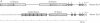Abstract
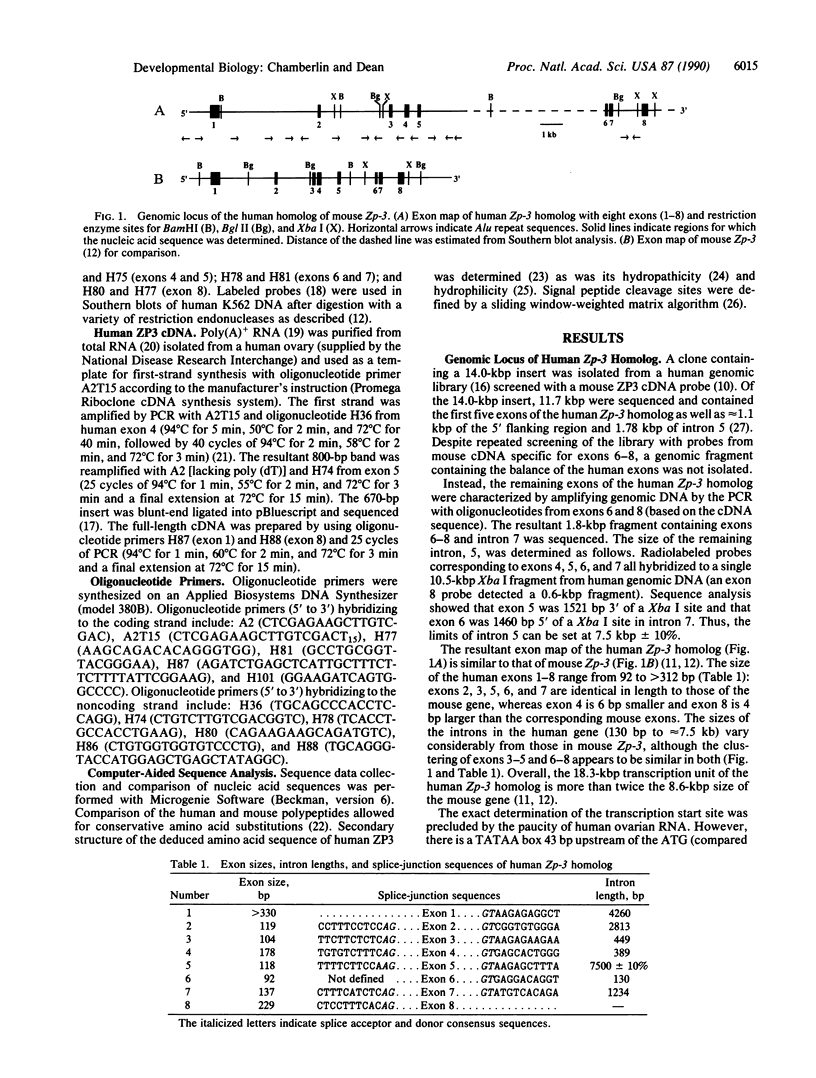
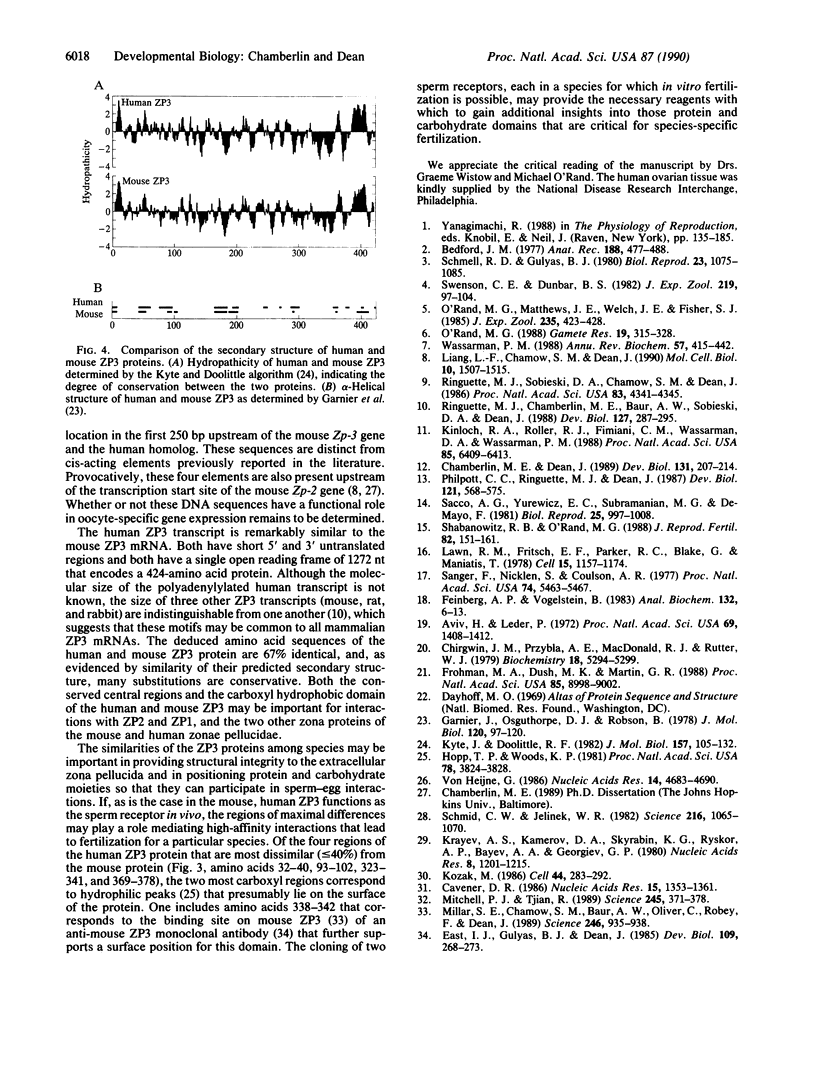
The human zona pellucida, composed of three glycoproteins (ZP1, ZP2, and ZP3), forms an extracellular matrix that surrounds ovulated eggs and mediates species-specific fertilization. The genes that code for at least two of the zona proteins (ZP2 and ZP3) cross-hybridize with other mammalian DNA. The recently characterized mouse sperm receptor gene (Zp-3) was used to isolate its human homolog. The human homolog spans approximately 18.3 kilobase pairs (kbp) (compared to 8.6 kbp for the mouse gene) and contains eight exons, the sizes of which are strictly conserved between the two species. Four short (8-15 bp) sequences within the first 250 bp of the 5' flanking region in the human Zp-3 homolog are also present upstream of mouse Zp-3. These elements may modulate oocyte-specific gene expression. By using the polymerase chain reaction, a full-length cDNA of human ZP3 was isolated from human ovarian poly(A)+ RNA and used to deduce the structure of human ZP3 mRNA. Certain features of the human and mouse ZP3 transcripts are conserved. Both have unusually short 5' and 3' untranslated regions, both contain a single open reading frame that is 74% identical, and both code for 424 amino acid polypeptides that are 67% the same. The similarity between the two proteins may define domains that are important in maintaining the structural integrity of the zona pellucida, while the differences may play a role in mediating the species-specific events of mammalian fertilization.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedford J. M. Sperm/egg interaction: the specificity of human spermatozoa. Anat Rec. 1977 Aug;188(4):477–487. doi: 10.1002/ar.1091880407. [DOI] [PubMed] [Google Scholar]
- Cavener D. R. Comparison of the consensus sequence flanking translational start sites in Drosophila and vertebrates. Nucleic Acids Res. 1987 Feb 25;15(4):1353–1361. doi: 10.1093/nar/15.4.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlin M. E., Dean J. Genomic organization of a sex specific gene: the primary sperm receptor of the mouse zona pellucida. Dev Biol. 1989 Jan;131(1):207–214. doi: 10.1016/s0012-1606(89)80052-1. