Abstract
Background
Delirium is an acute change in mental status characterized by sudden onset, fluctuating course, inattention, disorganized thinking, and abnormal level of consciousness. The objective of the randomized controlled trial “A Strategy to Reduce the Incidence of Postoperative Delirium in Elderly Patients”(STRIDE) is to assess the effectiveness of light versus heavy sedation on delirium and other outcomes in elderly patients undergoing hip fracture repair surgery. Our goal is to describe the design considerations and lessons learned in planning and implementing the STRIDE trial.
Methods
Discussed are challenges encountered including (1) how to ensure that we quickly identify, assess the eligibility of, and randomize traumatic hip fracture patients; (2) how to implement interventions that involve continuous monitoring and adjustment during the surgery; and (3) how to measure and ascertain the primary outcome, delirium.
Results
To address the first challenge, we monitored the operating room schedule more actively than anticipated. We constructed and organized eligibility assessment data collection forms by purpose and by source of information needed to complete them. We decided that randomization needs to take place in the operating room. To address the second challenge, we designed and implemented a treatment protocol, and covered the bispectral index monitor to prevent the Anesthesiologists/Anesthetists from being influenced by the bispectral index reading while administering the intervention. Finally, clinical assessment of delirium consisted of standardized interviews of the patient using validated instruments, interviews of those caring for the patient, and review of the medical record. A consensus panel made the final determination of a delirium diagnosis. We note that STRIDE is a single-center trial. The decisions we took may have different implications for multi-center trials.
Conclusions
Lessons learned are likely to provide useful information to others designing trials in emergency and surgical setting, and for those who are interested in unbiased assessment of delirium.
Keywords: Sedation, hip fracture repair, delirium, propofol, design considerations
Introduction
Delirium is an acute change in mental status characterized by sudden onset, fluctuating course, inattention, disorganized thinking, and abnormal level of consciousness.1 Mounting evidence has linked delirium with poor patient outcomes, including increased risk of death, institutionalization, and dementia.2 In addition, incident delirium is an important predictor of longer hospital stay and increased health care costs.3 Every year, 2–3 million elderly patients develop delirium during their hospital stay, accounting for more than 17.5 million inpatient days and more than $4 billion in Medicare expenditures.4
Rationale for the trial
The risk of delirium in elderly patients after major elective surgery is between 10% and 25%, and is as high as 50% following cardiac surgery and hip fracture repair.5–9 Because postoperative delirium is common, posing a heavy disease burden to the patient and the health care system, interventions that target modifiable risk factors are the mainstay of delirium prevention.10 One such risk factor may be sedative drugs where both drug selection and dosage can be modified. Sedative drugs, typically propofol, are administered in addition to anesthesia during the surgery to relieve anxiety, discomfort or pain, and diminish memory of the event. Although higher doses of intraoperative sedative drugs have been associated with higher risk of delirium in intensive care unit patients,11 intraoperative sedation level is not actively managed to prevent delirium in current clinical practice.
The principal objective of the randomized clinical trial “A Strategy to Reduce the Incidence of Postoperative Delirium in Elderly Patients”(STRIDE) is to assess the effectiveness of light versus heavy sedation in reducing the incidence of postoperative delirium and other outcomes in elderly patients undergoing hip fracture repair surgery. Light sedation was defined as an Observer’s Assessment of Alertness/Sedation (OAA/S) score of 3–5 and heavy sedation as an OAA/S score of 0–2. Our literature searches of the PubMed and The Cochrane Library at the time of planning STRIDE identified only one randomized controlled trial, a preliminary study performed by the same Principal Investigator (Dr. Frederick Sieber),12 and no systematic review or other randomized controlled trial that had addressed the main research question posted by STRIDE. The objective of this article is to describe important design considerations and decisions made in STRIDE.
Methods
Table 1 provides the design synopsis of STRIDE, more fully described in the protocol (online Appendix). In brief, STRIDE is a randomized, two-group, parallel, superiority trial in patients with traumatic hip fracture undergoing hip fracture repair surgery at the Johns Hopkins Bayview Medical Center. Participants were randomized at a 1:1 ratio, using a blocked random sequence stratified by age and dementia status, to light or heavy propofol sedation during surgery.
