To the Editor:
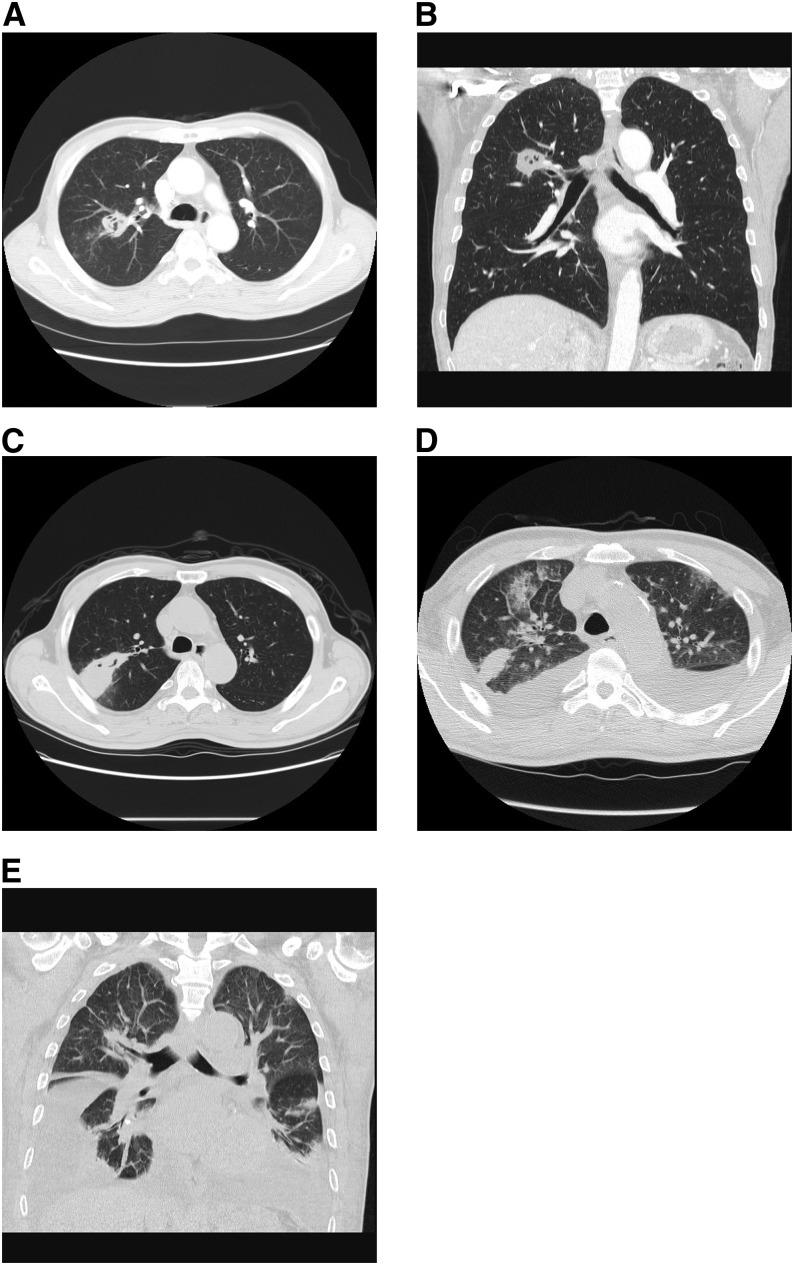
A 71-year-old man with two prior episodes of pulmonary tuberculosis (TB) in China (2008–2009) and San Francisco, California (2009–2010) presented in July 2013 with progressive shortness of breath. The patient immigrated to San Francisco in November 2008, at which time he was noted to have right upper lobe scarring with cavity formation (Figures 1A and 1B); three sputa were smear-negative and culture-negative for acid-fast bacilli (AFB). In July 2009, the man developed right upper lobe (RUL) consolidation in the area of the previously noted cavitary lesion, as well as pleural effusion (Figure 1C). Pan-susceptible Mycobacterium tuberculosis complex (MTBC) was isolated from both sputum and pleural biopsy. The patient completed standard short-course TB treatment in March 2010, when follow-up chest radiograph demonstrated interval decrease of the 3 × 2 cm RUL opacity; three sputa were again smear-negative and culture-negative. Persistent RUL opacity was noted at 12-month post-treatment follow-up in 2011, although sputum cultures remained negative. The patient returned intermittently to China and declined 18-month follow-up.
Figure 1.
Computed tomography of the lungs. Computed tomographic scans of the lungs without administration of contrast at time of immigration to the United States (A and B), second diagnosis of pulmonary tuberculosis in 2011 (C), and re-presentation in 2013 (current case report; D and E).
