Abstract
Here we review the neural correlates of cognitive control associated with bilingualism. We demonstrate that lifelong practice managing two languages orchestrates global changes to both the structure and function of the brain. Compared with monolinguals, bilinguals generally show greater gray matter volume, especially in perceptual/motor regions, greater white matter integrity, and greater functional connectivity between gray matter regions. These changes complement electroencephalography findings showing that bilinguals devote neural resources earlier than monolinguals. Parallel functional findings emerge from the functional magnetic resonance imaging literature: bilinguals show reduced frontal activity, suggesting that they do not need to rely on top-down mechanisms to the same extent as monolinguals. This shift for bilinguals to rely more on subcortical/posterior regions, which we term the bilingual anterior-to-posterior and subcortical shift (BAPSS), fits with results from cognitive aging studies and helps to explain why bilinguals experience cognitive decline at later stages of development than monolinguals.
Keywords: bilingualism, fMRI, EEG, brain structure, brain function
Introduction
It is well documented that both languages in a bilingual mind are jointly activated.1,2 Therefore, bilinguals must constantly manage attention to two languages that compete for selection,1 a situation that leads to neuroplastic changes in the brain.3 An emerging idea is that this lifelong experience managing linguistic conflict leads to domain-general cognitive changes to both the structure and function of the brain.4 Understanding how bilingualism contributes to neuroplasticity is especially important considering recent evidence that bilingualism protects against age-related cognitive decline. For example, Bialystok, Craik, and Freedman5 demonstrated that the onset of symptoms of dementia occurred 4 years later for bilinguals than monolinguals, a finding that has been replicated in different populations (for reviews, see Refs. 6 and 7). Other studies have shown that, among Alzheimer’s disease patients, bilinguals perform equivalently on cognitive tests, even when their brains show more disease-related atrophy than monolinguals.8 What remains unknown are the precise mechanisms affected by bilingual experience that reshape the brain and lead to these protective effects. Our proposal is that the changes in brain structure and function attributed to bilingualism lead to improved efficiency in domain-general cognitive processing.
There is substantial evidence that verbal and nonverbal cognitive tasks recruit overlapping brain networks and processes in bilinguals (for reviews, see Refs. 9 and 10), supporting the argument that domain-general resources are involved in learning a second language. Here, we review magnetic resonance imaging (MRI) and electroencephalography (EEG) studies to examine the bases for differences between monolinguals and bilinguals in domain-general cognitive processing. Contrary to some previous reviews,11 we argue that the literature is largely consistent, and we provide a theoretical framework to understand how second-language (L2) experience leads to the greater cognitive efficiency found in bilinguals.
We converge on five important findings. First, bilinguals generally show greater gray matter volume than monolinguals in multiple areas of the brain, especially in perceptual/motor regions. Second, increased L2 experience generally leads to greater white matter (WM) integrity, with the most consistent evidence appearing in studies that examine L2 proficiency within bilinguals and in longitudinal studies. Third, functional MRI (fMRI) studies show less frontal activation for bilinguals than monolinguals with equivalent performance on nonverbal executive control tasks; this effect is reversed in children who are just learning a new language. Fourth, functional connectivity between brain regions is generally stronger in bilinguals than monolinguals in nonverbal executive control tasks. This connectivity may have the effect of distributing effort across the network for bilinguals, whereas monolinguals rely more heavily on frontal regions for nonverbal cognitive tasks. Fifth, EEG studies reveal that bilinguals rely on earlier processes to complete control tasks than monolinguals for similar levels of performance. All of these findings support the interpretation that bilingualism leads to domain-general modifications of neural networks that allow the system to rely on more efficient processes for cognitive tasks.
Remodeling of gray matter
At the level of the cortex, bilinguals tend to have greater gray matter volume than monolinguals,12 particularly in the anterior cingulate cortex (ACC)13 and parietal lobes,14,15 parts of the frontoparietal network (FPN). Importantly, gray matter volume fluctuates as a function of proficiency and exposure to an L2. Abutalebi et al.,14 for example, reported that left inferior parietal lobule (IPL) volume correlated with L2 proficiency and that right IPL volume correlated with L2 exposure. Similar findings by Wei et al.15 led the authors to argue that increasing L2 exposure affects gray matter volume: earlier exposure correlates with right superior parietal lobule (SPL) expansion. However, the most consistent findings are the remodeling of the subcortical volumes of the basal ganglia. The basal ganglia are involved in motor and perceptual feedback via cortical–basal ganglia loops.16–18 The basal ganglia enable the selection of responses (motor programsb) from among competing alternatives,19 and increasing task conflict leads to greater recruitment of basal ganglia regions.20 This may explain why the basal ganglia regions are involved in bilingualism owing to the constant need for bilinguals to deal with competition between two languages.
The basal ganglia consist of the striatum, globus pallidus, substantia nigra, and subthalamic nuclei. As we shall see, the striatum has been posited to be particularly important for bilingualism. This structure includes the caudate nucleus, the putamen, and the ventral striatum. Both the caudate and putamen are well connected to the frontal lobes, and feedback loops might contribute to more efficient communication between these regions over time. The caudate connects to prefrontal cortical areas and is thought to “gate” access to these frontal regions.21 The putamen, in contrast, connects to sensorimotor regions and may help monitor cognitive and sensorimotor environments to determine whether initiation of motor programs is appropriate.19 Evidence for basal ganglia nuclei changes have been observed both with voxel-based morphometry (VBM; a widely available method of assessing whole-brain volume22,23) and, recently, with more sensitive Bayesian subcortical modeling procedures.23–25 Several of these studies also describe how the gray matter differences are modulated by proficiency and age of acquisition of the second language. Abutalebi et al.,22 for example, showed that bilinguals (collapsed across proficiency levels) had larger left putamens than monolinguals. They further showed that less proficient bilinguals recruited this structure more than proficient bilinguals to support task performance. Abutalebi et al.’s findings indicate that greater recruitment of the putamen is necessary when first learning a second language, and that over time this recruitment leads to greater volume and more efficient processing.
Burgaleta et al.24 used a subcortical Bayesian approach and found that bilinguals had larger basal ganglia and thalamic structures, including bilateral putamens and thalami and right globus pallidi and caudate nuclei, than monolinguals. Using continuous measures of language exposure and production, the authors showed that, as bilinguals became more balanced in terms of time spent listening to the second language, thalamus volumes increased. A comparable relationship was observed in the right caudate with production of the L2. Similarly, Pliastikas et al.25 examined the effects of immersion in an L2 on subcortical structures and found that more immersion was associated with bilateral expansion of the putamen, a structure necessary for monitoring articulation and phonological errors. In a group of participants with equal proficiency but who used their L2 infrequently, the authors reported expansion of bilateral caudate nuclei, structures involved in rule learning and regulating feedback to the frontal cortices. The basal ganglia coordinate the management of motor routines, for example in the globus pallidus, and perceptual experiences via the thalamus.26 Given that bilingualism modifies these structures, it is possible that the increased motor and perceptual processes here allow the system to rely less on top-down frontal regions to increase efficiency.
Another approach to investigating the effect of bilingualism on brain structure is through studies of language training, and these studies also show gray matter changes at both cortical and subcortical levels. Martensson et al.27 compared surface-based morphometry in a group of students learning a second language and a control group of cognitive science students. Both groups were scanned twice, once at the beginning of the semester and once at the end. At baseline, there were no structural differences between groups. After training, highly proficient L2 learners showed tissue expansion in the left superior temporal gyrus (STG) and right hippocampus relative to controls. Intriguingly, L2 learners who struggled in the coursec showed volume increases in the dorsal middle frontal gyrus, inferior frontal gyrus, and superior temporal gyrus. Furthermore, right hippocampal and left STG volume were predicted by L2 proficiency, whereas the medial frontal gyrus (MFG) correlated with instructor ratings of effort.
Another training study28 used VBM to examine native English-speaking students studying German in Switzerland. Over the 5-month study period, the authors observed that increasing language proficiency predicted increases in the left inferior frontal gyrus. Finally, in a study comparing multilingual adults and highly proficient bilinguals who were simultaneous interpreters, brain scans were analyzed using VBM and a region-of-interest (ROI) approach including regions previously implicated in distinguishing between monolinguals and bilinguals (cingulate gyrus, caudate nucleus, frontal operculum, inferior parietal lobe, and insula).20 In this case, more proficient bilinguals had reduced gray matter in the left middle ACC, bilateral insula, left supramarginal gyrus (SMG), bilateral pars triangularis, and left pars opercularis. Additionally, negative correlations between the number of hours of L2 practice and gray matter volume were found in the left pars triangularis, middle anterior cingulate gyrus, and bilateral caudate nuclei. Finally, we note that the reduction in bilateral caudate volumes reported by Stein et al.28 is also interesting from the perspective of the work by Pliastikas et al.,25 who reported that caudate remodeling occurred only in the less proficient bilingual participants.
A general finding across these studies is that bilingualism increases gray matter volume, with the most consistent changes in the basal ganglia. Expansion of tissue in parietal and ACC regions appears to depend on the level of L2 proficiency and exposure. The basal ganglia are generally larger in bilinguals than monolinguals, including the putamen, caudate nuclei, and thalami. The caudate nuclei appear to be remodeled only for less proficient bilinguals who are struggling to learn a second language. Similarly, more proficient bilinguals show volume reductions relative to less proficient bilinguals in many areas of the brain, including the ACC and striate nucleus. Therefore, the relationship between gray matter volume and language proficiency or expertise follows an inverted U shape: as bilinguals gain proficiency with a second language, tissue volume increases, particularly in frontostriatal regions. However, once bilinguals gain a high level of expertise, gray matter tissue becomes specialized and appears to reduce relative to bilingual non-experts.
These tissue modifications might contribute to a more efficient system for bilinguals. The basal ganglia in particular are enlarged for bilinguals compared with monolinguals, and these structural changes would allow bilinguals to rely more on perceptual/motor processes and less on frontal regions.
Greater integrity of white matter structures for bilinguals
WM integrity is another significant contributor to efficient communication between brain regions.29 Changes to WM structures with L2 experience may help to explain why bilinguals often outperform monolinguals on executive function tasks requiring fast response times.30 WM continues to develop throughout the life span, contrary to previous belief that the process stops after childhood.31 Increased theta-band activity generated from the ACC, a center critically involved in bilingualism,32 possibly contributes to increases in WM development.33 For example, Voelker et al.31 argued that theta rhythms lead to a release of a protease that influences dormant oligodendrocytes and results in increased WM integrity through myelination. This WM integrity in turn leads to increased motor efficiency. Semantic anomalies in sentence processing lead to power increases in the theta band,34 and these sorts of semantic anomalies typify the bilingual experience. Increases in theta activity are also associated with greater verbal working memory demands.35 Therefore, theta activity generated from the ACC might lead to greater WM integrity for bilinguals than monolinguals. The current evidence demonstrates that bilingualism modifies WM volume and integrity in important ways.