Table 1.
Study design synopsis
| Study Characteristics | Description |
|---|---|
| Population | 200 patients who are 65 years or older undergoing hip fracture repair with spinal anesthesia and propofol sedation. |
| Intervention | Light sedation, defined as an Observer’s Assessment of Alertness/Sedation score of 3–5. |
| Comparison intervention | Heavy sedation, defined as an Observer’s Assessment of Alertness/Sedation score of 0–2. |
| Outcomes | Primary outcome: in-hospital incidence of delirium during postoperative Day 1 to Day 5 or until hospital discharge, whichever occurs first. Delirium diagnosis was finalized by a Consensus Panel, based on Delirium Assessor’s standardized face-to-face interviews of the patient, family, friends and medical staff caring for the patient as well as review of medical record. Secondary outcomes: mortality at 12 months and risk of delirium at 1 month. Other outcomes: change in functional outcomes from baseline to 1- month and 12-month follow-up assessments, including activities of daily living, instrumental activities of daily living, grip strength, timed chair rise, and timed 3-meter walk; change in clinical dementia rating between baseline and 12-month follow-up; safety outcomes. |
| Setting | Johns Hopkins Bayview Medical Center, a tertiary hospital. |
| Duration | Follow up to 12 months after randomization. |
| Control of Biases | |
| Randomization | Participants were randomized to treatment group in a 1:1 ratio, using a computer generated, blocked random sequence stratified by age and dementia status. |
| Allocation concealment | The Study Anesthesiologist/Anesthetist requested the random sequence assignment in the operating room through the use of a web-based randomization assignment delivery system. The random assignment was concealed from the Study Anesthesiologist/Anesthetist until all eligibility criteria were assessed and entered. |
| Masking | The treatment assignment was only known to the Study Anesthesiologist/Anesthetist and the statistician. Study participants and outcome assessors were masked to the treatment assignment. |
| Missing data | STRIDE was designed to follow the recent recommendations and standards in the prevention and treatment of missing data [23, 24]. We followed all participants randomized regardless of adherence to the protocol unless consent was withdrawn. We pre-specified in the study protocol statistical methods and associated assumptions for dealing with missing data. |
We collected follow-up data in hospital and at 1 and 12 months post-surgery. The primary outcome was in-hospital incidence of delirium during post-operative Day 1 to Day 5 or to hospital discharge, whichever occurred first. The Johns Hopkins Medical Institutions Institutional Review Board approved the trial. Funding by the National Institute of Aging began on May 1, 2010; enrollment began on November 18, 2011. A trial protocol that follows the “Standard Protocol Items: Recommendations for Interventional Trials” (SPIRIT) statement13 is available in the online Appendix.
Results
Table 2 outlines the challenges and solutions implemented in STRIDE. Most of the anticipated challenges were identified by running the preliminary trial and through discussions with the clinicians, nurses, and family caring for hip fracture patients. We describe three challenges in detail below, including (1) how to ensure that we quickly identify, assess the eligibility of, and randomize traumatic hip fracture patients at a time when they are in great pain and require immediate surgery; (2) how to implement interventions that involve continuous monitoring and adjustment during the surgery; and (3) how to measure and ascertain the primary outcome, delirium, given the short hospital stay, changing nursing shifts, and the need for a verifiable and unbiased assessment.
Table 2.
