0.18 |
| Race | |||
| White, N (%) | 88 (87) | 93 (90) | 0.49 |
| Asian, N (%) | 6 (6) | 8 (8) | |
| Hispanic, N (%) | 5 (5) | 1 (1) | |
| African American, N (%) | 1 (1) | 0 | |
| Other, N (%) | 1 (1) | 1 (1) | |
| Tobacco use | |||
| Total, N (%) | 33 (33) | 52 (50) | 0.01 |
| Former smoker, N | 32 | 48 | |
| Current smoker, N | 1 | 4 | |
| Borne children, N (%) | 62 (63) | 79 (77) | 0.04 |
| Menarche, yr* | 13.3 (1.8) | 13.1 (1.6) | 0.29 |
| Menopause, yr* | 48.8 (6.4) | 47.4 (5.5) | 0.16 |
| Current hormone replacement therapy, N (%) | 28 (28) | 18 (17) | 0.08 |
Definition of abbreviation: N = number of subjects; pNTM = pulmonary nontuberculous mycobacteria.
Mean (SD).
TABLE 2.
PAST MEDICAL HISTORY
| Variable | Control Subjects (n = 101) | Patients with pNTM (n = 103) | P Value |
| “Self-reported” COPD, N (%) | 6 (6) | 14 (14) | 0.07 |
| Childhood respiratory diseases, N (%) | 19 (19) | 32 (32) | 0.04 |
| Sinusitis, N (%) | 17 (17) | 35 (34) | 0.01 |
| NTM-related lung complications | N/A | N/A | |
| Bronchiectasis, N (%)* | 103 (100) | ||
| Cavitary disease, N (%) | 19 (18) | ||
| History of hemoptysis, N (%) | 20 (19) | ||
| Prior lung resection, N (%) | 10 (10) | ||
| History of ≥2 pneumonias, N (%) | 49 (48) | ||
| Gastroesophageal reflux, N (%) | 41 (41) | 71 (69) | <0.0001 |
| BCG vaccination, N (%) | 9 (9) | 11 (11) | 0.67 |
| History of tuberculosis, N (%) | 0 (0) | 4 (4) | 0.01 |
| Hypertension, N (%) | 30 (30) | 33 (32) | 0.75 |
| Hyperlipidemia, N (%) | 31 (31) | 36 (35) | 0.52 |
| Osteopenia or osteoporosis, N (%) | 84 (83) | 73 (71) | 0.04 |
| Vitamin D level, ng/ml† | 39.36 (14.18) | 35.38 (13.82) | 0.08 |
| NSAID use, N (%) | 17 (17) | 21 (21) | 0.47 |
Definition of abbreviations: BCG = bacillus Calmette-Guérin; COPD = chronic obstructive pulmonary disease; N = number of subjects; N/A = not applicable; NSAID = nonsteroidal antiinflammatory drugs; NTM = nontuberculous mycobacteria; pNTM = pulmonary nontuberculous mycobacteria.
In the patients with pNTM, bronchiectasis was confirmed on CT scans. Only 20 control subjects had chest CT scans available, but none had a medical history compatible with bronchiectasis.
Mean (SD).
Microbiology
All mycobacterial isolates were identified using biochemical methods at NJH Mycobacterial Laboratory and 16S RIBOSOMAL RNA gene sequencing. MAC accounted for 78 (76%) of the NTM recovered. The second most common NTM recovered was M. abscessus, isolated from 20 (19%) patients. The remaining five patients had one of the following isolates: M. xenopi, M. terrae, M. lentiflavum, M. kansasii, and M. chelonae.
Body Morphotype Analysis
After linear regression analysis, which controlled for age and gender, patients with pNTM were found to be an average of 4.53 cm taller (Table 3) than control subjects (95% confidence interval [CI], 2.73–6.32 cm; P < 0.0001). The pNTM group had significantly lower BMI (22.06 vs. 23.98 kg/m2), percent body fat (28.46 vs. 31.28%), and total body fat (17.84 vs. 20.43 kg) than the control group (Table 3).
TABLE 3.
BODY MORPHOMETRIC ANALYSIS
| Variable | Control Subjects (n = 101)* | Patients with pNTM (n = 103)* | P Value |
| Height, cm | 161.83 (8.40) | 166.94 (7.62) | <0.0001 |
| Weight, kg | 63.12 (1.49) | 61.77 (1.23) | 0.48 |
| Arm circumference, cm | 27.65 (4.34) | 26.88 (3.30) | 0.16 |
| Waist circumference, cm | 83.12 (13.74) | 81.49 (12.76) | 0.38 |
| Hip circumference, cm | 92.49 (16.48) | 90.69 (8.92) | 0.34 |
| Biceps skin fold, cm | 6.30 (4.49) | 6.50 (4.61) | 0.75 |
| Triceps skin fold, cm | 16.87 (7.71) | 13.01 (5.73) | <0.0001 |
| Waist skin fold, cm | 20.16 (9.94) | 16.53 (7.19) | 0.003 |
| Hip skin fold, cm | 15.52 (11.22) | 12.02 (11.11) | 0.03 |
| Scapula skin fold, cm | 16.33 (9.73) | 12.80 (7.44) | 0.004 |
| Arm span, cm | 166.07 (9.88) | 169.04 (9.90) | 0.04 |
| Arm span/height ratio | 1.02 (0.04) | 1.01 (0.03) | 0.03 |
| Shoulder to hip distance, cm | 42.26 (3.92) | 44.29 (3.93) | 0.0005 |
| BMI, kg/m2 | 23.98 (5.07) | 22.06 (3.81) | 0.003 |
| Percent body fat, % | 31.28 (7.60) | 28.46 (7.42) | 0.008 |
| Total body fat, kg | 20.43 (8.82) | 17.84 (6.61) | 0.02 |
| Scoliosis, N (%)† | 10 (13.3) | 32 (31.1) | 0.006 |
| PEX, N (%)‡ | 13 (65) | 90 (87) | 0.01 |
Definition of abbreviations: BMI = body mass index; N = number of subjects; PEX = pectus excavatum; pNTM = pulmonary nontuberculous mycobacteria.