Coggins III, Kennedy, and Armstrong36 were the first to report that bilingualism modified a region of the corpus callosum, which is the largest WM structure in the brain, consisting of 200–300 million axons.37,38 Compared with monolinguals, middle-aged (~ 40 years old) bilinguals showed enhancement of the anterior midbody of the corpus callosum. Felton et al. found similar results in young adults.39 They examined the corpus callosum and found that bilinguals had greater volume in the middle-anterior and central regions than monolinguals. Diffusion tensor imaging (DTI) studies showed that bilingualism also leads to changes in fractional anisotropy (FA) in WM. Luk, Bialystok, Craik, and Grady40 demonstrated that older adult bilinguals had greater FA values than monolinguals in the corpus callosum and the superior and inferior longitudinal fasciculi. Similar FA findings and locations were reported for young adults,41 again with greater FA for bilinguals in the corpus callosum that extended bilaterally to the inferior fronto-occipital fasciculus, uncinate fasciculi, and superior longitudinal fasciculi. Olsen et al.12 showed40 that, in addition to greater FA, bilinguals also had greater WM volume in the frontal and temporal lobes. For both groups, greater WM in frontal regions was associated with faster reaction times (RTs) during the Stroop task.
Not all studies find this pattern. Gold et al.42 showed that older adult monolinguals had greater FA than bilinguals in the inferior longitudinal fasciculus, inferior fronto-occipital fasciculus, and multiple portions of the corpus callosum. There are several potential reasons for this discrepancy. First, it is possible that the sample of bilingual participants in Gold et al.’s study had higher rates of preclinical mild cognitive impairment (MCI) pathology than monolinguals, despite cognitively normal performance.42 If monolinguals with the same level of brain atrophy as bilinguals were excluded because they were being classified with MCI, but bilinguals remained cognitively intact, this pattern would explain Gold et al.’s findings. Second, it is possible that L2 experience modifies specific regions of WM structures in the brain, including different portions of the corpus callosum (e.g., anterior versus posterior). It is also sometimes the case that apparent increases in FA in areas with crossing fibers are not increases but reflect an inability to distinguish between axial diffusivity of one fiber tract and radial diffusivity of the crossing fiber tract.43 Thus, what is seen as an increase may be driven by a relative rather than an absolute change.
Finally, bilingualism is a complex experience that takes place in different environmental contexts44 that might themselves influence different portions of WM structures. For example, a dense code-switching environment44 in which individuals switch constantly between languages within a sentence might not enhance WM structures to the same degree as a dual-language context environment, which requires more control. Similar to the Gold et al.42 findings, Kuhl et al.45 found greater FA for young adult monolinguals than bilinguals in multiple WM tracts. However, unlike Gold and colleagues, who did not include a continuous measure of L2 practice/exposure within bilinguals, Kuhl et al. found that within Spanish–English bilinguals, time spent in the United States, as well as time spent listening and speaking English, led to greater increases in FA. These increases were evident in left corticospinal, left inferior fronto-occipital fasciculus, the left superior longitudinal fasciculus, and left inferior longitudinal fasciculus tracts. This pattern highlights the importance of looking at continuous measures, because group divisions potentially mask important effects of L2 experience.
Many linguistic factors are also likely to contribute to structural changes to WM integrity over time. Mohades et al.46 showed that monolingual children had greater FA in the anterior portion of the corpus callosum than bilingual children, but that bilinguals had greater FA in the left inferior fronto-occipital fasciculus. Thus, the different WM structures are modified in complex ways. It is interesting that monolinguals showed greater FA than bilinguals in the corpus callosum given that, among adults, bilinguals have typically shown greater FA in this region. The developmental course of WM structures might therefore hinge on language mastery and automaticity over the course of the life span. Furthermore, as we noted earlier, the corpus callosum is a large brain structure, and it is possible that collapsing across different portions of the tissue masks important group effects.
Cummine and Boliek47 provided further support for the idea that different regions of WM are modified by the bilingual experience. They showed that young adult bilinguals and monolinguals had qualitatively different relationships between FA values and RTs to name words. Monolinguals and bilinguals both exhibited faster RTs with increased FA in the parietal–occipital sulcus regions, but bilinguals also exhibited faster RTs with increased FA in the extreme capsule and near the caudate nucleus. In contrast, monolinguals showed faster RTs with greater FA near the supplementary motor area. This pattern again underscores the point that the brain–behavior relationship between WM integrity and RT is complex, and that L2 experience modifies this relationship. Nichols and Joanisse48 revealed that, with earlier age of L2 acquisition, young adult bilinguals had higher FA values in the inferior longitudinal fasciculus (ILF), the anterior midbody of the corpus callosum, and the arcuate fasciculus. L2 proficiency was also associated with greater FA in the ILF, the right arcuate fasciculus, and the forceps minor of the corpus callosum.
If WM is involved in L2 learning, then it might follow that greater WM integrity leads to greater ability to learn an L2. The evidence so far reveals that it does. Golestani et al.49 taught non-native speech sounds to a group of individuals and found that fast learners had greater WM density in the left Heschl’s gyrus and lingual gyri bilaterally than slow learners. More recently, Qi et al.50 showed that the most successful learners of a 4-week long Mandarin course had greater FA in both the right superior longitudinal fasciculus and the right inferior longitudinal fasciculus than less successful learners.
Compelling evidence for the idea that the corpus callosum is modified by L2 learning comes from a longitudinal study in which young adult university students enrolled in a 9-month L2 course.51 A group of control students with a similar course load did not undergo language training. Those who took the L2 course showed significant increases in FA in the corpus callosum over time, whereas controls did not. Similarly, Mohades et al.52 measured FA values for simultaneous bilinguals (L2 from birth), sequential bilinguals (L2 from age 3), and monolinguals (no L2) over a span of 2 years. First, they showed that simultaneous bilinguals and sequential bilinguals had greater FA than monolinguals in the left inferior fronto-occipital fasciculus at both times. Second, they demonstrated that sequential bilinguals had the greatest increases in FA over the 2-year period. The authors attributed the greater gains in WM integrity over the 2-year span for sequential bilinguals to their having the largest proportional change of time being bilingual (i.e., years being bilingual at T2/years being bilinguals at T1). Thus, the change in bilingual status was significantly related to WM integrity gains.
These changes to WM structures can in turn lead to more efficient communication between different areas of gray matter. Several recent studies with young adults have shown that bilingualism leads to more efficient communication between areas that are involved in language processing and control than monolinguals. One study examined WM connections between gray matter nodes using graph theory.53 They found greater connectivity for bilinguals than monolinguals among the left frontal and parietal/temporal regions, the left occipital and parietal/temporal regions, and the right superior frontal gyrus.
While most studies accept that mean diffusivity (MD) is a measure of cellulitis and edema, one study showed the surprising finding that increases in this measure were associated with performance gains. Bakhtiari, Boliek, and Cummine54 found that both monolinguals and bilinguals had faster reading times with greater mean diffusivity in the uncinate fasciculus, but that only bilinguals showed faster RTs with greater mean diffusivity in the arcuate fasciculus and superior longitudinal fasciculus. It is important to understand the relation between MD and FA to reconcile these findings. FA is the ratio of the first eigenvector along the axon’s gradient—axial diffusivity (AD)—to the second and third eigenvectors showing diffusion perpendicular and diagonal to the primary gradient—radial diffusivity (RD). MD, by contrast, is a simple average of the three eigenvectors. Greater MD could result from greater RD, which typically tracks axonal demyelination, or from greater AD, which increases with brain maturation and reduces with axonal injury. As such, any interpretation of the relationship between MD and FA requires information about RD and AD. Nonetheless, this study highlights yet another qualitative difference in WM connectivity between groups.
The most consistent pattern of WM findings is found in studies examining levels of proficiency and exposure within bilinguals and longitudinal studies examining WM changes over time. The regions most consistently reported in this regard include the corpus callosum and the inferior and superior longitudinal fasciculi. These structures generally become larger and show greater FA with increases in L2 experience. These WM integrity increases may contribute to delaying the onset of cognitive decline for bilinguals relative to monolinguals. For example, Douaud et al.43 demonstrated that conversion from MCI to Alzheimer’s disease was reliability indexed by declines in corpus callosum FA values. Importantly, WM adaptations due to bilingualism fit with a model of efficient cognitive processing: WM connects gray matter regions for more efficient functional communication.55,56
Bilingualism as a model of efficiency: the bilingual anterior-to-posterior and subcortical shift
Our interpretation of this literature is that bilingualism is associated with a model of efficient brain recruitment. The pattern takes the form of less recruitment of frontal and executive regions and greater recruitment of posterior/subcortical regions by bilinguals to manage nonverbal executive tasks than is found in monolinguals. In particular, bilinguals recruit the ACC and dorsolateral prefrontal cortices (DLPFC) less than their monolingual peers, but rely more than monolinguals on basal ganglia and posterior regions that are responsible for perceptual/motor functions. We use the phrase bilingual anterior-to-posterior and subcortical shift (BAPSS) (Fig. 1) to describe this pattern. We argue that this pattern reflects efficiency rather than dedifferentiation (i.e., lack of specificity of neural activation accompanying worse performance), because the contributing studies report either no behavioral differences between groups or, more impressively, matched behavior, allowing brain differences to be discussed in the absence of a behavioral confound. Many studies, however, examine behavioral correlates of activation. To the extent that bilinguals recruit frontal regions, they tend to show reduced performance.
Figure 1.
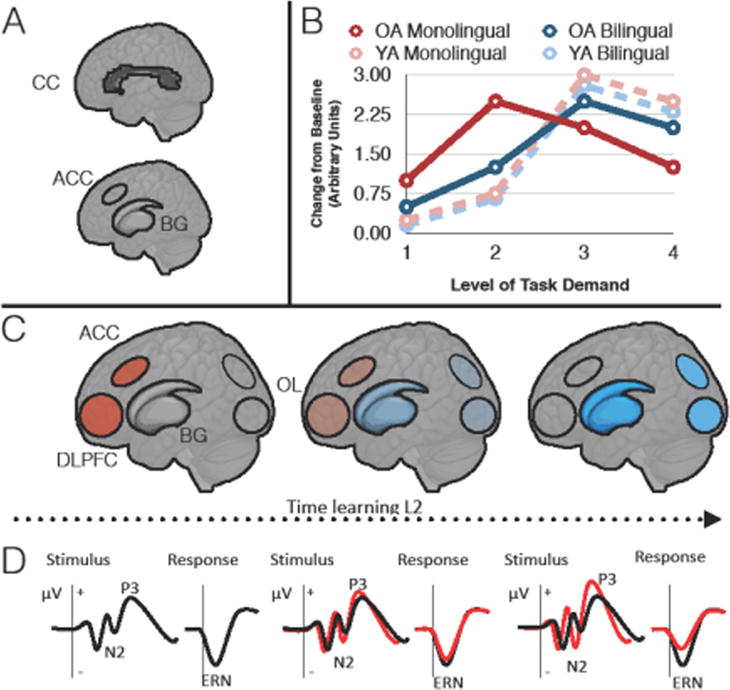
The bilingual anterior-to-posterior and subcortical shift (BAPSS). (A) Regions showing expansion of gray and white matter with L2 acquisition. (B) Hypothetical functional recruitment of frontal regions in response to task demand by language group and age. (C) Shift of functional recruitment from frontal to posterior and subcortical regions with L2 learning. Early frontal recruitment (red) gives way to posterior and subcortical regions at later stages of L2 acquisition (blue). (D) Hypothetical shift from controlled (late) to automatic (early) processes with L2 learning. This shift is indicated by the red lines. CC, corpus callosum; ACC, anterior cingulate cortex; DLPFC, dorsolateral prefrontal cortex; BG, basal ganglia; OL, occipital lobe; OA, older adult; YA, younger adult; ERN, error-related negativity.