Challenges encountered in STRIDE and strategies to address them
| Challenges | Strategies |
|---|---|
| Anticipated | |
| Identify and assess eligibility of traumatic hip fracture patients who require immediate surgery within a short time frame |
Check the operating room schedule manually; operating room staff and orthopedic residents page the study team; construct eligibility assessment forms for maximum efficiency |
| Randomize only those patients in whom placement of spinal anesthesia is successful |
Randomize patients after successful spinal anesthesia is achieved in the operating room through a web-based randomization delivery system |
| Implement study interventions to maintain sedation level |
Train Study Anesthesiologist/Anesthetist for treatment protocol; cover bispectral index monitor. |
| Maintain masking of outcome assessors and patients |
Keep all forms pertaining to study intervention separately in locked cabinets and enter data from these forms into database by a separate data entry team; control access to database tables; no contact between the Study Anesthesiologist/Anesthetist performing intervention and outcome assessment staff |
| Measure and ascertain the primary outcome in a standardized way |
Receive training for delirium assessment; use validated instruments; standardize measurement protocol; utilize a Delirium Consensus Panel for making the final delirium diagnosis |
| Follow-up of all randomized participants given short inpatient stay |
Call participants each month to build rapport; track medical complications and living arrangements following participant’s discharge |
| Prevent and analyze missing data | Define primary outcome for variable lengths of hospital stay, and thus it can be ascertained in all study participants and is estimable with minimal assumptions; inform participants of the commitment they are making during the consent process; follow all participants randomized; conduct sensitivity analysis for missing data to account for the uncertainty in missing data and examine the robustness of trial finding under plausible missing data scenarios. |
| Ensure patients are NOT delirious prior to randomization, particularly patients with significant cognitive impairment |
Exclude patients with any suggestion of new acute difficulties with confusion and attention |
| Unanticipated | |
| Preoperative cognitive status may change while patients are waiting for surgery |
Re-evaluate patient’s cognitive status to determine if s/he is still eligible (i.e. not delirious) if a patient is delayed for surgery over 24 hours. |
| Adverse event classification difficult because of co-morbidities |
Appoint Safety Officer affiliated with the National Institute of Aging who will work together with the Data and Safety Monitoring Board to review adverse events |
Assessing eligibility
The primary inclusion criteria for STRIDE were planned repair of acute primary traumatic hip fracture under spinal anesthesia; 65 years of age or older; Mini-mental Status Exam score of 15 or greater before surgery; ability to understand, speak, read, and write English; and successful spinal anesthesia in preparation for the hip repair surgery. In STRIDE, all participants received the same spinal anesthetic: 15 mg isobaric bupivacaine plus 25 mcg fentanyl. Exclusion criteria were contraindications to spinal anesthesia (e.g., severe aortic stenosis and anticoagulation); preoperative delirium; intubation pre-surgery; stage IV congestive heart failure; or severe chronic obstructive pulmonary disease. Also excluded were patients with a prescription for clopidogrel within 7 days prior to surgery, ticlopidine within 14 days prior to surgery, glycoprotein IIb/IIIa inhibitors, fondaparinux, or dabigatran within 48 hours prior to surgery. We estimated that 200 participants would be needed based on a 4-year recruitment period in a single clinical center setting.
Establish eligibility for the trial
Identifying and enrolling participants for STRIDE was viewed as potentially challenging because patients who have acute traumatic hip fracture go to surgery soon after the fracture occurs, and prospective patients needed to be identified and assessed rapidly and accurately to determine eligibility. The time window to collect all needed information is typically within 3–4 hours before surgery. The screening and recruitment process was further complicated because the eligibility information was to be obtained from multiple sources, including three electronic databases being used at Johns Hopkins Bayview Medical Center (e.g., MediTech, Electronic Patient Record, Centricity); discussion was required with the patient or legally authorized representative; time was needed to obtain the laboratory results; and the judgment of the Study Anesthesiologist/Anesthetist was necessary. Finally, the pre-operative delirium status might change while patients were waiting for surgery.