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- East I. J., Gulyas B. J., Dean J. Monoclonal antibodies to the murine zona pellucida protein with sperm receptor activity: effects on fertilization and early development. Dev Biol. 1985 Jun;109(2):268–273. doi: 10.1016/0012-1606(85)90454-3. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Frohman M. A., Dush M. K., Martin G. R. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8998–9002. doi: 10.1073/pnas.85.23.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinloch R. A., Roller R. J., Fimiani C. M., Wassarman D. A., Wassarman P. M. Primary structure of the mouse sperm receptor polypeptide determined by genomic cloning. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6409–6413. doi: 10.1073/pnas.85.17.6409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Krayev A. S., Kramerov D. A., Skryabin K. G., Ryskov A. P., Bayev A. A., Georgiev G. P. The nucleotide sequence of the ubiquitous repetitive DNA sequence B1 complementary to the most abundant class of mouse fold-back RNA. Nucleic Acids Res. 1980 Mar 25;8(6):1201–1215. doi: 10.1093/nar/8.6.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lawn R. M., Fritsch E. F., Parker R. C., Blake G., Maniatis T. The isolation and characterization of linked delta- and beta-globin genes from a cloned library of human DNA. Cell. 1978 Dec;15(4):1157–1174. doi: 10.1016/0092-8674(78)90043-0. [DOI] [PubMed] [Google Scholar]
- Liang L. F., Chamow S. M., Dean J. Oocyte-specific expression of mouse Zp-2: developmental regulation of the zona pellucida genes. Mol Cell Biol. 1990 Apr;10(4):1507–1515. doi: 10.1128/mcb.10.4.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar S. E., Chamow S. M., Baur A. W., Oliver C., Robey F., Dean J. Vaccination with a synthetic zona pellucida peptide produces long-term contraception in female mice. Science. 1989 Nov 17;246(4932):935–938. doi: 10.1126/science.2479101. [DOI] [PubMed] [Google Scholar]
- Mitchell P. J., Tjian R. Transcriptional regulation in mammalian cells by sequence-specific DNA binding proteins. Science. 1989 Jul 28;245(4916):371–378. doi: 10.1126/science.2667136. [DOI] [PubMed] [Google Scholar]
- O'Rand M. G., Matthews J. E., Welch J. E., Fisher S. J. Identification of zona binding proteins of rabbit, pig, human, and mouse spermatozoa on nitrocellulose blots. J Exp Zool. 1985 Sep;235(3):423–428. doi: 10.1002/jez.1402350314. [DOI] [PubMed] [Google Scholar]