The earliest researchers to make the bilingual neural efficiency claim were Abutalebi et al.32 in a study using the flanker task. The authors used a combination of structural (VBM) and functional (fMRI) methods to draw two conclusions. First, bilinguals had larger ACC volumes than monolinguals. Second, bilinguals recruited the ACC less than monolinguals, with better levels of behavioral performance. Their conclusion was that bilinguals relied on ACC tissue less than monolinguals for executive control. Rodriguez-Pujadas et al.57 replicated this reduction of ACC activation by bilinguals relative to monolinguals in a sample of Spanish–Catalan bilinguals and Spanish monolinguals while participants completed a stop-signal paradigm. Importantly, behavior was titrated so that all individuals had a 50% probability of stopping.
This pattern of efficiency is also observable in “late” bilinguals who acquired a second language between 9 and 17 years of age.58 Waldie et al.58 described a pattern consistent with BAPSS in English monolinguals and Macedonian–English bilinguals using a modified Stroop task. For the feature versus response conflict contrast on incongruent trials, bilinguals activated the left pons, left thalamus and left parahippocampus more than monolinguals. The first two of these regions are part of the striate nucleus (i.e., basal ganglia), which, as we shall see, is believed to gate information to the frontal lobes. The parahippocampal cortex is involved in spatial and object mapping, indicating a possible increased role for keeping the response mapping separate for bilinguals relative to monolinguals. For this contrast, monolinguals activated a host of frontal and temporal regions, including the right superior frontal, right middle frontal, right inferior frontal, left fusiform, left cingulate, and left lingual gyrus. This finding fits well with the proposed model of efficiency by bilingual brains and anticipates the role of the striatum, discussed next.
Some authors have argued that the shift from executive regions is paralleled by increased reliance on regions from the language control network.10,59,60 We see evidence of greater striatal involvement—and remodeling—in bilinguals relative to monolinguals, and there is now good evidence that the striate nuclei may be gating access to frontal structures.61,62 From this perspective, bilinguals do not need to draw upon frontal regions to deal with conflict, as this has largely been resolved earlier in the processing hierarchy. Stocco and Prat61 first described this finding empirically using two ROIs—one in the left striatum and one in the left frontal cortex. They showed that bilinguals increased striatal activity to manage conflict on a rapid-instructed task-learning paradigm relative to monolinguals. Rodriguez-Pujadas et al.63 reported similar findings using an “embedded critical trial design” with matched behavioral performance in a group of Spanish–Catalan bilinguals and Spanish monolinguals. Bilinguals recruited the left caudate and left inferior frontal gyrus (IFG) more than monolinguals to complete the paradigm. We note that this study only partially fits the BAPSS model because the relative increase of left inferior frontal gyrus by the bilinguals is anomalous. However, it does not necessarily follow that greater L2 proficiency within the bilingual group predicts greater recruitment of left IFG. Interestingly bilinguals also recruited the left caudate more than monolinguals. This greater caudate recruitment by bilinguals fits with the Stocco et al.61,62 proposal that increased processing by the striate nuclei is potentially the mechanism by which bilinguals gate access to the frontal lobes.
A shift between anterior regions and posterior and subcortical regions is also characteristic of changes with cognitive aging, although the direction is reversed (Fig. 1). Older adults typically show a shift from posterior to anterior processing relative to younger adults on simple tasks.64,65 For more difficult tasks, however, young adults also recruit additional frontal resources. Older adults are generally unable to perform these difficult tasks, possibly because there are no more frontal resources to call upon.66 If bilingualism leads to enhancements of posterior/subcortical regions, then the frontal regions in older adult bilinguals remain available for difficult cognitive tasks. These changes to neural recruitment may act to combat typical patterns of neural decline for bilinguals.
Two studies highlight these patterns. The first study67 matched participants behaviorally on intelligence, socioeconomic status, and cognitive ability. The authors then compared younger and older adult monolinguals and bilinguals on a nonlinguistic color–shape switching task administered in three blocks. For the critical switch trials, young adult performance was equivalent between language groups, and both groups were faster and more accurate than older adults. In the older adult sample, however, monolinguals showed significantly larger switch costs than bilinguals. ROI analyses of fMRI data revealed that monolingual older adults significantly overactivated the left DLPFC, left ventrolateral prefrontal cortex (VLPFC), and ACC compared with bilingual older adults and both sets of younger adults. The authors interpreted this finding in terms of greater efficiency by the older bilinguals.
A recent study provided a conceptual replication of these results using a Simon task.68 The participants were older adult French–English bilinguals and French monolinguals. Performance was equivalent between the groups, however, paralleling the results described by Gold et al.:67 only monolinguals showed the classic posterior-to-anterior shift with aging (PASA)64,65 pattern of activity in response to task demands. The BAPSS efficiency pattern thus maintains itself into older age.
Studies of linguistic processing also reveal findings in line with the BAPSS model in which frontal resources are drawn upon for L2 processing. Over time, this pattern of daily use leads to greater efficiency that is manifested by bilinguals on nonlinguistic tasks when compared with monolinguals. Managing two languages is taxing and draws upon broad language and executive control networks. Reverberi et al.69 contrasted intention to speak and the execution of speech in a group of young adult German–English bilinguals. Participants were cued to either prepare to respond in English or German or to subvocalize a response in English or German. During the subvocalization phase, the language network was recruited more when participants prepared to shift from one language to the other (regardless of the language). Notably, speaking (executing) the non-native language (English) resulted in greater ACC and caudate nucleus activation than speaking German. The authors argue that this finding underscores the demanding nature of managing two languages. Complementary findings were produced by another study70 that found that bilinguals recruited the right insula, ACC, and DLPFC to manage less proficient languages.
There is an exception to the BAPSS pattern from studies of children. In general, children tend to over-recruit those same regions that are later engaged more efficiently by bilingual adults. For example, Mohades et al.71 tested bilingual and monolingual children using the Simon and Stroop tasks and found that bilingual children recruited the bilateral cingulate cortex to a greater extent than monolinguals on conflict trials. Converging findings from two other studies72,73 show that bilingual children over-recruit frontal resources when completing theory-of-mind tasks and reading (though the latter is confounded by the linguistic nature of the task). Although the evidence from functional studies with children is limited, this over-recruitment may be a by-product of bilingual children attempting to master two languages at this stage of development. Evidence supporting this view comes from parallel studies of language processing where less proficient bilinguals devote more neural real estate to managing two languages than more proficient bilinguals.74,75 Thus, in the initial stages, learning to keep two languages in mind results in neural redundancy and over-recruitment. This pattern of over-recruitment ebbs once the brain recognizes the commonalities between the languages.
Modulation of functional connectivity in bilinguals and monolinguals
In the previous section, we argued that bilinguals have more efficient neural activity than monolinguals, particularly in frontal regions. In this section, we review evidence from functional connectivity studies. In general, bilinguals appear better able to modulate functional connectivity than monolinguals; specifically, during task-evoked brain activity, bilinguals show stronger connectivity with salience and frontoparietal network regions. These regions are involved in error detection, attention, shifting, and staying on task. These regions also substantially overlap with the language control networks. During rest, by contrast, bilinguals tend to show less connectivity with frontal task regions than monolinguals.
Luk et al.76 provided some of the earliest functional connectivity analyses of bilinguals and monolinguals. They used behavioral partial least squares (PLS) to examine networks of brain regions that covaried with RT during a flanker task. Importantly, behavioral performance did not differ between groups, but the relationship between performance and brain patterns was different for monolinguals and bilinguals. In the congruent–neutral contrast (facilitation), monolinguals and bilinguals drew upon a network including the right caudate nucleus, left superior frontal gyrus, and occipital regions. This network facilitated performance in both groups. In contrast, in the more difficult incongruent–neutral contrast (interference), bilinguals recruited a network including the bilateral thalami, ACC, and temporal and occipital regions, but monolinguals continued to use the same network from the easier condition. Thus, bilinguals are more able than monolinguals to adapt network connections in response to task demands.
Two further studies investigated whether bilinguals and monolinguals differentially modulated task versus rest functional connectivity.77,78 Grady et al.77 used the Luk et al.76 data set to conduct a seed–PLS analysis to extract the salience network (SN), FPN, and default mode (DMN) network. At rest, bilinguals had stronger connections to the FPN and the DMN than monolinguals. However, there was no interaction between task and rest functional connectivity by group, suggesting that bilinguals did not modulate functional connectivity more than monolinguals. A follow-up analysis examined whether FPN coupling across task and fixation conditions would predict task activity. They reported that FPN modulation across task/fixation states only predicted task activity in the bilinguals, with greater modulation of activity associated with greater activation changes.
A similar question was investigated by Li et al.78 using ROIs with bimodal bilinguals and monolinguals who performed a picture-naming task. The authors showed that bilinguals but not monolinguals were able to modulate connectivity with the dorsal ACC across task and rest states. Relative to monolinguals, bilinguals had stronger coupling with the ACC during the task and lower coupling with the ACC during rest. Increased ACC coupling was associated with slower RTs, an unsurprising result given the verbal nature of the task. Linguistic stimuli offer greater challenges for bilinguals than monolinguals, since in the former group both language representations must be managed. This conceivably requires greater ACC management and does not (necessarily) simply reflect better/faster performance. Complementary findings were reported by Costumero et al.,79 who defined the FPN and SN in Spanish monolinguals and Spanish–Catalan bilinguals who were performing a go/no-go task using independent component analysis. Echoing the other results described in this section, bilinguals had stronger functional connectivity during the task in the left FPN and SN than monolinguals. The authors reported that this increased modulation of the SN and FPN networks by bilinguals predicted better accuracy and faster RTs. No such relationship was found for monolinguals. Finally, Luk et al.40 used seed PLS focused on two regions (bilateral IFG) to examine language-group differences at rest. Monolinguals were found to have stronger resting-state connectivity within frontal lobes, while bilinguals expressed greater frontal-to-posterior connections. This last finding is intriguing and suggests that, even at rest, the functional organization of the bilingual brain differs in notable ways from that of monolinguals. Future studies comparing rest to task organization within subjects should be conducted to discover whether this innate difference is related to the pattern of greater bilingual modulation we described earlier.