Mean (SD), except for the last two rows.
Scoliosis assessed in 75 control subjects and 103 patients with pNTM.
Pectus excavatum assessed in 20 control subjects and 103 patients with pNTM.
The presence of scoliosis was assessed for all 103 patients with pNTM and the 75 control subjects who had chest X-rays available. Scoliosis was present in 32 (31%) patients with pNTM and in 10 (13%) control subjects (P = 0.006).
Using the Haller index to define PEX, 90 (87%) of 103 patients with pNTM met diagnostic criteria for PEX, whereas 13 (65%) of the 20 control subjects who had chest CT available had evidence of PEX (P = 0.01 for group comparisons). Among all subjects with PEX, 23 (26%) of 90 patients with pNTM had severe PEX, defined as Haller index greater than 3.5, and 5 (38%) of 13 control subjects had severe PEX.
Serum Adipokine Levels
When serum leptin was normalized for total body fat (kg), subjects with NTM had, on average, a 0.33 ng/ml/kg higher leptin concentration than control subjects (i.e., 1.25 ng/ml/kg vs. 0.92 ng/ml/kg, respectively; (95% CI, 0.02–0.64 ng/mL/kg; P = 0.03). The raw individual leptin levels for all subjects were then plotted as a function of their corresponding body fat, and a best-fit line was determined (Figure 1A). The leptin concentration in the control subjects increased by 1.88 ng/ml for every kg increase of body fat (P < 0.0001), which corresponded to a partial correlation coefficient (r) of 0.74. In contrast, for the pNTM group, leptin increased by only 0.16 ng/ml per kg of body fat (P = 0.51), which corresponded to a r value of 0.07. There was significant interaction between the groups (i.e., the slopes are significantly different [P < 0.0001]).
Figure 1.
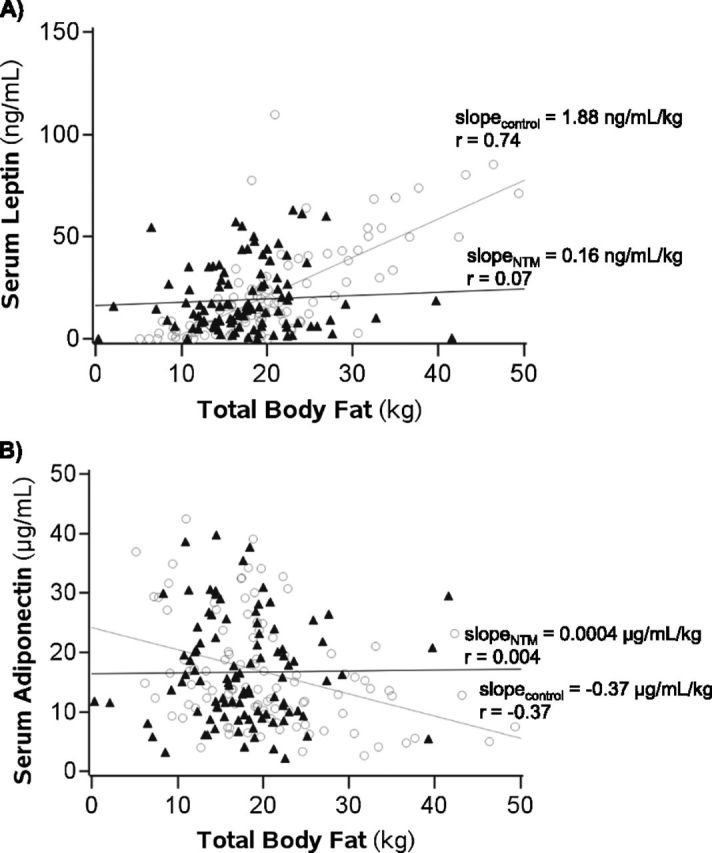
Serum leptin and adiponectin levels as a function of total body fat. (A) Serum leptin levels of control and patients with pulmonary nontuberculous mycobacteria (pNTM) plotted as a function of body fat (kg). Slope = rise in serum leptin concentration (ng/ml) per kg increase in body fat; P < 0.0001 for difference in slope of control subjects versus slope of patients with pNTM. (B) Serum adiponectin levels plotted as a function of body fat (kg). Slope = fall in serum adiponectin concentration (μg/ml) per kg increase in body fat; P = 0.02 for difference in slope of control subjects versus slope of patients with pNTM. See text for further discussion. Solid triangles = patients with pNTM; open circles = control subjects.
When serum adiponectin was normalized for total body fat (kg), subjects with NTM had, on average, a 0.52 μg/ml/kg higher adiponectin concentration than control subjects (i.e., 1.56 vs. 1.04 μg/ml/kg, respectively; 95% CI, −0.37 to 1.41; P = 0.25). The raw individual adiponectin levels were then plotted as a function of body fat, and a best-fit line was determined (Figure 1B). The adiponectin levels for the control group decreased by 0.37 μg/ml for each kg increase in body fat (P = 0.0001), which corresponded to a r value of –0.37. In contrast, for the pNTM group, adiponectin increased 0.0004 μg/ml for each kg increase in body fat (P = 0.997), which corresponded to a r value of 0.004. There was significant interaction between the groups (i.e., the slopes are significantly different [P = 0.02]).