In July 2013, the patient presented to our emergency department with 2 days of progressive shortness of breath without fevers, night sweats, cough, or weight loss. Computed tomography showed bilateral pleural effusions and persistent RUL opacification (Figures 1D and 1E). On hospital day 2, a sputum specimen sent for concentrated smear demonstrated few AFB. Xpert MTB/RIF (Cepheid, Sunnyvale, CA) testing of this specimen showed MTBC at very low quantity (mean cycle threshold, 30.6), with rifampin resistance detected within the region of probe A (see Figure E1 in the online supplement). On hospital day 3, rifampin, isoniazid, pyrazinamide, ethambutol, moxifloxacin, capreomycin, and linezolid were initiated pending confirmatory testing. Complete blood count was within normal limits, hemoglobin A1c level (15.7 mg/dL) indicated poorly controlled diabetes mellitus, HIV testing was negative, and blood cultures demonstrated no growth.
The concentrated sputum specimen tested by Xpert was sent to the Microbial Diseases Laboratory of the California Department of Public Health for pyrosequencing (1). The molecular target of IS6110 for MTBC identification and six loci, including rpoB for drug resistance detection, yielded no sequences, implying insufficient DNA or no MTBC present. Repeat sputum smears were AFB-negative. Diagnostic thoracentesis demonstrated transudative pleural fluid and no AFB on smear examination. On hospital day 8, the specimen tested by Xpert became culture-positive, with morphology consistent with non-TB mycobacteria (NTM). Repeat pyrosequencing and Xpert testing from the broth culture (BacT/ALERT 3D system; bioMerieux Inc., Durham, NC) were negative. The culture tested negative for MTBC twice, using AccuProbe (Hologic, Inc., San Diego, CA); was plated to Remel Middlebrook 7H11 Agar, where it demonstrated growth after 3 days; and was identified as a member of the Mycobacterium fortuitum-chelonae group. Three additional sputum cultures showed no growth of any organism. These data, along with low clinical suspicion for TB, prompted discontinuation of all anti-TB therapy on hospital day 14, and the patient was discharged. At 12-month follow-up, the patient remained asymptomatic without radiographic progression or clinical evidence of active TB, and three additional sputum specimens were smear-negative and culture-negative.
Our patient’s short interval of symptoms and evidence of volume overload strongly suggested a non-TB etiology of respiratory distress. In addition, reinfection with multidrug-resistant TB (MDR-TB) or acquisition of MDR-TB during treatment of pan-susceptible disease in California is rare (∼0.05%) (2). Nevertheless, population risk factors (geographic origin [3], recent travel, diabetes [4], and tobacco smoke [4, 5]), prior TB, and abnormal radiographic findings prompted his care providers to “rule out” TB and to initiate treatment in response to the positive Xpert result.
Ultimately, although active disease from acquired rifampin-resistance or reinfection by a rifampin-resistant strain were considered, the growth of NTM, the failure to detect MTBC by other molecular methods, and the atypical clinical presentation raised questions about the reliability of the Xpert results. Potential explanations included test error, mixed infection of MTB and NTM, or nonviable MTB from prior active disease. Xpert test errors in rifampin-resistance determination have been documented, in particular in the setting of low pretest probability (6, 7). The Xpert graphical read-out showing four of five probes with threshold-crossing cycle values and complete signal “drop-off” for probe A (indicating an unambiguous mutation present within the probe A detectable range [codons 507–512]) made test error less likely. With regard to the possibility of mixed infection, neither M. fortuitum nor M. chelonae has homology with MTBC within the core region of rpoB, and thus they do not result in false-positive Xpert results (6). Inability to determine M. tuberculosis viability is a well-known and important limitation of current-generation molecular tests (8). In a recent study, 27% of patients remained Xpert-positive after 26 weeks of therapy for pan-susceptible disease (9), and Xpert false-positivity has been noted more than 5 years after completion of prior TB treatment (10). Although Xpert is strongly recommended as an initial diagnostic test in the setting of suspected drug resistance (11), it remains unclear how previous TB episodes impact such testing.
Given the uncertainty regarding the relative sensitivity of Xpert versus pyrosequencing in a specimen containing a low quantity of AFB, we decided to sequence the rpoB core region from the initial 2009 isolate. Remarkably, the 511CCG mutation (within the rpoB region of probe A) was detected in the 2009 isolate. Strains harboring the 511CCG mutation are often phenotypically susceptible to rifampin (12). In the absence of clinical indication of recurrent TB, these findings strongly suggested shedding of genetic material from nonviable organisms related to the prior TB episode. Hypothetically, such longstanding shedding could relate to a reduced ability to clear MTBC organisms because of architectural distortion of the airways and/or diabetes-associated immunocompromise. Notably, inability to grow MTBC from culture in this case does not unequivocally prove shedding of nonviable MTBC, as overgrowth of NTM is known to hinder recovery of MTBC in culture. Nevertheless, at 12-month follow-up, the patient remains asymptomatic and without radiographic progression of disease, making active TB unlikely.
In conclusion, as use of automated nucleic acid amplification tests increase within TB control programs and hospitals in the United States, such tests will likely play an increasingly important role in the exclusion of TB among inpatients (13). Our case emphasizes the importance of clinical judgment and sequence-based molecular assays in evaluating seemingly contradictory molecular and phenotypic drug susceptibility test results (14). Limited resource settings, where confirmatory testing is typically unavailable, must balance the risk of potentially unnecessary treatment with patient and community morbidity and mortality, with the understanding that cases such as ours remain rare and case-reportable events (11).
