Abstract
Extracellular signal-regulated kinase 5 (ERK5) has been reported to regulate endothelial integrity and protect from vascular dysfunction under laminar flow. Previously reported research indicates that under laminar flow ERK5 is activated with production of atheroprotective molecules. However, the characterization of ERK5 activation and levels under different flow patterns has not been investigated.
Confluent HUVECs were serum-starved then seeded on glass slides. HUVECs incubated in 1% FBS were exposed to continuous laminar flow (CLF), to-and-fro flow (TFF), or pulsatile forward flow (PFF) in a parallel plate flow chamber. At the end of experimentation, cell lysates were immunoblotted with antibodies to phospho-ERK5 and total ERK5. ERK5 activation was assessed by the levels of phosphorylated ERK5. The densitometric mean ± SEM is calculated and analyzed by ANOVA. p < 0.05 is considered significant.
Levels of ERK5 decreased with all flow conditions with the largest decrease in TFF flow condition. TFF and CLF exhibited sustained ERK5 phosphorylation in HUVECs stimulated for up to 4 hours. PFF had transient phosphorylation of ERK5 at 2 hours, which then became undetectable at 4 hours of exposure to flow. Also, TFF and CLF both showed decreased levels at 4 hours, suggesting a decrease in activation for these flow conditions.
Exposure of HUVEC to different types of shear stress results in varying patterns of activation of ERK5. Activation of ERK5 with TFF suggests a role in the pathogenesis of atherosclerosis and vascular remodeling under disturbed flow conditions.
Keywords: atherosclerosis, shear stress, extracellular signal-regulated kinase 5, slowing of progression of atherosclerosis, atheroprotective molecules, vascular dysfunction, disturbed flow
Localized atherosclerotic plaque and regions exposed to the disturbed flow found at vessel curvatures, bifurcations, and branches have demonstrated a strong association with endothelium dysfunction.1 It has been hypothesized that the different types of mechanical forces may have different effects on endothelial cell (EC) function and dysfunction, which may promote atherogenesis.2 Interestingly, flow is a potent survival signal for ECs, via effects on multiple signaling pathways, which include phosphatidylinositol 3-kinase (PI3K), extracellular signal-regulated kinase 5 (ERK5), and NO.3 However, sustained laminar blood flow and unidirectional high shear stress are associated with atheroprotective properties such as, upregulation of antioxidant and growth-arrest genes in ECs.4
The anti-inflammatory and antiatherosclerotic effects of sustained laminar flow on ECs have been attributed to its effects on mitogen-activated protein kinase (MAPK) signaling.5 6 MAPK signaling is relatively complex and diverse; MAPK can engage in cross-talk with other MAPKs to regulate critical cell functions, such as proliferation, migration, and differentiation.7 Four distinct, classic MAPK cascades exist within mammalian cells: extracellular signal-regulated kinases (ERKs) 1/2, c-Jun NH2-terminal kinases (JNK) 1/2/3, p38MAPKα/β/γ/δ, and ERK5.7 The ERk1/2, JNK 1/2/3, p38, and ERK5 are activated via various hemodynamic forces, such as cyclic strain8 and shear stress.9 ERK5, the most recent member of MAPK family, has been evidenced to play a vital role in the mechanism of atheroprotective effects under sustained laminar flow that upregulates ERK5 activity.6 However, ERK5 expression is increased in atherogenic prone regions in mice, and comparison of the atheroprone region with the atheroprotected region showed no obvious difference in ERK5 subcellular localization.10 To evaluate this further, we investigated ERK5 activation and levels under different flow conditions, whether disturbed flow, continuous laminar flow (CLF), or pulsatile forward flow to determine whether spatial and temporal shear stress regulated ERK5 activation. We hypothesize that ERK5 activation in pulsatile uniform flow and CLF, but not in disturbed flow, as it is an atherogenic flow condition.