Stimulated Whole-Blood Cytokine Production
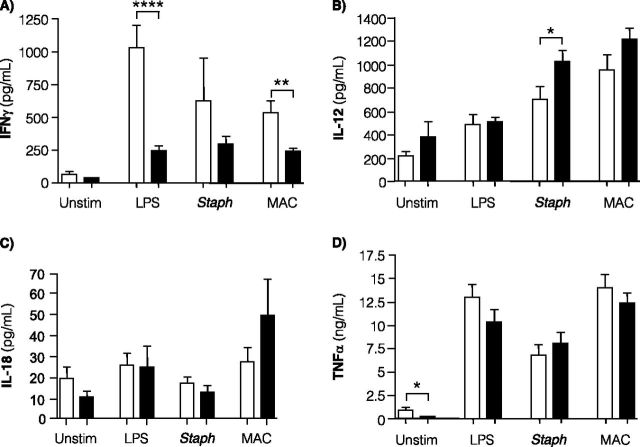
After stimulation of whole blood with LPS or live M. intracellulare, IFN-γ production was significantly decreased in patients with pNTM as compared with control subjects (Figure 2A). In the patients with pNTM, there was a trend toward less IFN-γ production in the unstimulated whole-blood cultures and cultures stimulated with S. epidermidis.
Figure 2.
IFN-γ, IL-12, and TNF-α levels in whole-blood cultures. Measured levels of (A) IFN-γ, (B) IL-12, (C) IL-18, and (D) TNF-α in diluted whole-blood cultures were quantified from 53 control subjects (open bars) and 47 patients with pulmonary nontuberculous mycobacteria (pNTM) (closed bars) for IFN-γ, IL-12, TNF-α, and 11 subjects from each group for IL-18. Whole-blood cultures were incubated for 18 hours in the presence of RPMI medium alone (Unstim), 20 ng/ml lipopolysaccharide (LPS), 6.2 μg of heat-killed Staphylococcus epidermidis protein/ml (Staph), or 5.5 × 106 live Mycobacterium intracellulare (MAC)/ml. Data shown are the mean ± SEM. *P < 0.05, **P < 0.01, and ****P < 0.0001 compared with corresponding control subjects. Statistical significance was unchanged after multivariate analysis adjusted for age, gender, body mass index, and height.
IL-12 is important in regulating IFN-γ synthesis. We determined the levels of IL-12 in the whole-blood cultures but found no significant differences between blood from patients with pNTM and control subjects exposed to RPMI alone, LPS, or live M. intracellulare (Figure 2B). However, in whole-blood cultures stimulated with S. epidermidis, IL-12 production was significantly increased in patients with pNTM compared with control subjects. Because IL-18 can induce IFN-γ production, we also determined whether there was differential IL-18 production in the whole blood of 11 randomly selected patients with pNTM and 11 randomly selected control subjects and found no significant difference (Figure 2C).
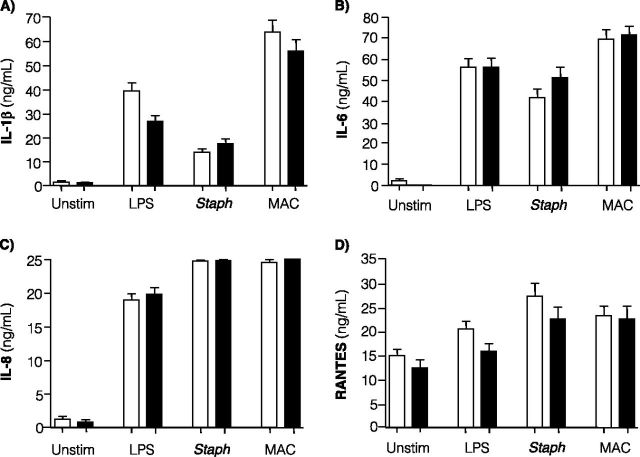
Unstimulated whole-blood cultures from patients with pNTM produced significantly less TNF-α compared with uninfected control subjects (Figure 2D). However, there was no difference in TNF-α production between patients with pNTM and control subjects in stimulated cultures. There was no significant difference in the baseline or stimulated levels of other proinflammatory cytokines, including IL-1β, IL-6, IL-8, and RANTES (“regulated and normal T cell expressed and secreted,” a T-cell–derived chemokine) (Figures 3A–3D).
Figure 3.
IL-1β, IL-6, IL-8, and RANTES levels in whole-blood cultures. Measured levels of (A) IL-1β, (B) IL-6, (C) IL-8, and (D) RANTES were measured in clarified supernatants from whole-blood cultures of 53 control subjects (open bars) and 47 patients with pulmonary nontuberculous mycobacteria (closed bars). Data shown are the mean ± SEM. Statistical significance was unchanged after multivariate analysis adjusted for age, gender, body mass index, and height. Whole-blood cultures were incubated for 18 hours in the presence of RPMI medium alone (Unstim), 20 ng/ml lipopolysaccharide (LPS), 6.2 μg of heat-killed Staphylococcus epidermidis protein/ml (Staph), or 5.5 × 106 live Mycobacterium intracellulare (MAC)/ml.
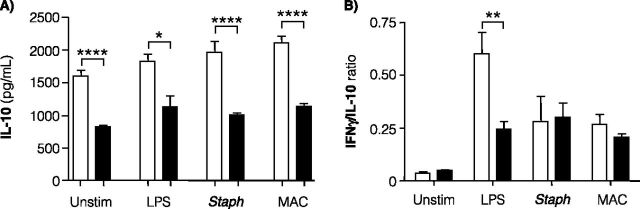
High levels of IL-10, an antiinflammatory cytokine, have been linked to pNTM disease (32). Unexpectedly, IL-10 levels were significantly lower in unstimulated and stimulated whole-blood cultures from patients with pNTM compared with control subjects (Figure 4A).
Figure 4.
IL-10 level and IFN-γ/IL-10 ratio in whole-blood cultures. (A) IL-10 was measured in the same clarified supernatants from whole-blood cultures used to quantify other cytokines. IL-10 was measured in 53 control subjects (open bars) and 47 patients with pulmonary nontuberculous mycobacteria (pNTM) (closed bars). Data shown are the mean ± SEM. Statistical significance was unchanged after multivariate analysis adjusted for age, gender, body mass index, and height. (B) The IFN-γ/IL-10 ratio was determined in 53 control subjects (open bars) and 47 patients with pNTM (closed bars). Data shown are the results as means ± SEM. *P < 0.05, **P < 0.01, and ****P < 0.0001 compared with corresponding control subjects. Whole-blood cultures were incubated for 18 hours in the presence of RPMI medium alone (Unstim), 20 ng/ml lipopolysaccharide (LPS), 6.2 μg of heat-killed Staphylococcus epidermidis protein/ml (Staph), or 5.5 × 106 live Mycobacterium intracellulare (MAC)/ml.