Earlier and more automatic processes in bilinguals
So far, we have shown that L2 experience leads to enhanced gray matter, WM, and functional connectivity, as well as a shift from reliance on anterior to subcortical and posterior brain regions. These changes might be related to a shift from more effortful, controlled processing to more automatic processing of stimuli, but fMRI alone cannot confirm this interpretation, owing to poor temporal resolution. Event-related potentials (ERPs) allow for the investigation of temporally rich neural processing at the level of millisecond resolution. The amplitude and latency of each ERP component provides information regarding the strength and timing of various cognitive processes and the interplay between automatic and controlled processing.80
Two of the most commonly reported electrophysiological markers for language processing are the N400 and the P600.81 The N400 is an index of several aspects of language processing and is especially sensitive to semantic integration of objects and representations.82 Bilinguals must work harder than monolinguals to integrate this information because they must consider information from two languages. The N400 is sensitive to activation from the non-target language during linguistic processing and is thus modified by the linguistic competition that bilinguals continually manage.83,84 The N400 is modulated by attentional control rather than being automatically produced in response to bottom-up linguistic input.85 N400 responses are often followed by another top-down, late positive component known as the P600, which appears approximately 600 ms after stimulus onset and is sensitive to syntactic violations.81 Critically, electrophysiological modulations at the N400 and the P600 in response to syntactic violations and grammatical processes are qualitatively different for monolinguals and bilinguals.86–88 Furthermore, language-switching studies show greater activation for language switch than non-switch trials at a related component known as the late positive component (LPC).89
Top-down executive control is thus heavily involved in language processing. Continual use of higher-order executive control centers has been shown to enhance early visual,90 auditory,91 and other sensory modality processes through feedback loops. Uncertainty and conflict can trigger a more in-depth analysis of visual features of stimuli. For example, the temporal parietal junction, an area responsible for early visual feature extraction,92 may receive feedback from higher-order control centers, such as the ACC, to enhance visual feature processing during conditions of uncertainty.93,94 Similarly, the conflict monitoring theory95–97 proposes that the ACC detects conflict, which in turn biases early perceptual processing toward task-relevant features and away from distractors. It is likely that lifelong use of higher-order cognitive processes leads to more efficient resource allocation and enhances more automatic, early processes to prepare the system for potential conflict. If L2 learning leads to domain-general cognitive changes, a prediction is that bilinguals will show enhanced automatic attentional allocation compared with monolinguals on nonverbal tasks. As we will see, this is precisely what the evidence shows. Although there are some exceptions, the overall picture is consistent: across a range of nonverbal cognitive tasks, L2 experience leads to larger and earlier ERPs at stimulus-locked components, such as the N2 and the P3, and reduced-amplitude ERPs in later windows, such as the stimulus-locked N450 and the response-locked error-related negativity (ERN).98,99
Evidence from N2
The N2 component is a frontocentral negative deflection that occurs approximately 200–300 ms after stimulus onset and is believed to be sensitive to automatic conflict detection.96 Language-switching studies have shown that N2 amplitudes are also linked to language-switching costs. For example, Jackson et al.89 found greater N2 amplitudes when switching between languages during a digit-naming task than when continuing with the same language. Similarly, Shao et al.100 showed that N2 effects depended on the degree of linguistic conflict present during picture naming. They recorded ERPs while participants named pictures with either high or low word agreement indicating the extent to which people agree on the name of the picture. The authors found larger N2 amplitudes in the low than in the high name-agreement pictures. The low word-agreement condition where there are alternative names parallels the bilingual extensive experience in selecting one word from among two competing languages. Given that N2 amplitudes increase when there is a high probability of encountering conflict,101 one might expect to find larger N2 amplitudes for bilinguals than monolinguals.
Several studies have demonstrated this effect, in which bilinguals have larger amplitude and shorter latency N2 responses during nonverbal cognitive tasks. Larger N2 amplitudes reflect more resources devoted to early conflict processing,102 and shorter latencies reflect faster (more automatic and efficient) processing.103 Fernandez et al.104 examined young adult monolinguals and bilinguals performing an auditory go/no-go task in which participants had to respond by making a button press to two subsequently presented high tones (go trials) and withhold responses to any other combination of high or low tones (no-go trials). There were no behavioral differences, but bilinguals showed larger N2 responses than monolinguals to no-go trials in which a motor response had to be withheld. L2 proficiency moderated this effect, such that higher proficiency was associated with larger N2 responses for no-go trials. Fernandez and colleagues105 further examined the modality specificity of these findings. Using a similar go/no-go paradigm but in both auditory and visual modalities, the authors found that bilinguals showed larger N2 responses than monolinguals in the auditory but not visual modality. This difference was once again moderated by L2 proficiency, with larger N2 responses on no-go trials for higher-proficiency bilinguals.
Other studies have also converged on the idea that bilinguals show enhanced N2 responses compared with monolinguals, even in the visual modality. Moreno et al.106 had young adults perform a visual go/no-go task in which participants were required to make a key press in response to white shapes (75% probability) and withhold responses to purple shapes (25% probability). Bilinguals showed larger N2 responses than two groups of monolinguals (i.e., musicians and non-musician controls) for no-go trials, despite no behavioral differences. The authors concluded that bilinguals were better at either detecting response competition or allocating resources to resolve conflict.
Sullivan et al.107 also used a go/no-go task with EEG to track the effect of early L2 experience. They tested English-speaking monolinguals enrolled in first-year Spanish or first-year psychology courses. Behavioral and EEG data were acquired at two time points—at the beginning of the term and after 6 months of instruction. Results revealed that, although the magnitude of the amplitude did not change, L2 learning led to earlier N2 latencies after training. This finding is consistent with more efficient and automatic conflict processing at the N2.
Finally, Barac, Moreno, and Bialystok108 administered a visual go/no-go task to 5-year-old children. Again, no group differences were observed for N2 amplitudes, but latency analyses revealed earlier N2 responses for bilingual than monolingual children, reflecting more efficient processing. In the behavioral results, bilinguals showed faster and more accurate responding than monolinguals. Furthermore, earlier latencies were associated with better performance in bilinguals but not monolinguals. This brain–behavior relationship illustrates how bilinguals take advantage of earlier N2 processing while monolinguals do not.
Another paradigm that has shown robust N2 differences between monolinguals and bilinguals is the AX version of the continuous performance task (AX-CPT).109 Morales et al.110 examined the electrophysiological correlates of proactive and reactive control111 as a function of L2 experience. Monolingual and bilingual young adults were instructed to respond to specific prime and probe combinations: to press “yes” when the letter X was preceded by the letter A and “no” for all other sequences. Target (AX) trials appeared 70% of the time and the other three combinations (AY, BX, BY) each appeared 10% of the time. AY trials consisted of an A prime followed by any letter other than X, BX trials consisted of primes that could be any letter other than A followed by an X letter probe, and BY trials were control trials in which neither the prime or the probe overlapped with target trials. On the most difficult AY trials, in which participants are primed to expect an X target and then withhold a prepotent (incorrect) response, bilinguals were more accurate and showed larger N2 amplitudes than monolinguals.
The AX-CPT task shares important features with the go/no-go task. AX trials are similar to go trials in that they occur most frequently and set up the participant to expect more trials of this sort. AY trials are similar to no-go trials because they occur less frequently, and some form of control is required to overcome the prepotent response. Thus, like the go/no-go task, bilingualism is associated with enhanced N2 on conflict trials in the AX-CPT task. This pattern was also found in a study of two groups of young adult bilinguals in which one group received practice in language switching; both groups performed the AX-CPT at two time intervals.112 ERPs were first recorded to get a measure of baseline performance. Following this, the experimental group received language-switching practice for 10 days, but the control group did not. The results showed larger prime-locked N2 amplitudes in the follow-up EEG recordings for the language-switching group but not for the control group. These results are consistent with the view that N2 amplitudes are enhanced by L2 learning and language-switching practice.
Most of the results described to this point have been consistent in demonstrating the effects of bilingualism on N2, but there is an exception to this pattern. Kousaie and Phillips113 tested monolingual and bilingual young adults on Stroop,114 Simon,115 and flanker116 tasks while EEG was recorded. They found no behavioral group differences on the three tasks but reported larger N2 amplitudes for monolinguals than bilinguals on the Stroop task. However, there are some important caveats regarding these findings. First, only the Stroop task showed larger N2 amplitudes for monolinguals, with no N2 group differences seen on the flanker or the Simon task. More importantly, the Stroop task is the only task that has a verbal component (word reading). Because bilinguals divide their time between two languages, they have less experience in each language than monolinguals.117–119 As a consequence, verbal tasks often disadvantage bilinguals, and resources typically devoted to earlier processing in bilinguals might be needed elsewhere for additional linguistic processing.
In sum, the overall pattern of findings is consistent with the view that bilinguals devote more early resources to the N2 than monolinguals while performing nonverbal cognitive tasks. Some have claimed that this reflects better inhibitory control by bilinguals,105 but this view has been challenged by others120 given that the N2 amplitude has been shown to become smaller with development.121 We agree with Paap et al.120 that it is best to not label this as better inhibitory control, but neither should it be labeled as worse control, as the authors argue, given that it becomes smaller with development. We instead propose that there are qualitative processing differences in attentional control between language groups when resolving conflict. Importantly, the amplitude of the N2 depends on both proactive and reactive conflict processing, so better control depends on the context. Grützmann et al.101 showed that the amplitude of the N2 gets smaller on immediate repetitions of conflict trials, but that it gets larger over the course of the block if many conflict trials are present, as opposed to few. Thus, bilinguals may be adopting a more proactive processing strategy than monolinguals, anticipating that many conflict trials will be present and allocating more early attentional resources to process these stimuli. This idea is consistent with the fact that bilinguals continually deal with linguistic conflict between two languages, while monolinguals do not. The temporal course of this information processing is especially interesting, and the evidence suggests that bilinguals process stimuli earlier at this conflict-sensitive N2 component. We argue that monolinguals adopt a different strategy in which later, more effortful control processes are used to reach the same behavioral outcome as bilinguals on conflict tasks.
Evidence from P3
Many of the studies that found earlier and larger-amplitude N2 components for bilinguals than monolinguals also showed group effects at the P3 component. The P3 appears around 300–400 ms after stimulus onset and may reflect stimulus categorization.122,123 Larger P3 amplitudes are associated with better working memory performance,124,125 and shorter P3 latencies are associated with faster stimulus-categorization time.122,123 Bilinguals generally show larger-amplitude and shorter-latency P3s than monolinguals on nonverbal tasks. Using a visual go/no-go task, Moreno et al.106 showed that bilinguals had larger P3 amplitudes than monolinguals in the conflict no-go trials but showed no difference in go trials. Sullivan et al.107 used a similar go/no-go paradigm and found that 6 months of enrollment in a Spanish-language university course led to larger P3 amplitudes over time, but no P3 changes in amplitude or latency were observed in a control group of participants enrolled in psychology. Similar findings were observed for children performing the go/no-go task, with larger P3 amplitudes for bilinguals than monolinguals.108 Barac et al.108 further demonstrated that bilingual children showed earlier P3 latencies, indexing faster stimulus-categorization times.