To address the above-mentioned challenges, we devised and revised several strategies for eligibility assessment in STRIDE. First, given the short time window prior to surgery, the protocol initially called for the Clinical Coordinator or the Study Anesthesiologist/Anesthetist to manually review the operating room schedule to identify prospective participants. The operating room front desk staff updated the operating room schedule on an hourly basis. The advantage to this approach was that the approach was relatively simple and STRIDE staff could monitor availability of potentially eligible patients. As the trial proceeded, we found that more active scrutiny of the operating room schedule was needed because STRIDE staff had other duties and could not always monitor the lists as closely as needed to maximize enrollment. Hence we modified the protocol to have the operating room staff page the Study Team whenever a hip fracture repair was posted on the operating room schedule. In addition, we encouraged the orthopedic residents to page the Study Team when asked to consult for a hip fracture, thus providing the Study Team with advanced notice about an upcoming surgery. The Clinical Coordinator entered all candidates scheduled for traumatic hip fracture repair surgery on a Participant Registration Log to track and record all candidates and their final eligibility. Any patient entered on the log was considered “screened”.14
Second, in order to streamline the workflow and improve the efficiency of screening, we constructed and organized eligibility assessment data collection forms by purpose and by source of information needed to complete them, and required forms and questions within forms to be completed in sequence. For example, all information needed to complete the first form, which included eligibility criteria concerning age, language, type of fracture, medication, lab test, and medical history, was already available in the patient’s medical record so that no interaction with the patient or legally authorized representative was required, and ineligible patients could be excluded right away based on existing information. This approach ensured that the reasons for exclusion were documented consistently and could easily be tabulated for reporting. Whenever a surgery was delayed for more than 24 hours, the Clinical Coordinator re-evaluated the patient’s cognitive status prior to surgery to establish whether s/he was still eligible.
The study question that the STRIDE aims to address (i.e., the relative effect of light versus heavy sedation on post-operative delirium) is relevant only among those patients in which spinal anesthesia is possible. Because spinal anesthesia is not always technically possible, as shown in the preliminary study,12 we required successful placement of spinal anesthesia as an eligibility criterion. This criterion can be assessed only after the patient was in the operating room and ready for surgery.
To prevent randomizing ineligible patients, we provided the Study Anesthesiologist/Anesthetist secured access to a web-based randomization assignment delivery system in the operating room. The Study Anesthesiologist/Anesthetist can request the randomization assignment after s/he confirms that the spinal anesthetic was optimize without leaving the operating room or interrupting the procedure.
Choice and implementation of study treatments
Propofol, the test intervention, was given for sedation. The Study Anesthesiologist/Anesthetist titrated propofol individually for each patient to achieve and maintain the depth of sedation required by that patient’s randomly assigned treatment group (“light” or “heavy” sedation). As noted earlier, we defined light sedation to be an OAA/S score of 3–5 and heavy sedation to be an OAA/S score of 0–2. The main advantage of choosing these two levels of sedation is to maximize the difference in the propofol comparators: light sedation is comparable to minimal or no drug sedation while heavy sedation is comparable to general anesthesia.
The utility of using electroencephalography to gauge the depth of sedation (e.g., bispectral index score) was initially considered but not adopted because the OAA/S score is a clinical measure and can be applied in almost all settings, whereas electroencephalography is not used in all operating rooms.15 More importantly, previous studies have shown that OAA/S score is better indicator of the level of sedation than electroencephalography.15,16
We discussed in depth the treatment protocol and how to follow it strictly as the OAA/S scores fluctuated during surgery. The final treatment protocol is described below, and in our view, it is generalizable to other centers. When the Study Anesthesiologist/Anesthetist determined the sedation level of a participant randomized to heavy sedation to be too light (an OAA/S score of 3 or greater), the infusion rate of propofol was increased by 10–20 mcg per kg per minute, and the sedation was reassessed 5 minutes later following the change in infusion rate. This sequence was repeated until a sedation level of 0–2 was obtained. Likewise, when the Study Anesthesiologist/Anesthetist determined the sedation level of a participant randomized to light sedation to be too heavy (an OAA/S score of less than 3), the propofol infusion rate was decreased in steps of 10–20 mcg per kg per minute at a time, with a 5-minute interval before repeating the sequence until an OAA/S score of 3 or greater was achieved.