Materials and Methods
Cell Culture
Primary cultures of human umbilical vein endothelial cells (HUVECs) (obtained from the laboratory of Dr. Jordan Pober, Department of Pathology, Yale School of Medicine) were cultured with M-199 medium enriched with 20% fetal bovine serum (FBS; Gemini Bio-Products, West Sacramento, CA), 10 μg/mL heparin, 50 μg/mL EC growth supplement (BD Biosciences, Bedford, MA), and penicillin-streptomycin antibiotic combination (both 100 μg/mL), in a 5% CO2 incubator at 37°C. After reaching confluence, 0.25% trypsin ethylene diamine tetra acetic acid was used for detachment, and passage 3 to 5 cells were seeded on fibronectin (BD Biosciences) coated glass slides (7 × 38 mm, Fisher Scientific, Pittsburgh, PA). After reaching confluence, HUVECs were serum-starved (SS) in 1% FBS media for 2 hours prior to experimentation. Before the experiment, cells were rinsed free of culture medium with HEPES-buffered saline solution (130 mmol/L NaCl, 5 mmol/L KCl, 1.5 mmol/L CaCl2, 1.0 mmol/L MgCl2, and 20 mmol/L HEPES, pH 7.4). This study was approved by the institutional review board.
Mechanical Stress Exposure
Serum-starved HUVECs were exposed to shear stress for 30 minutes, 1 hour, 2 hours, or 4 hours under different flow conditions utilizing a parallel-plate flow chamber system (Cytodyne, San Diego, CA), as previously described.11 12 Flow of the perfusion medium (M199 and 1% FBS) was regulated by a computer-controlled syringe pump (PHD 2000 and PHD Ultra Programmable, Harvard Apparatus, Holliston, MA). To generate PFF, an automated switch clamp (Auto-Fill valve box, Harvard Apparatus) is placed between the syringe pump and the flow chamber and between the syringe pump and the culture medium reservoir. Synchronous activation of both switch clamps with the cycle allows unidirectional flow. In TFF model, the switch clamp was not used, thus creating a form of disturbed flow. The flow chambers were directly attached to the flow loop circuit including the flow reservoir, which enabled culture medium in the flow chamber to be exchanged by every to-fro impulse. Approximately 1/10th of the culture media in the flow chamber was exchanged at least every 1 second. The shear stresses were 60 cycles per minute (cpm) of PFF, that is, a forward square wave impulse for 0.5 second alternating with no flow for 0.5 second; 60 cpm of TFF, that is, a forward square wave impulse for 0.5 second alternating with a backward square wave impulse for 0.5 second. CLF setup is a perfusion medium circulated in Norprene tubing (Masterflex, Court Vernon Hills, IL) using a roller pump to parallel plate flow chambers placed in the circuit. Small pulsations produced by the roller pump were dampened because of the long length of the tubing. The magnitude of shear was kept constant at 14 dyne/cm2 for all flow conditions. Each experiment was repeated at least three times.
Immunoblot for ERK5
After experimentation, cells were washed in ice-cold PBS (Hyclone, South Logan, UT) twice and scraped in RIPA lysis buffer containing 1 mM PMSF, 1% SDS, 2 mM sodium orthovanadate, cocktail of phosphatase inhibitor I and II (EMD4 Bioscience, San Diego, CA), and protease inhibitor (Roche Applied Science, Indianapolis, IN). Cell lysates were centrifuged for 15,000 g for 10 minutes, and supernatants were collected. Protein content was measured by Bio-Rad protein assay system (Bio-Rad, Hercules, CA). Laemmli sample buffer was added to equal amounts of each sample, and samples were boiled for 5 minutes. Samples were resolved on 10% SDS-PAGE, which were separate blots for total ERK5 and pERK5, and transferred to polyvinylidene fluoride membrane. The membranes were probed with phosphorylated (P-ERK5 and ERK5 (Cell Signaling Technology, Danvers, MA) as the primary antibody. P-ERK5 antibody was incubated in 10% BSA and 5% milk. Rabbit secondary antibody (Cell Signaling Technology, Danvers, MA) was added, as suggested by the manufacture, and detected using chemiluminescence (Lumiglo, Cell Signaling Technology, Danvers, MA). Membranes were developed on ChemiDoc MP Imager (Bio-Rad, Hercules, CA), and quantification of bands was performed by densitometric analysis using Image J software (http://rsbweb.nih.gov/ij/). Densitometric data were expressed as the fold induction compared with the static control level.