Acknowledgments
Acknowledgment
The authors thank this patient for consenting to share his story.
Footnotes
Author Contributions: All authors have contributed to drafting this letter.
This letter has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Lin SY, Rodwell TC, Victor TC, Rider EC, Pham L, Catanzaro A, Desmond EP. Pyrosequencing for rapid detection of extensively drug-resistant Mycobacteria tuberculosis in clinical isolates and clinical specimens. J Clin Microbiol. 2014;52:475–482. doi: 10.1128/JCM.01821-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Porco TC, Oh P, Flood JM. Antituberculosis drug resistance acquired during treatment: an analysis of cases reported in California, 1994–2006. Clin Infect Dis. 2013;56:761–769. doi: 10.1093/cid/cis989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization Global tuberculosis report 2014 [accessed 2014 Nov 14]Available from: http://www.who.int/tb/publications/global_report/en/
- 4.Hsu AH, Lee JJ, Chiang CY, Li YH, Chen LK, Lin CB. Diabetes is associated with drug-resistant tuberculosis in Eastern Taiwan. Int J Tuberc Lung Dis. 2013;17:354–356. doi: 10.5588/ijtld.11.0670. [DOI] [PubMed] [Google Scholar]
- 5.Ruddy M, Balabanova Y, Graham C, Fedorin I, Malomanova N, Elisarova E, Kuznetznov S, Gusarova G, Zakharova S, Melentyev A, et al. Rates of drug resistance and risk factor analysis in civilian and prison patients with tuberculosis in Samara Region, Russia. Thorax. 2005;60:130–135. doi: 10.1136/thx.2004.026922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Helb D, Jones M, Story E, Boehme C, Wallace E, Ho K, Kop J, Owens MR, Rodgers R, Banada P, et al. Rapid detection of Mycobacterium tuberculosis and rifampin resistance by use of on-demand, near-patient technology. J Clin Microbiol. 2010;48:229–237. doi: 10.1128/JCM.01463-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Rie A, Mellet K, John MA, Scott L, Page-Shipp L, Dansey H, Victor T, Warren R. False-positive rifampicin resistance on Xpert MTB/RIF: case report and clinical implications. Int J Tuberc Lung Dis. 2012;16:206–208. doi: 10.5588/ijtld.11.0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kennedy N, Gillespie SH, Saruni AO, Kisyombe G, McNerney R, Ngowi FI, Wilson S. Polymerase chain reaction for assessing treatment response in patients with pulmonary tuberculosis. J Infect Dis. 1994;170:713–716. doi: 10.1093/infdis/170.3.713. [DOI] [PubMed] [Google Scholar]
- 9.Friedrich SO, Rachow A, Saathoff E, Singh K, Mangu CD, Dawson R, Phillips PP, Venter A, Bateson A, Boehme CC, et al. Pan African Consortium for the Evaluation of Anti-tuberculosis Antibiotics (PanACEA) Assessment of the sensitivity and specificity of Xpert MTB/RIF assay as an early sputum biomarker of response to tuberculosis treatment. Lancet Respir Med. 2013;1:462–470. doi: 10.1016/S2213-2600(13)70119-X. [DOI] [PubMed] [Google Scholar]
- 10.Boyles TH, Hughes J, Cox V, Burton R, Meintjes G, Mendelson M. False-positive Xpert() MTB/RIF assays in previously treated patients: need for caution in interpreting results. Int J Tuberc Lung Dis. 2014;18:876–878. doi: 10.5588/ijtld.13.0853. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization Xpert MTB/RIF implementation manual. Technical and operational ‘how-to’: practical considerations Geneva, Switzerland: World Health Organization; 2014[accessed 2014 Oct 6]. Available from: http://apps.who.int/iris/bitstream/10665/112469/1/9789241506700_eng.pdf [Google Scholar]
- 12.Jureen P, Engstrand L, Eriksson S, Alderborn A, Krabbe M, Hoffner SE. Rapid detection of rifampin resistance in Mycobacterium tuberculosis by Pyrosequencing technology. J Clin Microbiol. 2006;44:1925–1929. doi: 10.1128/JCM.02210-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lippincott CK, Miller MB, Popowitch EB, Hanrahan CF, Van Rie A. Xpert(R) MTB/RIF shortens airborne isolation for hospitalized patients with presumptive tuberculosis in the United States. Clin Infect Dis. 2014;59:186–192. doi: 10.1093/cid/ciu212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Centers for Disease C. Prevention. Availability of an assay for detecting Mycobacterium tuberculosis, including rifampin-resistant strains, and considerations for its use - United States, 2013. MMWR Morb Mortal Wkly Rep. 2013;62:821–827. [PMC free article] [PubMed] [Google Scholar]