Implementing a protocol that required continuous monitoring and adjustment of the intervention presented challenges. First, the Study Anesthesiologist/Anesthetists were trained extensively in the treatment protocol before the initiation of recruitment. Each Study Anesthesiologist/Anesthetist read the treatment protocol and completed five study cases under the direction of the Lead Study Anesthesiologist (Dr. Frederick Sieber). They also received continued training as the trial progressed.
Furthermore, to document the implementation to treatment protocol, the Study Anesthesiologist/Anesthetist recorded the OAA/S score every 15 minutes on the Intraoperative Data Form, which was kept and stored separately from all other forms, at all times, so as to maintain masking of the treatment assignment from all other study personnel. On the same form, the Study Anesthesiologist/Anesthetist also recorded the start and stop times of propofol infusion, the rates of infusion, the time and amount of each propofol bolus during the intraoperative period. Sedatives other than propofol were not administered intraoperatively, nor were intravenous opioids administered. Time to surgery, duration of the surgery, amount of blood loss, and fluid replacement during surgery were collected on every patient.
The Study Anesthesiologists/Anesthetists also monitored patient blood pressure, pulse oximetry, and electrocardiography during surgery. The bispectral index monitor was used on all participants in STRIDE. The bispectral index monitor displays the bispectral index score, which can be viewed as a means of checking on the level of sedation achieved during surgery. The bispectral index score measures changes in a patient's level of sedation using a computer-generated algorithm through the bispectral index monitor, to analyze data from a patient's electroencephalogram during surgery. While the advantage of keeping track of bispectral index score is clear, it could influence and unmask the Study Anesthesiologist/Anesthetist. Thus, we decided that the bispectral index monitor readout should be covered throughout the surgery. In addition, bispectral index scores were kept and stored separately from all other forms, at all times, so as to maintain masking of the treatment assignment from all other study personnel. Our approach allows future comparison of the bispectral index data and OAA/S scores recorded intraoperatively.16
Considerations in measuring and ascertaining the primary outcome
The primary outcome of STRIDE was the in-hospital incidence of delirium during postoperative Day 1 to Day 5 or until hospital discharge, whichever occurs first. The mean hospital length of stay after hip fracture surgery at Johns Hopkins Bayview Medical Center is 5.7 days.17 The advantage to this more flexible outcome was that it accommodated the real world question related to in-hospital delirium. The disadvantage of this approach was that, if delirium occurred after discharge but within 5 days, we would not know.