Statistics
Calculation of the densitometric ± standard error of the mean of multiple experiments and statistical analysis utilizing ANOVA (analysis of variance) and post hoc test were performed using Minitab (State College, PA). A p value of < 0.05 was considered significant.
Results
Changes in ERK5 Levels when Exposed to Different Flow Conditions
ERK5 levels significantly decreased after 30 minutes of exposure to TFF compared with static conditions (n = 3). However, ERK5 levels did not change when exposed to CONT or PFF flow conditions after 30 minutes of stimulation (Fig. 1). ERK5 levels decreased to 53.3% in TFF conditions (Fig. 1C). After 1 hour of stimulation, ERK5 levels decreased in CONT, PFF, or TFF flow conditions (n = 3) compared with static conditions (Fig. 2). ERK5 levels decreased in CONT to 74.9%, PFF to 69.6%, and TFF to 53.3% compared with static conditions (Fig. 2C). On longer duration of experiments, all flow conditions exhibited decreased ERK5 levels compared with static conditions after 2 hours of stimulation (Fig. 3). ERK5 levels decreased in CONT to 49.5%, PFF to 65.7%, and TFF to 45.6% (n = 6) compared with static conditions (Fig. 3C). Also, after 4 hours of stimulation, ERK5 levels maintained a sustained decrease in all flow conditions (Fig. 4). ERK5 levels decreased in CONT to 48.6%, PFF to 55.8%, and TFF to 44.6% (n = 4) compared with static conditions (Fig. 4C).
Fig. 1.
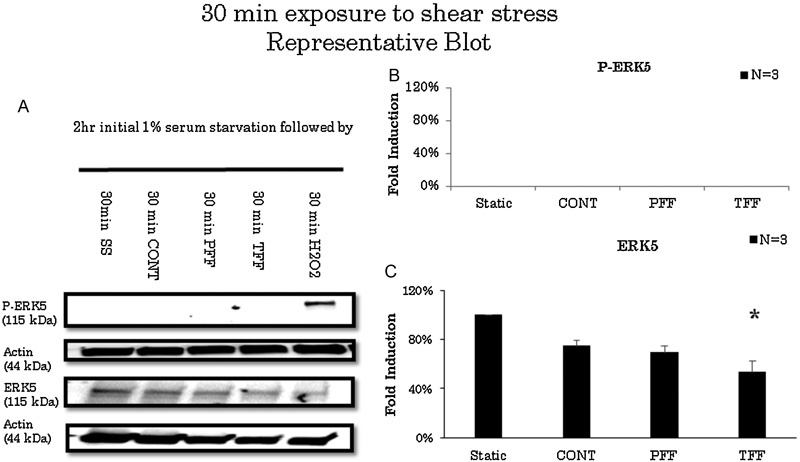
(A) Representative blot of extracellular signal-regulated kinase 5 (ERK5) activity in 30 minutes of various flow conditions. Cells were serum starved for 2 hours prior to experimentation. Hydrogen peroxide served as positive control. (B) Arbitrary densitometric measurements of P-ERK5 standardized to actin. There is no activation of ERK5 in any flow condition compared with static condition. (C) ERK5 standardized to actin showing decreased levels in to-and-fro flow (TFF) only. CONT; continuous laminar flow; PFF, pulsatile forward flow; SS, serum starvation. * p < 0.05.
Fig. 2.