The results reported by Kousaie and Phillips113 were less consistent than other studies, but still showed shorter P3 latencies by bilinguals than monolinguals on two of the three tasks (i.e., flanker and Simon), again demonstrating faster stimulus categorization for bilinguals. In contrast, the Stroop task showed greater P3 amplitudes for monolinguals than bilinguals. Similarly, Coderre et al.126 reported greater P3 amplitudes for monolinguals than bilinguals during a Stroop task. As described for the N2, the Stroop task requires linguistic processing, and this might lead to more distributed neural network recruitment for bilinguals than monolinguals and thus smaller P3 responses. Finally, larger P3 amplitudes for bilinguals than monolinguals on the AY conflict trials were reported during AX-CPT task described earlier.110
Taken together, the P3 findings demonstrate that, when group differences are observed on nonverbal conflict-resolution tasks, they are in the direction of shorter latencies and increased amplitudes for bilinguals. These findings might indicate that bilinguals devote more early neural resources to stimulus categorization than monolinguals on nonverbal cognitive tasks. We use the term “early” to reflect two things. First, earlier latencies for bilinguals suggest earlier stimulus categorization than monolinguals, not that the P3 is an early component. Second, even though P3 amplitudes are larger for bilinguals than monolinguals and the P3 is an attentional component, we provide evidence for the idea that later cognitive processes tend to be larger for monolinguals (e.g., N450 and response-locked ERN—see below). More resource deployment at early stages of processing (e.g., N2) for bilinguals than monolinguals might also facilitate categorization at the P3 in order to lessen the requirements for later cognitive processing. In this sense, the P3 is part of set of components that allow more automatic and efficient processing of stimuli for bilinguals than monolinguals.
N450 and the late, sustained negative-going potentials
The N450 is a late negative-going component that appears approximately 450 ms after stimulus onset and is sensitive to interference control before response selection during the Stroop task.127 The sustained negative-going potential, which appears around 550–700 ms after stimulus onset and follows the N450, is believed to index conflict-resolution and response-selection processes.128,129 Heidlmayr et al.130 had monolingual and bilingual young adults perform a Stroop task that combined elements of negative priming. The authors found ERP Stroop effects (incongruent minus congruent) for monolinguals but not bilinguals at both the N450 and the late sustained potential, despite no behavioral differences. These ERP effects suggest that monolinguals might be devoting more resources at late stages of processing (interference control and response selection) than bilinguals to deal with conflict. In another study, Coderre et al.126 examined the N450 and found that bilinguals had smaller ERP Stroop effects than monolinguals with equal behavioral performance, again demonstrating that monolinguals are devoting more resources at late stages of processing compared with bilinguals.
It is important to note that all of these studies reporting language-group differences on the late components used a Stroop task and therefore required linguistic processing. Thus, it is unclear if monolinguals are devoting more resources at these late stages of processing to perform the task equivalently to bilinguals or if bilinguals are distributing their resources elsewhere for the linguistic demands of the task. Future research will be needed to separate these competing possibilities.
Evidence from error-related negativity
Given that behavioral performance with young adults is often similar across language groups in these studies and that fMRI findings generally show greater activation in frontal regions (e.g., the ACC) for monolinguals than bilinguals, it is possible that monolinguals are deploying more control later in processing. Preliminary evidence from the error-related negativity (ERN) supports this interpretation. The ERN is a response-locked ERP that appears following error trials and is believed to index the need for more control after response execution.131 The response-locked ERN and the stimulus-locked N2 are tightly linked in that both respond to conflict; more control at the ERN is required when control at the N2 is not sufficient.131 Moreover, both the ERN and the N2 have generators in the ACC,132 which is critically involved in distinguishing between monolinguals and bilinguals performing conflict-resolution tasks. Furthermore, the ERN is believed to be involved in both verbal and nonverbal error processing.133 If monolinguals devote fewer resources than bilinguals at the N2 and more resources later, then it follows that monolinguals should show greater ERN amplitudes than bilinguals to signal the need for more control following errors.
Only two studies have examined the ERN in monolingual and bilingual participants, but the evidence is consistent with our speculations. First, Kousaie and Phillips113 reported a larger ERN for monolinguals than bilinguals during the Stroop task, despite equivalent behavioral performance for both RT and accuracy. Second, using the AX-CPT task, Morales et al.110 showed that monolinguals had larger ERN amplitudes than bilinguals across all trial types. They concluded that bilinguals showed more adaptive control mechanisms than monolinguals and that their findings were in line with other research reporting smaller ERNs for more efficient self-monitoring systems.134 In the Morales et al. study, bilinguals had fewer errors than monolinguals; this is important because errors are negatively correlated with the size of the ERN.135 Larger ERN amplitudes reflect more controlled response strategies.136 Thus, in the Morales study, bilingual ERNs are likely enhanced owing to more accurate responding than monolinguals, and, with equivalent behavioral performance, the ERN distinctions between groups would be even larger.
Additionally, Festman and Münte134 conducted a study in which they divided groups into switcher and non-switcher bilinguals according to their ability to stay in the intended language (non-switchers) or switch to the unintended language (switchers) during picture naming. Following this classification, the two groups performed a flanker task while EEG was recorded. Accuracy was equivalent between groups, but non-switchers were faster on incongruent trials and showed smaller ERN amplitudes than switchers across all trials. This suggests that better language control results in smaller ERN amplitudes on nonverbal executive function tasks.d Given that bilinguals have a lifetime of experience managing two languages and monolinguals do not, one would expect ERN differences to emerge between the groups.
In summation, the limited amount of evidence suggests that L2 experience leads to smaller ERN amplitudes. We suggest that this may be the result of a lifetime of committing errors during language learning and consequently opting to rely on earlier automatic processes that make the system more efficient. Future studies should examine this possibility.
Conclusions
The literature reviewed above indicates that bilingualism modifies both structural and functional aspects of the brain and that these changes contribute to domain-general cognition. When first learning a new language, bilinguals devote more frontal resources to help them deal with competition between the two languages. Over time, drawing upon these resources becomes more efficient by enlarging important gray matter and WM structures and facilitating communication between anterior (cognitive) and subcortical/posterior (motor/sensory/perceptual) regions. However, with increasing experience and specialization, some structures are optimally remodeled. This remodeling sometimes manifests in the form of selective volume reductions. Compared with monolinguals, over time bilinguals devote fewer resources to anterior regions and more resources to subcortical/posterior regions (BAPSS), corresponding to a shift from more demanding, late, top-down processing, to more automatic processing of stimuli during nonverbal executive control tasks.
The BAPSS framework may help explain why bilinguals show delayed cognitive decline associated with aging compared with monolinguals.137 There is a well-documented PASA64,65 in functional activity. Older adults recruit more frontal regions to complete cognitive tasks at the same level of performance as younger adults. If bilinguals rely less on frontal regions, as the evidence suggests, and more on subcortical/posterior regions than monolinguals, this efficiency would stave off cognitive decline associated with older age. The BAPSS framework is the first attempt to unite the EEG, structural MRI, fMRI, and aging literatures to account for the effects of bilingualism on cognition across the life span. It is now well accepted that experience has the capacity to lead to neuroplastic changes in brain structure and function. The research summarized in this review suggests that bilingualism is one such experience.
Acknowledgments
Preparation of this paper was funded by Grants R01HD052523 and R21AG048431 from the U.S. National Institutes of Health and Grant A2559 from the Natural Sciences and Engineering Research Council of Canada to E.B.
Footnotes
Competing interests
The authors declare no competing interests.
There are three well-recognized motor circuits involved in speech. The first is the motor circuit, which, together with the supplementary motor area, initiates speech motor programs. The second, the prefrontal circuit, likely involves a working memory component for speech acting to help buffer and order incoming sounds. Third is the cingulate circuit, which governs the motivation to speak and involves projections from the thalamus to premotor/ACC regions.
Martenssson et al. defined “struggle” as the instructor’s assessment on a 9-point Likert scale of “the amount of effort needed to stay at the academy.” Proficiency was assessed using the participant’s grades on a mid-year oral and written exam. What is unclear is whether participants who struggled more eventually gained equal levels of proficiency to those who did not. The authors note that no one failed or dropped out of the program, suggesting that all participants met a basic level of proficiency.
Notice the similarity between the conceptualization of “switchers” and “non-switchers” with Green’s and Abutalebi’s44 concepts of “dense code-switching” and “dual-language” bilinguals from the adaptive control hypothesis. They posit that different control mechanisms are required for different language contexts and that more control processes are generally required for dual-language learners than other interactional contexts. Green’s and Abutalebi’s theory highlights the importance of defining the language context in which bilinguals are immersed when comparing neuroimaging results between monolinguals and bilinguals. Null results at various ERP components may be the product of comparing monolinguals to dense code–switching bilinguals rather than to dual language–context bilinguals.