In addition, no diagnostic physiologic measures of delirium exist; and in practice, the diagnosis is based on clinical judgment after examining the patient, collecting information from the informant sources, and reviewing the medical record. This presents a challenge for trials utilizing delirium as an outcome because measurement errors are likely.
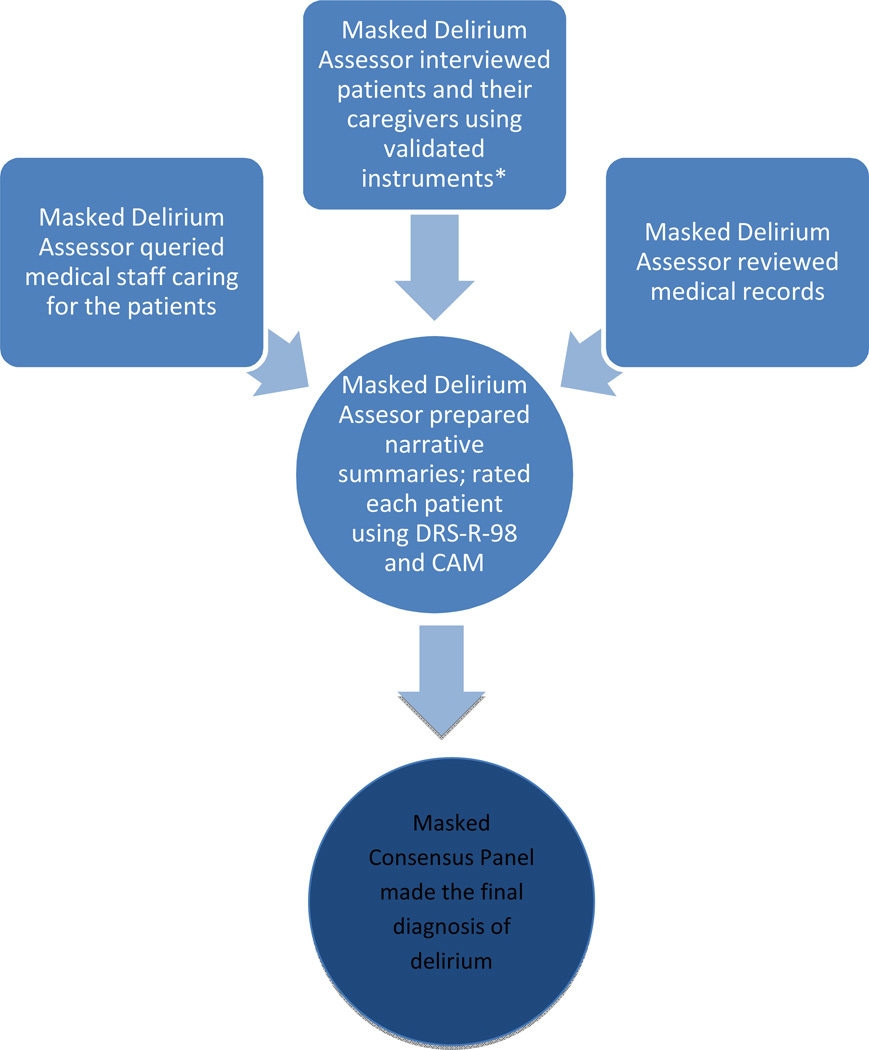
To overcome this challenge, we reviewed the literature and brought in experts in geriatrics, psychiatry, anesthesiology, clinical trial, and statistics. We decided that, in STRIDE, clinical assessment of delirium, performed by delirium assessors masked to the treatment assignment, would consist of standardized face-to-face interviews of the patient using validated instruments, interviews of family, friends and medical staff caring for the patient, and review of the medical record for the foregoing 24 hours (Figure 1). In addition, we convened a consensus panel who made the final determination of a delirium diagnosis. An advantage to this approach was that it was likely to be a thorough, standardized, and unbiased assessment of the presence of delirium. We believed that this was necessary to provide reliable estimates of treatment effect that could impact medical practice if an effect was found. A disadvantage of our approach is that it is not “real world” and would be time consuming and costly to implement in the trial.
Figure 1. Delirium assessment and diagnosis.
Cognition tests: Mini Mental State Examination,15 Abbreviated Digit Span Test,16 Informant Questionnaire on Cognitive Decline in the Elderly,17 as well as testing of attention. DRS-R-98 (Delirium Rating Scale Revised 98)
CAM (Confusion Assessment Method)
The patient interview by delirium assessor comprised a series of questions about the patient’s sleep–wake cycle, perceptual experiences, recent account about their stay in the hospital, and the patient’s current pain rating. The delirium assessor also tested the patient’ cognition using the Mini Mental State Examination,18 Abbreviated Digit Span Test,19 and attention by asking the patient to recite the months of the year, or days of the week, in reverse order. The delirium assessor interviewed family members or friends using the Short Form of the Informant Questionnaire on Cognitive Decline in the Elderly20 to establish the patient’s cognitive function prior to hospitalization. Family members or friends also commented on the patient’s current function on the baseline interview, specifically probing for any information regarding an acute change in thinking or confusion consistent with a delirium diagnosis. The delirium assessor queried medical staff, including nurses and physicians caring for the patient, regarding the signs and symptoms of delirium at baseline and on each post-operative day of assessment. The delirium assessor reviewed the medical record each day, noting any references made by the clinical staff to patient confusion, agitation, somnolence, disrupted sleep-wake cycle, and perceptual disturbances.