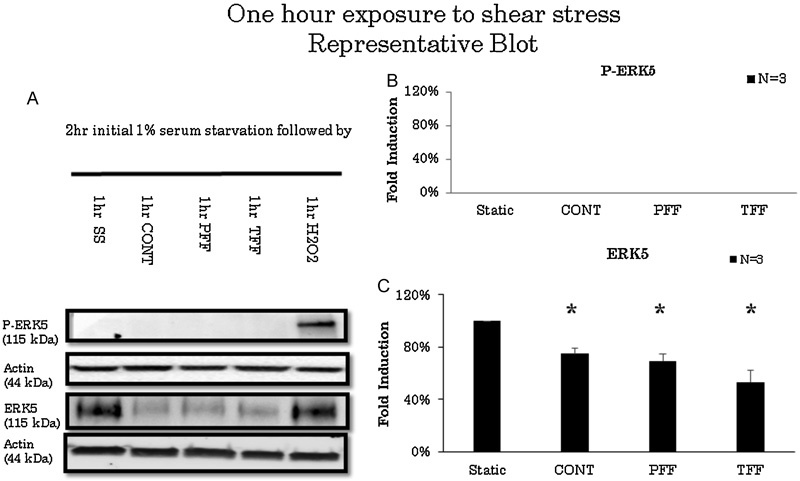
(A) Representative blot of extracellular signal-regulated kinase 5 (ERK5) activation in 1 hour of various flow conditions. Cells were serum starved for 2 hours prior to experimentation. Hydrogen peroxide served as s positive control. (B) Arbitrary densitometric measurements of P-ERK5 standardized to actin. . There is no activation of ERK5 in any flow condition compared with static condition. (C) ERK5 standardized to actin showing decreased levels in all flow conditions. CONT; continuous laminar flow; PFF, pulsatile forward flow; SS, serum starvation; TFF, to-and-fro flow. * p < 0.05.
Fig. 3.
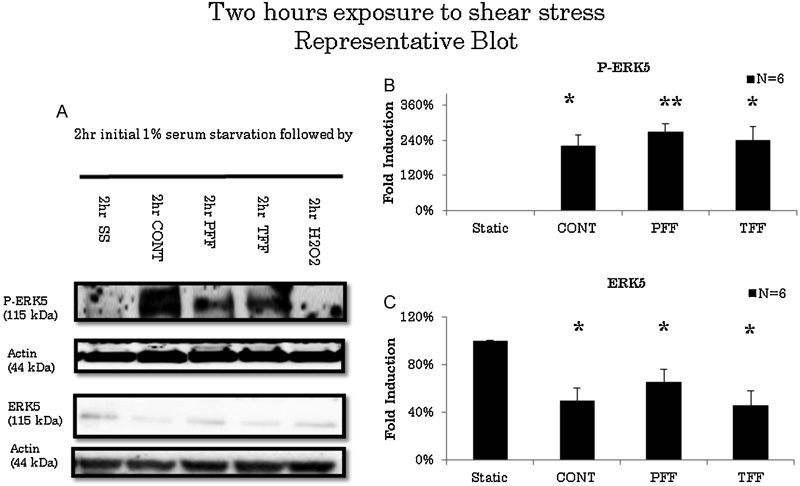
(A) Representative blot of extracellular signal-regulated kinase 5 (ERK5) activation in 2 hours of various flow conditions. Cells were serum starved for 2 hours prior to experimentation. There is activation of ERK5 in all flow conditions. (B) P-ERK5 standardized to actin. There are increased levels of P-ERK5 in all flow conditions except in static and 2-hour H2O2 media for 2 hours. (C) Arbitrary densitometric measurements of ERK5 standardized to actin. There is decrease in ERK5 in all flow conditions. CONT; continuous laminar flow; PFF, pulsatile forward flow; SS, serum starvation; TFF, to-and-fro flow. * p < 0.05, ** p < 0.001.
Fig. 4.