Because a low IFN-γ/IL-10 ratio has been associated with disease severity in TB (37), we determined the ratio of these two cytokines for both cohorts. There was no significant difference in the mean IFN-γ/IL-10 ratio comparing patients with pNTM and uninfected subjects in unstimulated whole-blood cultures or cultures exposed to S. epidermidis or M. intracellulare (Figure 4B). However, the IFN-γ/IL-10 ratio was significantly reduced in the LPS-stimulated whole-blood cultures of patients with pNTM (Figure 4B).
Discussion
This study is the first to examine body morphotype, adipokines, and blood cytokine levels in patients with pNTM and control subjects with similar demographics in regard to age, gender, and race. Our finding that patients with pNTM have lower BMI and less body fat supports the hypothesis that thin individuals are more susceptible to NTM lung disease (14, 16, 20). This finding is notable because both the pNTM and control groups had substantially lower percent body fat than similarly aged women in the National Health and Nutrition Examination Survey (NHANES) cohort (42.5% body fat for women 60–79 yr of age) (38). We speculate that the use of a more “average” control group may have resulted in even more striking differences in adipokine levels. Although it is plausible that reduced body fat was a result of the NTM infection, Kim and colleagues showed that self-reported BMI before pNTM diagnosis and current BMI were significantly lower than NHANES control subjects (16). Furthermore, they found that patients with pNTM had lower BMI than patients with disseminated NTM (16).
The present findings show that patients with pNTM have an increased frequency of the anthropometric features (taller stature, thin body habitus, scoliosis, and PEX) seen in Marfan syndrome (MFS) (15, 16). The prevalence of PEX as determined by the Haller index was likely overly sensitive because PEX was significantly greater in the pNTM and control cohorts than the estimated 1% occurrence in the general population (39). Nevertheless, PEX was significantly more common in patients with pNTM than in control subjects. Although the patients with pNTM in this study do not fulfill diagnostic criteria for classic MFS, the high frequency of Marfan-like features suggests that such individuals may have a forme fruste of MFS. In classic MFS, FIBRILLIN-1 gene mutations may cause lung disease (emphysema and bronchiectasis) and elevated tissue transforming growth factor (TGF)-β (40–44). Because most individuals with pNTM have bronchiectasis and because elevated TGF-β increases susceptibility to TB (45, 46) and MAC (47, 48), it is plausible that one or more of the approximately 600 FIBRILLIN-1 gene mutations identified (49) may predispose to pNTM. pNTM disease was recently reported in a patient with congenital contractural arachnodactyly due to FIBRILLIN-2 gene mutation (50). This disorder shares many clinical features with MFS, including tall and slender body habitus, scoliosis, and PEX (50). The authors speculated that mutation in FIBRILLIN-2 led to abnormal TGF-β metabolism, increasing the patient’s susceptibility to NTM (50).
In patients with pNTM, the typical direct relationship between body fat and serum leptin level was essentially absent. Although the mean serum leptin normalized for body fat was slightly higher in the patients with pNTM, this is likely due to the fact that patients with pNTM with very low body fat had higher leptin levels than control subjects with similar body fat (Figure 1A). For example, at 10 kg of body fat, the mean leptin level was 16.23 ng/ml higher (95% CI, 9.52–22.95 ng/ml higher) in the pNTM group compared with the control group (P < 0.0001) (Figure 1A). In contrast, at 30 kg body fat, the mean serum leptin concentration was 18.23 ng/ml lower (95% CI, 10.13–26.33 ng/ml lower) in the pNTM group compared with control group (P < 0.0001) (Figure 1A). One hypothesis for this finding is that thin subjects with pNTM may have more severe disease and the resulting increased inflammation enhanced leptin production because TNF-α and IL-1β have been shown to induce leptin in experimental animals (19, 51–53); however, this is controversial in humans (18, 19, 54). Although there was no positive correlation between baseline whole-blood TNF-α and leptin levels (n = 47; r = 0.14; P = 0.35), there was significant correlation between baseline IL-1β and leptin in patients with pNTM (n = 47; r = 0.40; P = 0.006). However, because there was no significant difference in whole-blood IL-1β levels between pNTM and control subjects, the contribution of IL-1β to elevated leptin in patients with pNTM with low body fat is unknown.
We expected to find elevated serum adiponectin in the pNTM cohort because adiponectin increases as body fat decreases. The patients with pNTM did have elevated mean serum adiponectin after normalization for body fat, although this difference was not statistically significant. The typically inverse relationship between body fat and adiponectin was absent in the patients with pNTM (Figure 1B). Our findings do not replicate the work of Tasaka and colleagues (24), who showed that although adiponectin was elevated and leptin was reduced in patients with pNTM, there was no difference in the slopes of the linear regression lines between the groups. This may be due to the fact that they found no difference in BMI between the two cohorts. Because the ratio of leptin to adiponectin concentration may have biological significance (55), we calculated the mean leptin:adiponectin ratio and found no significant difference, although there was a trend toward decreased leptin/adiponectin ratio in the pNTM group (1.68 ± 0.20 ng/μg) compared with the control group (2.30 ± 0.39 ng/μg). Nevertheless, loss of the direct relationship between leptin versus body fat as well as the loss of the inverse relationship between adiponectin versus body fat in patients with pNTM indicate that these immune-modulating adipokines are abnormally regulated in them.