References
- 1.Kroll JF, Dussias PE, Bogulski CA, Kroff JRV. Psychology of Learning and Motivation. Vol. 56. Elsevier; 2012. Juggling Two Languages in One Mind; pp. 229–262. 2012. [Google Scholar]
- 2.Kroll JF, Bobb SC, Hoshino N. Two Languages in Mind: Bilingualism as a Tool to Investigate Language, Cognition, and the Brain. Curr Dir Psychol Sci. 2014;23(3):159–163. doi: 10.1177/0963721414528511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bialystok E, Craik FIM, Luk G. Bilingualism: consequences for mind and brain. Trends Cogn Sci. 2012;16(4):240–250. doi: 10.1016/j.tics.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Costa A, Sebastián-Gallés N. How does the bilingual experience sculpt the brain? Nat Rev Neurosci. 2014;15(5):336–345. doi: 10.1038/nrn3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bialystok E, Craik FIM, Freedman M. Bilingualism as a protection against the onset of symptoms of dementia. Neuropsychologia. 2007;45(2):459–464. doi: 10.1016/j.neuropsychologia.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 6.Gold BT. Lifelong bilingualism and neural reserve against Alzheimer’s disease: A review of findings and potential mechanisms. Behav Brain Res. 2015;281:9–15. doi: 10.1016/j.bbr.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guzmán-Vélez E, Tranel D. Does bilingualism contribute to cognitive reserve? Cognitive and neural perspectives. Neuropsychology. 2015;29(1):139–150. doi: 10.1037/neu0000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schweizer TA, Ware J, Fischer CE, Craik FIM, Bialystok E. Bilingualism as a contributor to cognitive reserve: Evidence from brain atrophy in Alzheimer’s disease. Cortex. 2012;48(8):991–996. doi: 10.1016/j.cortex.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 9.Abutalebi J, Green DW. Neuroimaging of language control in bilinguals: neural adaptation and reserve. Biling Lang Cogn. 2016;19(4):689–698. doi: 10.1017/S1366728916000225. [DOI] [Google Scholar]
- 10.Pliatsikas C, Luk G. Executive control in bilinguals: A concise review on fMRI studies. Biling Lang Cogn. 2016;19(4):699–705. doi: 10.1017/S1366728916000249. [DOI] [Google Scholar]
- 11.García-Pentón L, García YF, Costello B, Duñabeitia JA, Carreiras M. The neuroanatomy of bilingualism: how to turn a hazy view into the full picture. Lang Cogn Neurosci. 2016;31(3):303–327. doi: 10.1080/23273798.2015.1068944. [DOI] [Google Scholar]
- 12.Olsen RK, Pangelinan MM, Bogulski C, et al. The effect of lifelong bilingualism on regional grey and white matter volume. Brain Res. 2015;1612:128–139. doi: 10.1016/j.brainres.2015.02.034. [DOI] [PubMed] [Google Scholar]
- 13.Abutalebi J, Guidi L, Borsa V, et al. Bilingualism provides a neural reserve for aging populations. Neuropsychologia. 2015;69:201–210. doi: 10.1016/j.neuropsychologia.2015.01.040. [DOI] [PubMed] [Google Scholar]
- 14.Abutalebi J, Canini M, Della Rosa PA, Green DW, Weekes BS. The neuroprotective effects of bilingualism upon the inferior parietal lobule: A structural neuroimaging study in aging Chinese bilinguals. J Neurolinguistics. 2015;33:3–13. doi: 10.1016/j.jneuroling.2014.09.008. [DOI] [Google Scholar]
- 15.Wei M, Joshi AA, Zhang M, et al. How age of acquisition influences brain architecture in bilinguals. J Neurolinguistics. 2015;36:35–55. doi: 10.1016/j.jneuroling.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990;13(7):266–271. doi: 10.1016/0166-2236(90)90107-l. doi: http://dx.doi.org/10.1016/0166-2236(90)90107-L. [DOI] [PubMed] [Google Scholar]
- 17.Alexander GE, DeLong MR, Strick PL. Parallel Organization of Functionally Segregated Circuits Linking Basal Ganglia and Cortex. Annu Rev Neurosci. 1986;9(1):357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- 18.Middleton FA, Strick PL. Basal Ganglia Output and Cognition: Evidence from Anatomical, Behavioral, and Clinical Studies. Brain Cogn. 2000;42(2):183–200. doi: 10.1006/brcg.1999.1099. [DOI] [PubMed] [Google Scholar]
- 19.Mink JW. The basal ganglia: Focused selection and inhibition of competing motor programs. Prog Neurobiol. 1996;50(4)(96):381–425. 00042–1. doi: 10.1016/S0301-0082. [DOI] [PubMed] [Google Scholar]
- 20.Frank MJ. Hold your horses: A dynamic computational role for the subthalamic nucleus in decision making. Neural Netw. 2006;19(8):1120–1136. doi: 10.1016/j.neunet.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 21.Haber SN. Corticostriatal circuitry. Dialogues Clin Neurosci. 2016;18(1):7. doi: 10.31887/DCNS.2016.18.1/shaber. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abutalebi J, Rosa PAD, Castro Gonzaga AK, Keim R, Costa A, Perani D. The role of the left putamen in multilingual language production. Brain Lang. 2013;125(3):307–315. doi: 10.1016/j.bandl.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 23.Pliatsikas C, Johnstone T, Marinis T. Grey matter volume in the cerebellum is related to the processing of grammatical rules in a second language: A structural voxel-based morphometry study. Cerebellum. 2014;13(1):55–63. doi: 10.1007/s12311-013-0515-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burgaleta M, Sanjuán A, Ventura-Campos N, Sebastian-Galles N, Ávila C. Bilingualism at the core of the brain. Structural differences between bilinguals and monolinguals revealed by subcortical shape analysis. NeuroImage. 2016;125:437–445. doi: 10.1016/j.neuroimage.2015.09.073. [DOI] [PubMed] [Google Scholar]
- 25.Pliatsikas C, DeLuca V, Moschopoulou E, Saddy JD. Immersive bilingualism reshapes the core of the brain. 2016 doi: 10.1007/s00429-016-1307-9. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grillner S, Robertson B. The Basal Ganglia Over 500 Million Years. Curr Biol. 2016;26(20):R1088–R1100. doi: 10.1016/j.cub.2016.06.041. [DOI] [PubMed] [Google Scholar]
- 27.Mårtensson J, Eriksson J, Bodammer NC, et al. Growth of language-related brain areas after foreign language learning. NeuroImage. 2012;63(1):240–244. doi: 10.1016/j.neuroimage.2012.06.043. [DOI] [PubMed] [Google Scholar]
- 28.Stein M, Federspiel A, Koenig T, et al. Structural plasticity in the language system related to increased second language proficiency. Cortex. 2012;48(4):458–465. doi: 10.1016/j.cortex.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 29.Zhang K, Sejnowski TJ. A universal scaling law between gray matter and white matter of cerebral cortex. Proc Natl Acad Sci. 2000;97(10):5621–5626. doi: 10.1073/pnas.090504197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hilchey MD, Klein RM. Are there bilingual advantages on nonlinguistic interference tasks? Implications for the plasticity of executive control processes. Psychon Bull Rev. 2011;18(4):625–658. doi: 10.3758/s13423-011-0116-7. [DOI] [PubMed] [Google Scholar]
- 31.Voelker P, Piscopo D, Weible AP, et al. How changes in white matter might underlie improved reaction time due to practice. Cogn Neurosci. 2016 Apr;2016:1–7. doi: 10.1080/17588928.2016.1173664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abutalebi J, Della Rosa PA, Green DW, et al. Bilingualism tunes the anterior cingulate cortex for conflict monitoring. Cereb Cortex. 2012;22(9):2076–2086. doi: 10.1093/cercor/bhr287. [DOI] [PubMed] [Google Scholar]
- 33.Cahn BR, Polich J. Meditation states and traits: EEG, ERP, and neuroimaging studies. Psychol Bull. 2006;132(2):180. doi: 10.1037/0033-2909.132.2.180. [DOI] [PubMed] [Google Scholar]
- 34.Kielar A, Meltzer JA, Moreno S, Alain C, Bialystok E. Oscillatory Responses to Semantic and Syntactic Violations. J Cogn Neurosci. 2014;26(12):2840–2862. doi: 10.1162/jocn_a_00670. [DOI] [PubMed] [Google Scholar]
- 35.Sauseng P, Klimesch W, Schabus M, Doppelmayr M. Fronto-parietal EEG coherence in theta and upper alpha reflect central executive functions of working memory. Int J Psychophysiol. 2005;57(2):97–103. doi: 10.1016/j.ijpsycho.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 36.Coggins PE, III, Kennedy TJ, Armstrong TA. Bilingual corpus callosum variability. Brain Lang. 2004;89(1):69–75. doi: 10.1016/S0093-934X(03)00299-2. [DOI] [PubMed] [Google Scholar]
- 37.Aboitiz F, Scheibel AB, Fisher RS, Zaidel E. Fiber composition of the human corpus callosum. Brain Res. 1992;598:1–2. 143–153. doi: 10.1016/0006-8993(92)90178-C. [DOI] [PubMed] [Google Scholar]
- 38.Tomasch J. Size, distribution, and number of fibres in the human Corpus Callosum. Anat Rec. 1954;119(1):119–135. doi: 10.1002/ar.1091190109. [DOI] [PubMed] [Google Scholar]
- 39.Felton A, Vazquez D, Ramos-Nunez AI, et al. Bilingualism influences structural indices of interhemispheric organization. J Neurolinguistics. 2017;42:1–11. doi: 10.1016/j.jneuroling.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luk G, Bialystok E, Craik FIM, Grady CL. Lifelong bilingualism maintains white matter integrity in older adults. J Neurosci. 2011;31(46):16808–16813. doi: 10.1523/JNEUROSCI.4563-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pliatsikas C, Moschopoulou E, Saddy JD. The effects of bilingualism on the white matter structure of the brain. Proc Natl Acad Sci. 2015;112(5):1334–1337. doi: 10.1073/pnas.1414183112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gold BT, Johnson NF, Powell DK. Lifelong bilingualism contributes to cognitive reserve against white matter integrity declines in aging. Neuropsychologia. 2013;51(13):2841–2846. doi: 10.1016/j.neuropsychologia.2013.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Douaud G, Jbabdi S, Behrens TEJ, et al. DTI measures in crossing-fibre areas: Increased diffusion anisotropy reveals early white matter alteration in MCI and mild Alzheimer’s disease. NeuroImage. 2011;55(3):880–890. doi: 10.1016/j.neuroimage.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Green DW, Abutalebi J. Language control in bilinguals: The adaptive control hypothesis. J Cogn Psychol. 2013;25(5):515–530. doi: 10.1080/20445911.2013.796377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kuhl PK, Stevenson J, Corrigan NM, van den Bosch JJF, Can DD, Richards T. Neuroimaging of the bilingual brain: Structural brain correlates of listening and speaking in a second language. Brain Lang. 2016;162:1–9. doi: 10.1016/j.bandl.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 46.Mohades SG, Struys E, Van Schuerbeek P, Mondt K, Van De Craen P, Luypaert R. DTI reveals structural differences in white matter tracts between bilingual and monolingual children. Brain Res. 2012;1435:72–80. doi: 10.1016/j.brainres.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 47.Cummine J, Boliek CA. Understanding white matter integrity stability for bilinguals on language status and reading performance. Brain Struct Funct. 2013;218(2):595–601. doi: 10.1007/s00429-012-0466-6. [DOI] [PubMed] [Google Scholar]
- 48.Nichols ES, Joanisse MF. Functional activity and white matter microstructure reveal the independent effects of age of acquisition and proficiency on second-language learning. NeuroImage. 2016;143:15–25. doi: 10.1016/j.neuroimage.2016.08.053. [DOI] [PubMed] [Google Scholar]