- O'Rand M. G. Sperm-egg recognition and barriers to interspecies fertilization. Gamete Res. 1988 Apr;19(4):315–328. doi: 10.1002/mrd.1120190402. [DOI] [PubMed] [Google Scholar]
- Philpott C. C., Ringuette M. J., Dean J. Oocyte-specific expression and developmental regulation of ZP3, the sperm receptor of the mouse zona pellucida. Dev Biol. 1987 Jun;121(2):568–575. doi: 10.1016/0012-1606(87)90192-8. [DOI] [PubMed] [Google Scholar]
- Ringuette M. J., Chamberlin M. E., Baur A. W., Sobieski D. A., Dean J. Molecular analysis of cDNA coding for ZP3, a sperm binding protein of the mouse zona pellucida. Dev Biol. 1988 Jun;127(2):287–295. doi: 10.1016/0012-1606(88)90315-6. [DOI] [PubMed] [Google Scholar]
- Ringuette M. J., Sobieski D. A., Chamow S. M., Dean J. Oocyte-specific gene expression: molecular characterization of a cDNA coding for ZP-3, the sperm receptor of the mouse zona pellucida. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4341–4345. doi: 10.1073/pnas.83.12.4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacco A. G., Yurewicz E. C., Subraminian M. G., DeMayo F. J. Zona pellucida composition: species cross reactivity and contraceptive potential of antiserum to a purified pig zona antigen (PPZA). Biol Reprod. 1981 Dec;25(5):997–1008. doi: 10.1095/biolreprod25.5.997. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmell E. D., Gulyas B. J. Mammalian sperm-egg recognition and binding in vitro. I. Specificity of sperm interactions with live and fixed eggs in homologous and heterologous inseminations of hamster, mouse, and guinea pig oocytes. Biol Reprod. 1980 Dec;23(5):1075–1085. doi: 10.1095/biolreprod23.5.1075. [DOI] [PubMed] [Google Scholar]
- Schmid C. W., Jelinek W. R. The Alu family of dispersed repetitive sequences. Science. 1982 Jun 4;216(4550):1065–1070. doi: 10.1126/science.6281889. [DOI] [PubMed] [Google Scholar]
- Shabanowitz R. B., O'Rand M. G. Characterization of the human zona pellucida from fertilized and unfertilized eggs. J Reprod Fertil. 1988 Jan;82(1):151–161. doi: 10.1530/jrf.0.0820151. [DOI] [PubMed] [Google Scholar]
- Swenson C. E., Dunbar B. S. Specificity of sperm-zona interaction. J Exp Zool. 1982 Jan 10;219(1):97–104. doi: 10.1002/jez.1402190112. [DOI] [PubMed] [Google Scholar]
- Wassarman P. M. Zona pellucida glycoproteins. Annu Rev Biochem. 1988;57:415–442. doi: 10.1146/annurev.bi.57.070188.002215. [DOI] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]