It remains controversial whether patients with pNTM have defects in cytokine production (16, 27–31). We found that in patients with pNTM, whole blood stimulated with LPS or M. intracellulare produced significantly less IFN-γ, and these patients showed a trend toward decreased IFN-γ production after S. epidermidis stimulation. Because the levels of other proinflammatory cytokines were unchanged, the defect in IFN-γ production is likely downstream of Toll-like receptor-2, Toll-like receptor-4, and perhaps other pattern-recognition receptors and may be relatively specific for IFN-γ production. Because leptin can induce IFN-γ (20), we analyzed whether there was a correlation between leptin and basal blood IFN-γ in the pNTM cohort but found none (data not shown). Although the mechanisms responsible for the suppressed IFN-γ remain to be elucidated, our experimental findings indicate that the defect is not due to IL-12 or IL-18 deficiency in the patients with pNTM. Although there was a significantly reduced level of IFN-γ seen in the blood of patients with pNTM compared with control subjects after ex vivo stimulation, the IFN-γ level was not zero. Thus, it is quite plausible that the ability to produce IFN-γ, albeit at reduced level, is one reason why such patients do not manifest disseminated NTM or other opportunistic infections.
Prior studies examining stimulated PBMCs from patients with pNTM showed elevated IL-10 production compared with control subjects (31–33). Increased IL-10 in stimulated PBMCs has been linked to more severe pNTM disease (33). At baseline and across all stimuli, we found less IL-10 in the whole blood of patients with pNTM compared with control subjects. Because the inverse relationship between adiponectin and body fat was absent in the pNTM cohort, one explanation for the unexpected reduction in IL-10 might be a defect in the induction of IL-10 by adiponectin. In addition, our study used stimulated whole blood, whereas prior studies reporting elevated IL-10 in subjects with pNTM used PBMC cultures (31–33). Using whole blood for cytokine assays better reflects the in vivo microenvironment because soluble substances that modulate cytokine production are preserved (56).
Because IFN-γ and IL-10 levels were reduced in stimulated whole blood of patients with pNTM, an imbalance between immune-activating IFN-γ and immunosuppressive IL-10 may predispose slender individuals with abnormal body habitus to pNTM infection. It has been reported that a very low stimulated whole-blood IFN-γ/IL-10 ratio correlated with severe or disseminated TB and, conversely, a higher IFN-γ/IL-10 ratio was associated with less severe localized TB (37). We found no difference in the IFN-γ/IL-10 ratio between the whole blood of patients with pNTM and control subjects basally or after stimulation with S. epidermidis or M. intracellulare. Based on this finding, we speculate that a “preserved” IFN-γ/IL-10 ratio may have allowed the NTM infection to be confined to the lungs and helped prevent other opportunistic infections from developing. However, the IFN-γ/IL-10 ratio was significantly lower in the patients with pNTM after LPS stimulation compared with control subjects. Although this low ratio may indicate an intrinsic predisposition to LPS-containing gram-negative bacteria and supports the clinical observation that many patients with pNTM are coinfected with these organisms, the vulnerability may also be due to impaired ability to clear airway secretions due to the bronchiectasis itself or to selection pressure from repeated courses of antibiotics (57).
Because several antibiotics, including the macrolides, have antiinflammatory properties and inhibit cytokine production (58), we analyzed the cytokine data comparing patients with NTM who were on antibiotics at the time of enrollment with those who were not. Overall, there was no difference in whole-blood basal and stimulated cytokine levels between patients with NTM on current therapy and those not on therapy except for a modest reduction in IFN-γ production after LPS stimulation in patients on antibiotics (data not shown). However, there was no difference in IFN-γ production in those on or off therapy after stimulation of their blood with S. epidermidis or MAC. Importantly, basal and stimulated IFN-γ production was suppressed in patients with NTM, regardless of antibiotic status, when compared with control subjects. We also compared the mean levels of leptin and adiponectin in patients with NTM on and off antibiotics and found no differences (data not shown). We were not able to find evidence in the literature that antibiotic therapy affect adipokine levels.
Several limitations of this study should be considered in future work. Osteoporosis may be a confounder in our study because adipokine and cytokine levels may be altered in these individuals (59). However, because both the control and pNTM groups had a high prevalence of osteoporosis, we do not believe it significantly affected the results. Additionally, it would be interesting to compare body morphotype and immunophenotype as a function of severity of NTM lung disease. Although most patients with pNTM in our study had severe disease, consistent with the fact that nearly all patients with pNTM seen at NJH are referred because of recalcitrant disease, we did not conduct a formal, quantifiable measure of disease severity. Furthermore, prospectively analyzing percent body fat, adipokine levels, and relevant cytokine levels before and after anti-NTM treatment may help decipher what components of the abnormal immunophenotype are reversible upon successful treatment of the lung infection. Correlating leptin and adiponectin levels with lymphocyte counts as well as lymphocyte subtypes may reveal additional insights into the relationship between peripheral blood cell differential and levels of adipokines. Future studies should consider examining an additional control group, such as patients with idiopathic bronchiectasis without NTM infection. However, these patients may require bronchoalveolar lavage to definitively exclude current NTM infection. Furthermore, it is possible that these patients are also at high risk of contracting NTM lung disease but have not yet experienced a significant environmental exposure of organisms. Although it would be interesting to compare the body morphotype and immunophenotype of patients with NTM with active TB cases, patients with TB are frequently younger and foreign born, whereas patients with pNTM at NJH are predominantly elderly, white women. Although significantly more patients with pNTM had a history of tobacco use, we were unable to determine whether cigarette smoke exposure is a risk factor for pNTM disease because only one control subject and four patients with pNTM were current smokers at the time of enrollment. Future studies should examine estrogen levels because this hormone may play a role in host defense against mycobacteria (60). However, it has been shown that 6 months of hormone replacement therapy in postmenopausal women did not affect leptin levels (61). DEXA scans may provide a more accurate assessment of body fat than the caliper method. In the future, other Marfanoid features that can be objectively measured, such as lumbosacral dural ectasia, can be assessed (62, 63). Lung-specific immune cells should also be examined to evaluate the local immunophenotype, including the expression of T regulatory cells and TGF-β.