- 49.Golestani N, Molko N, Dehaene S, LeBihan D, Pallier C. Brain Structure Predicts the Learning of Foreign Speech Sounds. Cereb Cortex. 2007;17(3):575–582. doi: 10.1093/cercor/bhk001. [DOI] [PubMed] [Google Scholar]
- 50.Qi Z, Han M, Garel K, San Chen E, Gabrieli JDE. White-matter structure in the right hemisphere predicts Mandarin Chinese learning success. J Neurolinguistics. 2015;33:14–28. doi: 10.1016/j.jneuroling.2014.08.004. [DOI] [Google Scholar]
- 51.Schlegel AA, Rudelson JJ, Peter UT. White matter structure changes as adults learn a second language. J Cogn Neurosci. 2012;24(8):1664–1670. doi: 10.1162/jocn_a_00240. [DOI] [PubMed] [Google Scholar]
- 52.Mohades SG, Van Schuerbeek P, Rosseel Y, Van De Craen P, Luypaert R, Baeken C. White-matter development is different in bilingual and monolingual children: a longitudinal DTI study. PloS One. 2015;10(2):e0117968. doi: 10.1371/journal.pone.0117968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.García-Pentón L, Pérez Fernández A, Iturria-Medina Y, Gillon-Dowens M, Carreiras M. Anatomical connectivity changes in the bilingual brain. NeuroImage. 2014;84:495–504. doi: 10.1016/j.neuroimage.2013.08.064. [DOI] [PubMed] [Google Scholar]
- 54.Bakhtiari R, Boliek C, Cummine J. Investigating the contribution of ventral-lexical and dorsal-sublexical pathways during reading in bilinguals. Front Hum Neurosci. 2014;8 doi: 10.3389/fnhum.2014.00507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.He Y, Dagher A, Chen Z, et al. Impaired small-world efficiency in structural cortical networks in multiple sclerosis associated with white matter lesion load. Brain. 2009;132(12):3366–3379. doi: 10.1093/brain/awp089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Duong M-VA, Audoin B, Boulanouar K, et al. Altered functional connectivity related to white matter changes inside the working memory network at the very early stage of MS. J Cereb Blood Flow Metab. 2005;25(10):1245–1253. doi: 10.1038/sj.jcbfm.9600122. [DOI] [PubMed] [Google Scholar]
- 57.Rodríguez-Pujadas A, Sanjuán A, Fuentes P, Ventura-Campos N, Barrós-Loscertales A, Ávila C. Differential neural control in early bilinguals and monolinguals during response inhibition. Brain Lang. 2014;132:43–51. doi: 10.1016/j.bandl.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 58.Waldie KE, Badzakova-Trajkov G, Miliivojevic B, Kirk IJ. Neural activity during Stroop colour-word task performance in late proficient bilinguals: A functional Magnetic Resonance Imaging study. Psychol Neurosci. 2009;2(2):125–136. doi: 10.3922/j.psns.2009.2.004. [DOI] [Google Scholar]
- 59.Coderre EL, Smith JF, Van Heuven WJB, Horwitz B. The functional overlap of executive control and language processing in bilinguals*. Bilingualism. 2016;2016 doi: 10.1017/S1366728915000188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marian V, Chabal S, Bartolotti J, Bradley K, Hernandez AE. Differential recruitment of executive control regions during phonological competition in monolinguals and bilinguals. Brain Lang. 2014;139:108–117. doi: 10.1016/j.bandl.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stocco A, Prat CS. Bilingualism trains specific brain circuits involved in flexible rule selection and application. Brain Lang. 2014;137:50–61. doi: 10.1016/j.bandl.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 62.Stocco A, Yamasaki B, Natalenko R, Prat CS. Bilingual brain training: A neurobiological framework of how bilingual experience improves executive function. Int J Biling. 2014;18(1):67–92. [Google Scholar]
- 63.Rodríguez-Pujadas A, Sanjuán A, Ventura-Campos N, et al. Bilinguals Use Language-Control Brain Areas More Than Monolinguals to Perform Non-Linguistic Switching Tasks. PLoS One. 2013;8(9) doi: 10.1371/journal.pone.0073028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Grady CL, Maisog JM, Horwitz B, et al. Age-related changes in cortical blood flow activation during visual processing of faces and location. J Neurosci. 1994;14(3):1450–1462. doi: 10.1523/JNEUROSCI.14-03-01450.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Davis SW, Dennis NA, Daselaar SM, Fleck MS, Cabeza R. Qué PASA? The Posterior–Anterior Shift in Aging. Cereb Cortex. 2008;18(5):1201–1209. doi: 10.1093/cercor/bhm155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Reuter-Lorenz PA, Cappell KA. Neurocognitive Aging and the Compensation Hypothesis. Curr Dir Psychol Sci. 2008;17(3):177–182. doi: 10.1111/j.1467-8721.2008.00570.x. [DOI] [Google Scholar]
- 67.Gold BT, Kim C, Johnson NF, Kryscio RJ, Smith CD. Lifelong Bilingualism Maintains Neural Efficiency for Cognitive Control in Aging. J Neurosci. 2013;33(2):387–396. doi: 10.1523/JNEUROSCI.3837-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ines Ansaldo Ana, Ladan Ghazi-Saidi, Daniel Adrover-Roig. Interference control in elderly bilinguals: Appearances can be misleading. J Clin Exp Neuropsychol. 2015;37(5):455–470. doi: 10.1080/13803395.2014.990359. [DOI] [PubMed] [Google Scholar]
- 69.Reverberi C, Kuhlen A, Abutalebi J, et al. Language control in bilinguals: Intention to speak vs. execution of speech. Brain Lang. 2015;144:1–9. doi: 10.1016/j.bandl.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 70.Hernandez AE, Meschyan G. Executive function is necessary to enhance lexical processing in a less proficient L2: Evidence from fMRI during picture naming. Bilingualism. 2006;9(2):177–188. doi: 10.1017/S1366728906002525. [DOI] [Google Scholar]
- 71.Mohades SG, Struys E, Van Schuerbeek P, Baeken C, Van De Craen P, Luypaert R. Age of second language acquisition affects nonverbal conflict processing in children: An fMRI study. Brain Behav. 2014;4(5):626–642. doi: 10.1002/brb3.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kobayashi C, Glover GH, Temple E. Switching language switches mind: Linguistic effects on developmental neural bases of “Theory of Mind”. Soc Cogn Affect Neurosci. 2008;3(1):62–70. doi: 10.1093/scan/nsm039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jasinska KK, Petitto LA. How age of bilingual exposure can change the neural systems for language in the developing brain: A functional near infrared spectroscopy investigation of syntactic processing in monolingual and bilingual children. Dev Cogn Neurosci. 2013;6:87–101. doi: 10.1016/j.dcn.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Perani D, Abutalebi J. The neural basis of first and second language processing. Curr Opin Neurobiol. 2005;15(2):202–206. doi: 10.1016/j.conb.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 75.Abutalebi J, Della Rosa PA, Ding G, Weekes B, Costa A, Green DW. Language proficiency modulates the engagement of cognitive control areas in multilinguals. Cortex. 2013;49(3):905–911. doi: 10.1016/j.cortex.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 76.Luk G, Anderson JAE, Craik FIM, Grady C, Bialystok E. Distinct neural correlates for two types of inhibition in bilinguals: Response inhibition versus interference suppression. Brain Cogn. 2010;74(3):347–357. doi: 10.1016/j.bandc.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 77.Grady CL, Luk G, Craik FIM, Bialystok E. Brain network activity in monolingual and bilingual older adults. Neuropsychologia. 2015;66:170–181. doi: 10.1016/j.neuropsychologia.2014.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li L, Abutalebi J, Zou L, et al. Bilingualism alters brain functional connectivity between “control” regions and “language” regions: Evidence from bimodal bilinguals. Neuropsychologia. 2015;71:236–247. doi: 10.1016/j.neuropsychologia.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 79.Costumero V, Rodríguez-Pujadas A, Fuentes-Claramonte P, Ávila C. How bilingualism shapes the functional architecture of the brain: A study on executive control in early bilinguals and monolinguals: Effect of Bilingualism on Neural Control. Hum Brain Mapp. 2015;36(12):5101–5112. doi: 10.1002/hbm.22996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Luck SJ, Kappenman ES. The Oxford Handbook of Event-Related Potential Components. Oxford University Press; 2011. 2011. [Google Scholar]
- 81.Kutas M, Moreno E, Wicha N. Code-switching and the brain. In: Bullock BE, Toribio AJ, editors. The Cambridge Handbook of Linguistic Code-Switching. Vol. 2009. Cambridge: Cambridge University Press; 2009. pp. 289–306. http://ebooks.cambridge.org/ref/id/CBO9780511576331A032. Accessed September 29, 2016. [Google Scholar]
- 82.Kutas M, Federmeier KD. Thirty Years and Counting: Finding Meaning in the N400 Component of the Event-Related Brain Potential (ERP) Annu Rev Psychol. 2011;62(1):621–647. doi: 10.1146/annurev.psych.093008.131123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Thierry G, Wu YJ. Brain potentials reveal unconscious translation during foreign-language comprehension. Proc Natl Acad Sci. 2007;104(30):12530–12535. doi: 10.1073/pnas.0609927104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wu YJ, Thierry G. Event-Related Brain Potential Investigation of Preparation for Speech Production in Late Bilinguals. Front Psychol. 2011;2 doi: 10.3389/fpsyg.2011.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.McCarthy G, Nobre AC. Modulation of semantic processing by spatial selective attention. Electroencephalogr Clin Neurophysiol Potentials Sect. 1993;88(3):210–219. doi: 10.1016/0168-5597(93)90005-A. [DOI] [PubMed] [Google Scholar]
- 86.Hahne A, Mueller JL, Clahsen H. Morphological Processing in a Second Language: Behavioral and Event-related Brain Potential Evidence for Storage and Decomposition. J Cogn Neurosci. 2006;18(1):121–134. doi: 10.1162/089892906775250067. [DOI] [PubMed] [Google Scholar]
- 87.Moreno S, Bialystok E, Wodniecka Z, Alain C. Conflict resolution in sentence processing by bilinguals. J Neurolinguistics. 2010;23(6):564–579. doi: 10.1016/j.jneuroling.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mueller JL, Hahne A, Fujii Y, Friederici AD. Native and Nonnative Speakers’ Processing of a Miniature Version of Japanese as Revealed by ERPs. J Cogn Neurosci. 2005;17(8):1229–1244. doi: 10.1162/0898929055002463. [DOI] [PubMed] [Google Scholar]
- 89.Jackson GM, Swainson R, Cunnington R, Jackson SR. ERP correlates of executive control during repeated language switching. Biling Lang Cogn. 2001;4(2):169–178. [Google Scholar]
- 90.Gilbert CD, Li W. Top-down influences on visual processing. Nat Rev Neurosci. 2013;14(5):350–363. doi: 10.1038/nrn3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Poghosyan V, Ioannides AA. Attention Modulates Earliest Responses in the Primary Auditory and Visual Cortices. Neuron. 2008;58(5):802–813. doi: 10.1016/j.neuron.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 92.Lamb MR, Robertson LC, Knight RT. Attention and interference in the processing of global and local information: Effects of unilateral temporal-parietal junction lesions. Neuropsychologia. 1989;27(4):471–483. doi: 10.1016/0028-3932(89)90052-3. [DOI] [PubMed] [Google Scholar]