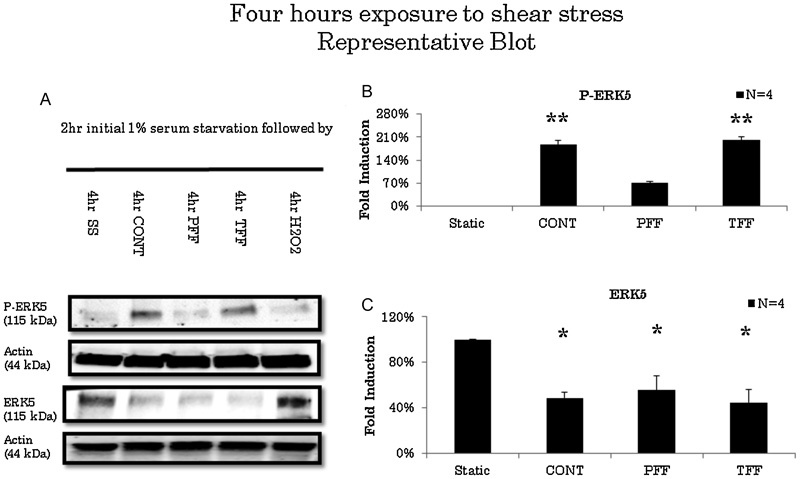
(A) Representative blot of extracellular signal-regulated kinase 5 (ERK5) activation in 4 hours of various flow conditions. Cells were serum starved for 2 hours prior to experimentation. There is activation of ERK5 in nonpulsatile uniform flow and disturbed flow conditions. (B) Arbitrary densitometric measurements of P-ERK5 standardized to actin. There is activation of ERK5 in continuous laminar and disturbed flow conditions. (C) ERK5 standardized to actin showing decreased levels in all flow conditions. CONT; continuous laminar flow; PFF, pulsatile forward flow; SS, serum starvation; TFF, to-and-fro flow. * p < 0.05, ** p < 0.001.
Significant Differences in ERK5 Level among Different Flow Conditions within Same Time Point
There were differences in reduction in ERK5 levels between all flow conditions after each time point experiment (Fig. 5). ERK5 levels decreased more in TFF than in CONT flow conditions after 30 minutes of exposure (ANOVA test, p < 0.05). After 1 hour of stimulation, there was no change in reduction in ERK5 levels between the different flow conditions. However, after 2 hours of stimulation, PFF exhibited least decrease in levels much lower compared with TFF and CONT flow conditions. Also, after 4 hours of stimulation, TFF decreased compared with CONT and PFF. There was no statistical significance between different time point experiments within same or different flow conditions.
Fig. 5.
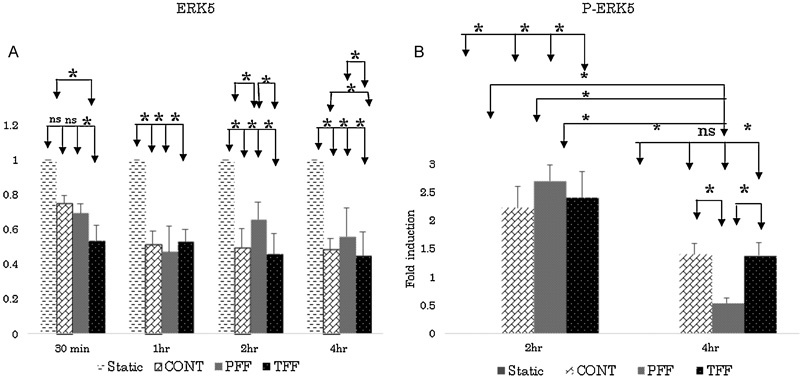
(A) ERK5 activity in response to different types of flow. ERK5 levels significantly decreased in disturbed flow compared with continuous laminar flow, after 4 hours of stimulation both, continuous and disturbed flow decreased compared with pulsatile flow. (B) Activation of ERK5 increased in continuous and disturbed flow compared with pulsatile uniform flow. CONT, continuous laminar flow; PFF, pulsatile forward flow; TFF, to-and-fro flow. * p < 0.05, ANOVA analysis with post hoc test (Fischer's test).
ERK5 Activation in Different Flow Conditions
There was no activation of ERK5 levels in all flow conditions for up to 1 hour of stimulation (n = 3). Hydrogen peroxide stimulation served as a positive control whereas static conditions served as negative controls (Fig. 1A, 2A). However, after 2 hours of activation, compared with static conditions, ERK5 is significantly activated in CONT, PFF, and TFF flow conditions (Fig. 2A), and it exhibits a 2.2-, 2.7-, and 2.4-fold increase (Fig. 2B). Static conditions and hydrogen peroxide, known to be a short-time activator of ERK5, served as negative controls.13 After 4 hours of stimulation, only CONT and TFF conditions were significantly activated (Fig. 4A). CONT and TFF exhibited a 1.8- and 2-fold increase, respectively, whereas PFF did not exhibit any significant activation (Fig. 4B). P-ERK5 levels in PFF conditions significantly decreased from 2 to 4 hours of stimulation compared with CONT, PFF, and TFF flow conditions. Also, after 4 hours of stimulation P-ERK5 levels is significantly low compared with TFF and CONT (Fig. 5B).