In conclusion, we found that, compared with a control group with similar demographics, patients and pNTM were taller and thinner, and had more thoracic cage abnormalities. Serum leptin and adiponectin levels were abnormally regulated in the patients with pNTM. After whole-blood stimulation, IFN-γ and IL-10 levels were significantly suppressed in the patients with pNTM. Our findings warrant future studies to explore a possible genetic basis for the abnormal immunophenotype we observed and to clarify the molecular mechanisms responsible for the abnormal induction of leptin, adiponectin, IFN-γ, and IL-10 in patients with NTM lung disease.
Acknowledgments
The authors thank Dr. Leonid Heifets for providing the M. intracellulare isolate used in the ex vivo experiments, Eric Schonteich for help with some of the assays, and Dr. Mary Bessesen for providing Dr. Kartalija with a Training Grant from the Denver Veterans Affairs Medical Center.
Footnotes
This work was supported by Fern and Phil Leitman of Non-tuberculous Mycobacteria Info and Research (NTMir) of Miami, Florida and by National Institutes of Health/National Center for Research Resources Colorado CTSI grant no. UL1 RR025780.
Author Contributions: Conception, hypothesis delineation, and design: M.K., A.R.O., M.S.R., M.M., M.D.I., L.S., E.D.C. Acquisition of data, analysis, and interpretation: M.K., A.R.O., C.L.B., G.B.P., G.F., J.T., M.J.S., X.B., P.R., V.N., A.R.L., A.F.-A., P.C.G., L.S., E.D.C. Writing of article and/or substantial revision: M.K., A.R.O., G.F., J.T., M.J.S., J.K., M.D.I., L.S., E.D.C.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201206-1035OC on November 9, 2012
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Iseman MD, Marras TK. The importance of nontuberculous mycobacterial lung disease. Am J Respir Crit Care Med 2008;178:999–1000. [DOI] [PubMed] [Google Scholar]
- 2.Marras TK, Daley CL. Epidemiology of human pulmonary infection with nontuberculous mycobacteria. Clin Chest Med 2002;23:553–567. [DOI] [PubMed] [Google Scholar]
- 3.Prevots DR, Shaw PA, Strickland D, Jackson LA, Raebel MA, Blosky MA, Montes de Oca R, Shea YR, Seitz AE, Holland SM, et al. . Nontuberculous mycobacterial lung disease prevalence at four integrated health care delivery systems. Am J Respir Crit Care Med 2010;182:970–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cassidy PM, Hedberg K, Saulson A, McNelly E, Winthrop KL. Nontuberculous mycobacterial disease prevalence and risk factors: a changing epidemiology. Clin Infect Dis 2009;49:e124–e129. [DOI] [PubMed] [Google Scholar]
- 5.Winthrop KL, McNelley E, Kendall B, Marshall-Olson A, Morris C, Cassidy M, Saulson A, Hedberg K. Pulmonary nontuberculous mycobacterial disease prevalence and clinical features: an emerging public health disease. Am J Respir Crit Care Med 2010;182:977–982. [DOI] [PubMed] [Google Scholar]
- 6.Falkinham JO. Nontuberculous mycobacteria from household plumbing of patients with nontuberculous mycobacteria disease. Emerg Infect Dis 2011;17:419–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, Holland SM, Horsburgh R, Huitt G, Iademarco MF, et al. . An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 2007;175:367–416. [DOI] [PubMed] [Google Scholar]
- 8.Glassroth J. Pulmonary disease due to nontuberculous mycobacteria. Chest 2008;133:243–251. [DOI] [PubMed] [Google Scholar]
- 9.Okumura M, Iwai K, Ogata H, Ueyama M, Kubota M, Aoki M, Kokuto H, Tadokoro E, Uchiyama T, Saotome M, et al. . Clinical factors on cavitary and nodular bronchiectatic types in pulmonary Mycobacterium avium complex disease. Intern Med 2008;47:1465–1472. [DOI] [PubMed] [Google Scholar]
- 10.Palmer CE, Jablon S, Edwards PQ. Tuberculosis morbidity of young men in relation to tuberculin sensitivity and body build. Am Rev Tuberc 1957;76:517–539. [DOI] [PubMed] [Google Scholar]
- 11.Tverdal A. Height, weight and incidence of tuberculosis. Bull Int Union Tuberc Lung Dis 1988;63:16–18. [PubMed] [Google Scholar]
- 12.Tverdal A. Body mass index and incidence of tuberculosis. Eur J Respir Dis 1986;69:355–362. [PubMed] [Google Scholar]
- 13.Leung CC, Lam TH, Chan WM, Yew WW, Ho KS, Leung G, Law WS, Tam CM, Chan CK, Chang KC. Lower risk of tuberculosis in obesity. Arch Intern Med 2007;167:1297–1304. [DOI] [PubMed] [Google Scholar]
- 14.Iseman MD. Mycobacterium avium and slender women: an unrequited affair. Trans Am Clin Climatol Assoc 1998;109:199–204. [PMC free article] [PubMed] [Google Scholar]
- 15.Iseman MD, Buschman DL, Ackerson LM. Pectus excavatum and scoliosis: thoracic anomalies associated with pulmonary disease caused by Mycobacterium avium complex. Am Rev Respir Dis 1991;144:914–916. [DOI] [PubMed] [Google Scholar]
- 16.Kim RD, Greenberg DE, Ehrmantraut ME, Guide SV, Ding L, Shea Y, Brown MR, Chernick M, Steagall WK, Glasgow CG, et al. . Pulmonary nontuberculous mycobacterial disease: prospective study of a distinct preexisting syndrome. Am J Respir Crit Care Med 2008;178:1066–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reich JM, Johnson RE. Mycobacterium avium complex pulmonary disease presenting as an isolated lingular or middle lobe pattern: the Lady Windermere syndrome. Chest 1992;101:1605–1609. [DOI] [PubMed] [Google Scholar]