- 93.Grundy JG, Benarroch MFF, Woodward TS, Metzak PD, Whitman JC, Shedden JM. The bivalency effect in task switching: event-related potentials. Hum Brain Mapp. 2013;34(5):999–1012. doi: 10.1002/hbm.21488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Grundy JG, Shedden JM. Support for a history-dependent predictive model of dACC activity in producing the bivalency effect: An event-related potential study. Neuropsychologia. 2014;57:166–178. doi: 10.1016/j.neuropsychologia.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 95.Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001;108(3):624–652. doi: 10.1037/0033-295X.108.3.624. [DOI] [PubMed] [Google Scholar]
- 96.Yeung N, Botvinick MM, Cohen JD. The Neural Basis of Error Detection: Conflict Monitoring and the Error-Related Negativity. Psychol Rev. 2004;111(4):931–959. doi: 10.1037/0033-295X.111.4.931. [DOI] [PubMed] [Google Scholar]
- 97.Clayson PE, Larson MJ. Conflict adaptation and sequential trial effects: Support for the conflict monitoring theory. Neuropsychologia. 2011;49(7):1953–1961. doi: 10.1016/j.neuropsychologia.2011.03.023. [DOI] [PubMed] [Google Scholar]
- 98.Falkenstein M, Hohnsbein J, Hoormann J, Blanke L. Effects of crossmodal divided attention on late ERP components. II. Error processing in choice reaction tasks. Electroencephalogr Clin Neurophysiol. 1991;78(6):447–455. doi: 10.1016/0013-4694(91)90062-9. [DOI] [PubMed] [Google Scholar]
- 99.Gehring WJ, Goss B, Coles MGH, Meyer DE, Donchin E. A Neural System for Error Detection and Compensation. Psychol Sci. 1993;4(6):385–390. doi: 10.1111/j.1467-9280.1993.tb00586.x. [DOI] [Google Scholar]
- 100.Shao Z, Roelofs A, Acheson DJ, Meyer AS. Electrophysiological evidence that inhibition supports lexical selection in picture naming. Brain Res. 2014;1586:130–142. doi: 10.1016/j.brainres.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 101.Grützmann R, Riesel A, Klawohn J, Kathmann N, Endrass T. Complementary modulation of N2 and CRN by conflict frequency. Psychophysiology. 2014;51(8):761–772. doi: 10.1111/psyp.12222. [DOI] [PubMed] [Google Scholar]
- 102.Donkers FCL, van Boxtel GJM. The N2 in go/no-go tasks reflects conflict monitoring not response inhibition. Brain Cogn. 2004;56(2):165–176. doi: 10.1016/j.bandc.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 103.Correa Á, Rao A, Nobre AC. Anticipating conflict facilitates controlled stimulus-response selection. J Cogn Neurosci. 2009;21(8):1461–1472. doi: 10.1162/jocn.2009.21136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fernandez M, Tartar JL, Padron D, Acosta J. Neurophysiological marker of inhibition distinguishes language groups on a non-linguistic executive function test. Brain Cogn. 2013;83(3):330–336. doi: 10.1016/j.bandc.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 105.Fernandez M, Acosta J, Douglass K, Doshi N, L Tartar J, Division of Social and Behavioral Sciences, Nova Southeastern University, Ft. Lauderdale, FL,33314 USA Email: Mercedes.fernandez@nova.edu; Tel: 1-954-262-7804 Speaking Two Languages Enhances an Auditory but Not a Visual Neural Marker of Cognitive Inhibition. AIMS Environ Sci. 2014;1(2):145–157. doi: 10.3934/Neuroscience.2014.2.145. [DOI] [Google Scholar]
- 106.Moreno S, Wodniecka Z, Tays W, Alain C, Bialystok E. Inhibitory Control in Bilinguals and Musicians: Event Related Potential (ERP) Evidence for Experience-Specific Effects. In: Di Russo F, editor. PLoS ONE. 4. Vol. 9. 2014. p. e94169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sullivan MD, Janus M, Moreno S, Astheimer L, Bialystok E. Early stage second-language learning improves executive control: Evidence from ERP. Brain Lang. 2014;139:84–98. doi: 10.1016/j.bandl.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 108.Barac R, Moreno S, Bialystok E. Behavioral and Electrophysiological Differences in Executive Control Between Monolingual and Bilingual Children. Child Dev. 2016;87(4):1277–1290. doi: 10.1111/cdev.12538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rosvold HE, Mirsky AF, Sarason I, Bransome ED, Jr, Beck LH. A continuous performance test of brain damage. J Consult Psychol. 1956;20(5):343–350. doi: 10.1037/h0043220. [DOI] [PubMed] [Google Scholar]
- 110.Morales J, Yudes C, Gómez-Ariza CJ, Bajo MT. Bilingualism modulates dual mechanisms of cognitive control: Evidence from ERPs. Neuropsychologia. 2015;66:157–169. doi: 10.1016/j.neuropsychologia.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 111.Braver TS. The variable nature of cognitive control: a dual mechanisms framework. Trends Cogn Sci. 2012;16(2):106–113. doi: 10.1016/j.tics.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhang H, Kang C, Wu Y, Ma F, Guo T. Improving proactive control with training on language switching in bilinguals. NeuroReport. 2015;26(6):354–359. doi: 10.1097/WNR.0000000000000353. [DOI] [PubMed] [Google Scholar]
- 113.Kousaie S, Phillips NA. Conflict monitoring and resolution: Are two languages better than one? Evidence from reaction time and event-related brain potentials. Brain Res. 2012;1446:71–90. doi: 10.1016/j.brainres.2012.01.052. [DOI] [PubMed] [Google Scholar]
- 114.Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychol. 1935;18(6):643. [Google Scholar]
- 115.Simon JR. The Effects of an Irrelevant Directional CUE on Human Information Processing. In: Reeve RWP, T G, editors. Advances in Psychology. Vol. 65. Vol. 1990. North-Holland: 1990. pp. 31–86. (Advances in Psychology). http://www.sciencedirect.com/science/article/pii/S0166411508612182. Accessed November 11, 2016. [Google Scholar]
- 116.Eriksen BA, Eriksen CW. Effects of noise letters upon the identification of a target letter in a nonsearch task. Percept Psychophys. 1974;16(1):143–149. doi: 10.3758/BF03203267. [DOI] [Google Scholar]
- 117.Gollan TH, Montoya RI, Fennema-Notestine C, Morris SK. Bilingualism affects picture naming but not picture classification. Mem Cognit. 2005;33(7):1220–1234. doi: 10.3758/BF03193224. [DOI] [PubMed] [Google Scholar]
- 118.Gollan TH, Montoya RI, Cera C, Sandoval TC. More use almost always means a smaller frequency effect: Aging, bilingualism, and the weaker links hypothesis. J Mem Lang. 2008;58(3):787–814. doi: 10.1016/j.jml.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gollan TH, Sandoval T, Salmon DP. Cross-Language Intrusion Errors in Aging Bilinguals Reveal the Link Between Executive Control and Language Selection. Psychol Sci. 2011;22(9):1155–1164. doi: 10.1177/0956797611417002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.R Paap K, M Sawi O, Dalibar C, Darrow J, A Johnson H, Language, Attention, & Cognitive Engineering (LACE) Lab, Department of Psychology, San Francisco State University, San Francisco, CA, USA Beyond Panglossian Optimism: Larger N2 Amplitudes Probably Signal a Bilingual Disadvantage in Conflict Monitoring. AIMS Neurosci. 2015;2(1):1–6. doi: 10.3934/Neuroscience.2015.1.1. [DOI] [Google Scholar]
- 121.Lamm C, Zelazo PD, Lewis MD. Neural correlates of cognitive control in childhood and adolescence: Disentangling the contributions of age and executive function. Neuropsychologia. 2006;44(11):2139–2148. doi: 10.1016/j.neuropsychologia.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 122.Polich J. Updating P300: An integrative theory of P3a and P3b. Clin Neurophysiol. 2007;118(10):2128–2148. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Polich J. Neuropsychology of P300. Oxf Handb Event-Relat Potential Compon. 2012;2012:159–188. [Google Scholar]
- 124.Steiner GZ, Brennan ML, Gonsalvez CJ, Barry RJ. Comparing P300 modulations: Target-to-target interval versus infrequent nontarget-to-nontarget interval in a three-stimulus task: Comparing P300 modulations: TTI versus infrequent NNI. Psychophysiology. 2013;50(2):187–194. doi: 10.1111/j.1469-8986.2012.01491.x. [DOI] [PubMed] [Google Scholar]
- 125.Donchin E. Surprise!… surprise? Psychophysiology. 1981;18(5):493–513. doi: 10.1111/j.1469-8986.1981.tb01815.x. [DOI] [PubMed] [Google Scholar]
- 126.Coderre EL, van Heuven WJB. Electrophysiological Explorations of the Bilingual Advantage: Evidence from a Stroop Task. In: Alain C, editor. PLoS ONE. 7. Vol. 9. 2014. p. e103424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.West R, Alain C. Effects of task context and fluctuations of attention on neural activity supporting performance of the Stroop task. Brain Res. 2000;873(1)(00):102–111. 02530–0. doi: 10.1016/S0006-8993. [DOI] [PubMed] [Google Scholar]
- 128.West R. Neural correlates of cognitive control and conflict detection in the Stroop and digit-location tasks. Neuropsychologia. 2003;41(8):1122–1135. doi: 10.1016/S0028-3932(02)00297-X. [DOI] [PubMed] [Google Scholar]
- 129.Hanslmayr S, Pastötter B, Bäuml KH, Gruber S, Wimber M, Klimesch W. The Electrophysiological Dynamics of Interference during the Stroop Task. J Cogn Neurosci. 2008;20(2):215–225. doi: 10.1162/jocn.2008.20020. [DOI] [PubMed] [Google Scholar]
- 130.Heidlmayr K, Hemforth B, Moutier S, Isel F. Neurodynamics of executive control processes in bilinguals: evidence from ERP and source reconstruction analyses. Front Psychol. 2015;6 doi: 10.3389/fpsyg.2015.00821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gehring WJ, Liu Y, Orr JM, Carp J. The error-related negativity (ERN/Ne) Oxf Handb Event-Relat Potential Compon. 2012;2012:231–291. [Google Scholar]
- 132.Nieuwenhuis S, Yeung N, van den Wildenberg W, Ridderinkhof KR. Electrophysiological correlates of anterior cingulate function in a go/no-go task: Effects of response conflict and trial type frequency. Cogn Affect Behav Neurosci. 2003;3(1):17–26. doi: 10.3758/CABN.3.1.17. [DOI] [PubMed] [Google Scholar]
- 133.Ganushchak L, Christoffels I, Schiller NO. The use of electroencephalography in language production research: a review. Lang Sci. 2011;2:208. doi: 10.3389/fpsyg.2011.00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Festman J, Münte TF. Cognitive Control in Russian–German Bilinguals. Front Psychol. 2012;3 doi: 10.3389/fpsyg.2012.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Herrmann MJ, Römmler J, Ehlis A-C, Heidrich A, Fallgatter AJ. Source localization (LORETA) of the error-related-negativity (ERN/Ne) and positivity (Pe) Cogn Brain Res. 2004;20(2):294–299. doi: 10.1016/j.cogbrainres.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 136.Pailing PE, Segalowitz SJ, Dywan J, Davies PL. Error negativity and response control. Psychophysiology. 2002;39(2):198–206. doi: 10.1017/S0048577202010247. [DOI] [PubMed] [Google Scholar]
- 137.Craik FIM, Bialystok E, Freedman M. Delaying the onset of Alzheimer disease Bilingualism as a form of cognitive reserve. Neurology. 2010;75(19):1726–1729. doi: 10.1212/WNL.0b013e3181fc2a1c. [DOI] [PMC free article] [PubMed] [Google Scholar]