Discussion
Laminar flow activates MEK5/ERK5 pathway in ECs, which results in an overall protective phenotype characterized by increased apoptosis resistance and a decreased angiogenic, migratory, and proinflammatory potential.9 10 14 15 16 17 18 However, ERK5 also mediates noxious pathways, such tumor genesis and proinflammatory pathways.19 20 Although ectopic expression of ERK5 in the prostate cancer cell line PC3 increased proliferation, RNAi knockdown did not decrease proliferation. This suggests that the role of ERK5 in sustaining the proliferative signal may be cancer-cell-line- or tumor-specific, and at this time, it is unknown what makes a cancer cell sensitive to ERK5 inhibition.21 This known association of ERK5 with sustain proliferative signaling coincidences with the MEK5/ERK5 cascade has been shown to protect cells from apoptotic stimuli.22 Furthermore, both MEK5 and ERK5 knockout mice rapidly die due to disruption of the vascular integrity.23 24 25 Previous studies have demonstrated that ERK5 plays an essential role in the ability of shear stress to prevent EC apoptosis and plays a key role in flow-mediated anti-inflammatory effects.23 26
Our observation of ERK5 activation in CLF conditions is a validated observation based on previous studies.17 18 However, the ERK5 levels change in different flow conditions, and ERK5 activation in disturbed flow and transit activation in PFF has not been shown before. As these flow conditions, CLF and disturbed flow, are not natural physiologic flow conditions that EC cells are accustomed to, this observation further illustrates that disturbed flow is an apoptotic stimulus requiring ERK5 activation. Also, previous experiments have shown that ERK5 is activated in other apoptotic conditions such as high concentration hydrogen peroxide concentration.26 However, whether activation of ERK5 by CLF has the same MEK5/ERK5 cascade as disturbed flow requires further analysis. It has been shown that the ERK5 cascade in ECs exposed to disturbed flow in vivo is different from exposure to laminar flow, in which disturbed flow-induced SUMOylation of proteins, including p53 and ERK5 occurs and is thought to be important in atherosclerosis formation.27
Further experiments are required to explain the observation of ERK5 level changes in different flow conditions. Our hypothesis is structural changes, or ubiquitination of ERK5 under these conditions may have caused these observations. It has been observed that in vivo studies ERK5 levels are increased in atherogenic areas of the vasculature compared with areas exposed to laminar flow.10 Our in vitro model has shown differences of ERK5 levels among different flow conditions. Further studies are required to identify the causation of increase in ERK5 levels in vivo under disturbed flow whereas ERK5 levels decrease in vitro under the same flow condition. Our hypothesis is that in vivo conditions cells are not serum-starved and are able to replenish ERK5 levels whereas in vitro they are incapable to do so, and thus levels decrease.
The phenomena of temporal and spatial components of shear stress may have independent or interposing effects; for example, low steady shear stress is the major impetus for differential gene expression and cell proliferation, whereas disturbed flow regulates monocyte adhesion.28 Thus, further experiments are required to identify the different components of shear stress independent effects and its signaling pathway.
Conclusion
In our study, different patterns of flow induce decrease in ERK5 levels with the most decrease in TFF. ERK5 is an atheroprotective molecule that under uniform flow conditions induces downstream anti-inflammatory and antimigratory effects on ECs. However, disturbed flow induces ERK5 dysfunction. Our findings may aid to decipher in the understanding of the progression of atherosclerosis. Further studies investigating ERK-5 effect on intranuclear gene expression are required.