- 18.Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol 2005;115:911–919. [DOI] [PubMed] [Google Scholar]
- 19.Fantuzzi G, Faggioni R. Leptin in the regulation of immunity, inflammation, and hematopoiesis. J Leukoc Biol 2000;68:437–446. [PubMed] [Google Scholar]
- 20.Chan ED, Iseman MD. Slender, older women appear to be more susceptible to nontuberculous mycobacterial lung disease. Gend Med 2010;7:5–18. [DOI] [PubMed] [Google Scholar]
- 21.Raso GM, Pacilio M, Esposito E, Coppola A, Di Carlo R, Meli R. Leptin potentiates IFN-gamma-induced expression of nitric oxide synthase and cyclo-oxygenase-2 in murine macrophage J774A.1. Br J Pharmacol 2002;137:799–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Crevel R, Karyadi E, Netea MG, Verhoef H, Nelwan RHH, West CE, van der Meer JWM. Decreased plasma leptin concentrations in tuberculosis patients are associated with wasting and inflammation. J Clin Endocrinol Metab 2002;87:758–763. [DOI] [PubMed] [Google Scholar]
- 23.Cakir B, Yonem A, Guler S, Odabasi E, Demirbas B, Gursoy G, Aral Y. Relation of leptin and tumor necrosis factor alpha to body weight changes in patients with pulmonary tuberculosis. Horm Res 1999;52:279–283. [DOI] [PubMed] [Google Scholar]
- 24.Tasaka S, Hasegawa N, Nishimura T, Yamasawa W, Kamata H, Shinoda H, Kimizuka Y, Fujiwara H, Hirose H, Ishizaka A. Elevated serum adiponectin level in patients with Mycobacterium avium-intracellulare complex pulmonary disease. Respiration 2010;79:383–387. [DOI] [PubMed] [Google Scholar]
- 25.Ordway D, Henao-Tamayo M, Smith E, Shanley C, Harton M, Troudt JL, Bai X, Basaraba RJ, Orme IM, Chan ED. Animal model of Mycobacterium abscessus lung infection. J Leukoc Biol 2008;83:1502–1511. [DOI] [PubMed] [Google Scholar]
- 26.Wieland CW, Florquin S, Chan ED, Leemans JC, Weijer S, Verbon A, Fantuzzi G, van der Poll T. Pulmonary Mycobacterium tuberculosis infection in leptin-deficient ob/ob mice. Int Immunol 2005;17:1399–1408. [DOI] [PubMed] [Google Scholar]
- 27.Greinert U, Schlaak M, Rüsch-Gerdes S, Flad HD, Ernst M. Low in vitro production of interferon-gamma and tumor necrosis factor-alpha in HIV-seronegative patients with pulmonary disease caused by nontuberculous mycobacteria. J Clin Immunol 2000;20:445–452. [DOI] [PubMed] [Google Scholar]
- 28.Kwon YS, Kim EJ, Lee S-H, Suh GY, Chung MP, Kim H, Kwon OJ, Koh W-J. Decreased cytokine production in patients with nontuberculous mycobacterial lung disease. Lung 2007;185:337–341. [DOI] [PubMed] [Google Scholar]
- 29.Safdar A, Atrmstrong D, Murray HW. A novel defect in interferon-γ secretion in patients with refractory nontuberculous pulmonary mycobacteriosis. Ann Intern Med 2003;138:521. [DOI] [PubMed] [Google Scholar]
- 30.Safdar A, White DA, Stover D, Armstrong D, Murray HW. Profound interferon gamma deficiency in patients with chronic pulmonary nontuberculous mycobacteriosis. Am J Med 2002;113:756–759. [DOI] [PubMed] [Google Scholar]
- 31.Vankayalapati R, Wizel B, Samten B, Griffith DE, Shams H, Galland MR, Von Reyn CF, Girard WM, Wallace RJ, Barnes PF. Cytokine profiles in immunocompetent persons infected with Mycobacterium avium complex. J Infect Dis 2001;183:478–484. [DOI] [PubMed] [Google Scholar]
- 32.Lim A, Allison C, Price P, Waterer G. Susceptibility to pulmonary disease due to Mycobacterium avium-intracellulare complex may reflect low IL-17 and high IL-10 responses rather than Th1 deficiency. Clin Immunol 2010;137:296–302. [DOI] [PubMed] [Google Scholar]
- 33.Emori M. Production of cytokines in patients with primary pulmonary Mycobacterium avium-intracellulare complex disease. Kurume Med J 2004;51:133–139. [DOI] [PubMed] [Google Scholar]
- 34.Chan ED, Ovrutsky A, Bryan C, Levin A, Pott G, Rothman M, McDermott M, Bai X, Iseman M, Ramamoorthy P, et al. . Patients with nontuberculous mycobacterial lung disease have abnormal serum adipokine profiles [abstract]. Am J Respir Crit Care Med 2012;185:A4040. [Google Scholar]
- 35.Kartalija M, Ovrutsky A, Bryan C, Pott G, Rothman M, McDermott M, Bai X, Iseman M, Fantuzzi G, Shapiro L, et al. . Immunophenotypes associated with nontuberculous mycobacterial lung disease [abstract]. Am J Respir Crit Care Med 2011;183:A3318. [Google Scholar]
- 36.Haller JA, Kramer SS, Lietman SA. Use of CT scans in selection of patients for pectus excavatum surgery: a preliminary report. J Pediatr Surg 1987;22:904–906. [DOI] [PubMed] [Google Scholar]
- 37.Jamil B, Shahid F, Hasan Z, Nasir N, Razzaki T, Dawood G, Hussain R. Interferon gamma/IL10 ratio defines the disease severity in pulmonary and extra pulmonary tuberculosis. Tuberculosis (Edinb) 2007;87:279–287. [DOI] [PubMed] [Google Scholar]
- 38.Li C, Ford ES, Zhao G, Balluz LS, Giles WH. Estimates of body composition with dual-energy X-ray absorptiometry in adults. Am J Clin Nutr 2009;90:1457–1465. [DOI] [PubMed] [Google Scholar]
- 39.Williams AM, Crabbe DC. Pectus deformities of the anterior chest wall. Paediatr Respir Rev 2003;4:237–242. [DOI] [PubMed] [Google Scholar]
- 40.Foster ME, Foster DR. Bronchiectasis and Marfan's syndrome. Postgrad Med J 1980;56:718–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Judge DP, Dietz HC. Marfan's syndrome. Lancet 2005;366:1965–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Neptune ER, Frischmeyer PA, Arking DE, Myers L, Bunton TE, Gayraud B, Ramirez F, Sakai LY, Dietz HC. Dysregulation of TGF-beta activation contributes to pathogenesis in Marfan syndrome. Nat Genet 2003;33:407–411. [DOI] [PubMed] [Google Scholar]
- 43.Teoh PC. Bronchiectasis and spontaneous pneumothorax in Marfan's syndrome. Chest 1977;72:672–673. [DOI] [PubMed] [Google Scholar]
- 44.Wood JR, Bellamy D, Child AH, Citron KM. Pulmonary disease in patients with Marfan syndrome. Thorax 1984;39:780–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hirsch CS, Yoneda T, Averill L, Ellner JJ, Toossi Z. Enhancement of intracellular growth of Mycobacterium tuberculosis in human monocytes by transforming growth factor β1. J Infect Dis 1994;170:1229–1237. [DOI] [PubMed] [Google Scholar]
- 46.Roberts T, Beyers N, Aguirre A, Walzl G. Immunosuppression during active tuberculosis is characterized by decreased interferon-gamma production and CD25 expression with elevated forkhead box P3, transforming growth factor-beta, and interleukin-4 mRNA levels. J Infect Dis 2007;195:870–878. [DOI] [PubMed] [Google Scholar]
- 47.Champsi J, Young LS, Bermudez LE. Production of TNF-alpha, IL-6 and TGF-beta, and expression of receptors for TNF-alpha and IL-6, during murine Mycobacterium avium infection. Immunology 1995;84:549–554. [PMC free article] [PubMed] [Google Scholar]
- 48.Denis M, Ghadirian E. Transforming growth factor beta (TGF-β1) plays a detrimental role in the progression of experimental Mycobacterium avium infection: in vivo and in vitro evidence. Microb Pathog 1991;11:367–372. [DOI] [PubMed] [Google Scholar]
- 49.Hilhorst-Hofstee Y, Hamel BC, Verheij JB, Rijlaarsdam ME, Mancini GM, Cobben JM, Giroth C, Ruivenkamp CA, Hansson KB, Timmermans J, et al. . The clinical spectrum of complete FBN1 allele deletions. Eur J Hum Genet 2011;19:247–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paulson ML, Olivier KN, Holland SM. Pulmonary non-tuberculous mycobacterial infection in congenital contractural arachnodactyly. Int J Tuberc Lung Dis 2012;16:561–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Faggioni R, Fantuzzi G, Fuller J, Dinarello CA, Feingold KR, Grunfeld C. IL-1 beta mediates leptin induction during inflammation. Am J Physiol 1998;274:R204–R208. [DOI] [PubMed] [Google Scholar]
- 52.Grunfeld C, Zhao C, Fuller J, Pollack A, Moser A, Friedman J, Feingold KR. Endotoxin and cytokines induce expression of leptin, the ob gene product, in hamsters. J Clin Invest 1996;97:2152–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sarraf P, Frederich RC, Turner EM, Ma G, Jaskowiak NT, Rivet DJ, Flier JS, Lowell BB, Fraker DL, Alexander HR. Multiple cytokines and acute inflammation raise mouse leptin levels: potential role in inflammatory anorexia. J Exp Med 1997;185:171–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carlson GL, Saeed M, Little RA, Irving MH. Serum leptin concentrations and their relation to metabolic abnormalities in human sepsis. Am J Physiol 1999;276:E658–E662. [DOI] [PubMed] [Google Scholar]
- 55.Kotani K, Sakane N. Leptin:adiponectin ratio and metabolic syndrome in the general Japanese population. Korean J Lab Med 2011;31:162–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pott GB, Chan ED, Dinarello CA, Shapiro L. Alpha-1-antitrypsin is an endogenous inhibitor of pro-inflammatory cytokine production in whole blood. J Leukoc Biol 2009;85:886–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moulton BC, Barker AF. Pathogenesis of bronchiectasis. Clin Chest Med 2012;33:211–217. [DOI] [PubMed] [Google Scholar]
- 58.Chmura K, Bai X, Nakamura M, Kandasamy P, McGibney M, Kuronuma K, Mitsuzawa H, Voelker DR, Chan ED. Induction of IL-8 by Mycoplasma pneumoniae membrane in BEAS-2B cells. Am J Physiol Lung Cell Mol Physiol 2008;295:L220–L230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pino AM, Rios S, Astudillo P, Fernandez M, Figueroa P, Seitz G, Rodriguez JP. Concentration of adipogenic and proinflammatory cytokines in the bone marrow supernatant fluid of osteoporotic women. J Bone Miner Res 2010;25:492–498. [DOI] [PubMed] [Google Scholar]
- 60.Tsuyuguchi K, Suzuki K, Matsumoto H, Tanaka E, Amitani R, Kuze F. Effect of oestrogen on Mycobacterium avium complex pulmonary infection in mice. Clin Exp Immunol 2001;123:428–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Di Carlo C, Tommaselli GA, De Rosa N, Fabozzi A, Santoro R, Bifulco G, Sparice S, Nappi C. Plasma leptin and adiponectin levels in hormone replacement therapy and contraception: effects of different progesterones. Fertil Steril 2011;96:214–219. [DOI] [PubMed] [Google Scholar]
- 62.Pyeritz RE, Fishman EK, Bernhardt BA, Siegelman SS. Dural ectasia is a common feature of the Marfan syndrome. Am J Hum Genet 1988;43:726–732. [PMC free article] [PubMed] [Google Scholar]
- 63.De Paepe A, Devereux RB, Dietz HC, Hennekam RC, Pyeritz RE. Revised diagnostic criteria for the Marfan syndrome. Am J Med Genet 1996;62:417–426. [DOI] [PubMed] [Google Scholar]