References
- 1.VanderLaan P A, Reardon C A, Getz G S. Site specificity of atherosclerosis: site-selective responses to atherosclerotic modulators. Arterioscler Thromb Vasc Biol. 2004;24(1):12–22. doi: 10.1161/01.ATV.0000105054.43931.f0. [DOI] [PubMed] [Google Scholar]
- 2.Frangos S G, Gahtan V, Sumpio B. Localization of atherosclerosis: role of hemodynamics. Arch Surg. 1999;134(10):1142–1149. doi: 10.1001/archsurg.134.10.1142. [DOI] [PubMed] [Google Scholar]
- 3.Hahn C, Schwartz M A. Mechanotransduction in vascular physiology and atherogenesis. Nat Rev Mol Cell Biol. 2009;10(1):53–62. doi: 10.1038/nrm2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berk B C. Atheroprotective signaling mechanisms activated by steady laminar flow in endothelial cells. Circulation. 2008;117(8):1082–1089. doi: 10.1161/CIRCULATIONAHA.107.720730. [DOI] [PubMed] [Google Scholar]
- 5.Sumpio B E, Yun S, Cordova A C. et al. MAPKs (ERK1/2, p38) and AKT can be phosphorylated by shear stress independently of platelet endothelial cell adhesion molecule-1 (CD31) in vascular endothelial cells. J Biol Chem. 2005;280(12):11185–11191. doi: 10.1074/jbc.M414631200. [DOI] [PubMed] [Google Scholar]
- 6.Berk B C Abe J I Min W Surapisitchat J Yan C Endothelial atheroprotective and anti-inflammatory mechanisms Ann N Y Acad Sci 200194793–109., discussion 109–111 [DOI] [PubMed] [Google Scholar]
- 7.Chang L Karin M Mammalian MAP kinase signalling cascades Nature 2001410(6824):37–40. [DOI] [PubMed] [Google Scholar]
- 8.Azuma N, Duzgun S A, Ikeda M. et al. Endothelial cell response to different mechanical forces. J Vasc Surg. 2000;32(4):789–794. doi: 10.1067/mva.2000.107989. [DOI] [PubMed] [Google Scholar]
- 9.Yan C, Takahashi M, Okuda M, Lee J D, Berk B C. Fluid shear stress stimulates big mitogen-activated protein kinase 1 (BMK1) activity in endothelial cells. Dependence on tyrosine kinases and intracellular calcium. J Biol Chem. 1999;274(1):143–150. doi: 10.1074/jbc.274.1.143. [DOI] [PubMed] [Google Scholar]
- 10.Nigro P, Abe J, Woo C H. et al. PKCzeta decreases eNOS protein stability via inhibitory phosphorylation of ERK5. Blood. 2010;116(11):1971–1979. doi: 10.1182/blood-2010-02-269134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abe R, Yamashita N, Rochier A. et al. Pulsatile to-fro flow induces greater and sustained expression of tissue factor RNA in HUVEC than unidirectional laminar flow. Am J Physiol Heart Circ Physiol. 2011;300(4):H1345–H1351. doi: 10.1152/ajpheart.01197.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Azuma N, Akasaka N, Kito H. et al. Role of p38 MAP kinase in endothelial cell alignment induced by fluid shear stress. Am J Physiol Heart Circ Physiol. 2001;280(1):H189–H197. doi: 10.1152/ajpheart.2001.280.1.H189. [DOI] [PubMed] [Google Scholar]
- 13.Suzaki Y, Yoshizumi M, Kagami S. et al. Hydrogen peroxide stimulates c-Src-mediated big mitogen-activated protein kinase 1 (BMK1) and the MEF2C signaling pathway in PC12 cells: potential role in cell survival following oxidative insults. J Biol Chem. 2002;277(11):9614–9621. doi: 10.1074/jbc.M111790200. [DOI] [PubMed] [Google Scholar]
- 14.Ohnesorge N, Viemann D, Schmidt N. et al. Erk5 activation elicits a vasoprotective endothelial phenotype via induction of Kruppel-like factor 4 (KLF4) J Biol Chem. 2010;285(34):26199–26210. doi: 10.1074/jbc.M110.103127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamik A, Lin Z, Kumar A. et al. Kruppel-like factor 4 regulates endothelial inflammation. J Biol Chem. 2007;282(18):13769–13779. doi: 10.1074/jbc.M700078200. [DOI] [PubMed] [Google Scholar]
- 16.McCormick S M, Eskin S G, McIntire L V. et al. DNA microarray reveals changes in gene expression of shear stressed human umbilical vein endothelial cells. Proc Natl Acad Sci U S A. 2001;98(16):8955–8960. doi: 10.1073/pnas.171259298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Komaravolu R K, Adam C, Moonen J R, Harmsen M C, Goebeler M, Schmidt M. Erk5 inhibits endothelial migration via KLF2-dependent down-regulation of PAK1. Cardiovasc Res. 2015;105(1):86–95. doi: 10.1093/cvr/cvu236. [DOI] [PubMed] [Google Scholar]
- 18.Parmar K M, Larman H B, Dai G. et al. Integration of flow-dependent endothelial phenotypes by Kruppel-like factor 2. J Clin Invest. 2006;116(1):49–58. doi: 10.1172/JCI24787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gavine P R, Wang M, Yu D. et al. Identification and validation of dysregulated MAPK7 (ERK5) as a novel oncogenic target in squamous cell lung and esophageal carcinoma. BMC Cancer. 2015;15:454. doi: 10.1186/s12885-015-1455-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoshizumi M, Kyotani Y, Zhao J. et al. Role of big mitogen-activated protein kinase 1 (BMK1)/extracellular signal-regulated kinase 5 (ERK5) in the pathogenesis and progression of atherosclerosis. J Pharmacol Sci. 2012;120(4):259–263. doi: 10.1254/jphs.12r11cp. [DOI] [PubMed] [Google Scholar]
- 21.Lochhead P A, Gilley R, Cook S J. ERK5 and its role in tumour development. Biochem Soc Trans. 2012;40(1):251–256. doi: 10.1042/BST20110663. [DOI] [PubMed] [Google Scholar]
- 22.Wang X, Merritt A J, Seyfried J. et al. Targeted deletion of mek5 causes early embryonic death and defects in the extracellular signal-regulated kinase 5/myocyte enhancer factor 2 cell survival pathway. Mol Cell Biol. 2005;25(1):336–345. doi: 10.1128/MCB.25.1.336-345.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pi X, Yan C, Berk B C. Big mitogen-activated protein kinase (BMK1)/ERK5 protects endothelial cells from apoptosis. Circ Res. 2004;94(3):362–369. doi: 10.1161/01.RES.0000112406.27800.6F. [DOI] [PubMed] [Google Scholar]
- 24.Sohn S J, Sarvis B K, Cado D, Winoto A. ERK5 MAPK regulates embryonic angiogenesis and acts as a hypoxia-sensitive repressor of vascular endothelial growth factor expression. J Biol Chem. 2002;277(45):43344–43351. doi: 10.1074/jbc.M207573200. [DOI] [PubMed] [Google Scholar]
- 25.Hayashi M, Kim S W, Imanaka-Yoshida K. et al. Targeted deletion of BMK1/ERK5 in adult mice perturbs vascular integrity and leads to endothelial failure. J Clin Invest. 2004;113(8):1138–1148. doi: 10.1172/JCI19890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li L, Tatake R J, Natarajan K. et al. Fluid shear stress inhibits TNF-mediated JNK activation via MEK5-BMK1 in endothelial cells. Biochem Biophys Res Commun. 2008;370(1):159–163. doi: 10.1016/j.bbrc.2008.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heo K S, Chang E, Le N T. et al. De-SUMOylation enzyme of sentrin/SUMO-specific protease 2 regulates disturbed flow-induced SUMOylation of ERK5 and p53 that leads to endothelial dysfunction and atherosclerosis. Circ Res. 2013;112(6):911–923. doi: 10.1161/CIRCRESAHA.111.300179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Conway D E, Williams M R, Eskin S G, McIntire L V. Endothelial cell responses to atheroprone flow are driven by two separate flow components: low time-average shear stress and fluid flow reversal. Am J Physiol Heart Circ Physiol. 2010;298(2):H367–H374. doi: 10.1152/ajpheart.00565.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]