The delirium assessor synthesized the information gathered in a summary narrative. The narrative described the patient’s appearance, behavior, attention, level of arousal, overall performance, environment in which interviewed, and any salient events that occurred during the interview. The delirium assessor then rated the patient using the 13-item severity scale “Delirium Rating Scale Revised 98” and the “Confusion Assessment Method”.21–23 Because delirium symptoms fluctuate throughout the day, the delirium assessor attempted to see the patient at approximately the same time each day in the postoperative period.
Training of the Delirium Assessor
The Delirium Assessor, experienced with standardized psychiatric interviewing skills, was supervised by the Study Psychiatrist. Training for each Delirium Assessor prior to the start of the study included 3 hours of didactic lectures on the clinical characteristics of delirium and dementia and 2.5 hours of video viewing of examples of patients with delirium for rating purposes using Delirium Rating Scale Revised 98 and Confusion Assessment Method assessment forms. Afterwards, each Delirium Assessor was trained with volunteer patients (with or without delirium) not involved in the study. The Delirium Assessor and the Study Psychiatrist independently completed their own ratings on the volunteer patients. Agreement between the Delirium Assessor and Study Psychiatrist ratings was compared, discussed, and documented after each training case. Delirium Assessors were allowed to begin evaluating patients in STRIDE once they had completed co-rated training interviews that were in 100% agreement for Confusion Assessment Method diagnosis and +/− 2 points on the Delirium Rating Scale total severity score (range 0 – 39) for 5 consecutive cases. The number of training cases completed prior to the start of the study ranged from 5 to 12 for the 3 Delirium Assessors.
Consensus panel for delirium diagnosis
A consensus panel, consisting of a geriatrician and two psychiatrists who have expertise in delirium assessment, masked to individual treatment assignment, reviewed all delirium assessments. To reach consensus on the delirium diagnosis, the consensus panel reviewed all information, including the assessors’ narrative description of the patient interview, data on patient performance on all of the cognitive tests, and collateral information. Each consensus panel member independently rated the patient against diagnostic criteria for delirium according to the Diagnostic and Statistical Manual for Mental Disorders, 4th Edition for each foregoing 24-hour period. There are three possible ratings: No delirium, no criteria met; No delirium, but 1 or 2 criteria met; and Delirium, all 3 criteria met. The consensus panel discussed each case until consensus was achieved, that is, all raters agreed to one of the three ratings. Consensus was achieved prospectively and ratings for previous days were not changed in light of information revealed for the subsequent days.
Discussion
The design and conduct of the STRIDE trial posed significant challenges. Although most of the challenges were anticipated, we had to revisit and modify the design decisions as needed to improve the efficiency of the trial and to minimize biases. Understanding the threats to the validity is the key to bring all investigators and research staff on board to implement the changes.
In the preliminary study,12 several randomized patients did not receive the study intervention because of technical difficulty in inserting the spinal needle. To minimize the possibility of randomizing ineligible patients, we decided that, in STRIDE, randomization had to take place in the operating room. In addition, during the eligibility assessment, the preoperative cognitive status of the patient may change while s/he is waiting for surgery. We modified the design to require a re-assessment of the patient’s cognitive status (i.e., whether delirious or not) if s/he is delayed for surgery over 24 hours. These changes ensured that only eligible patients were randomized and randomization was not compromised by knowledge of the next assignment until all eligibility criteria were assessed and entered into the study database, thus minimizing selection bias.
To minimize performance bias (i.e., systematic deviation from the intended intervention), we designed a treatment protocol and trained the Study Anesthesiologist/Anesthetist for treatment protocol. We covered bispectral index monitor so that the sedation level was determined based on OAA/S assessment alone, independent of the bispectral index reading. OAA/S is a validated clinical measure of sedation level.15,16 In using the OAA/S assessment, we also hope to generalize our results to settings where bispectral index monitor is unavailable in the operating room.
To minimize information bias (i.e., bias arising from systematic measurement error), the treatment assignment was known only to the Study Anesthesiologist/Anesthetist and the statistician. Study participants and outcome assessors were masked to the treatment assignment. To achieve this level of masking, all forms pertaining to study intervention were kept separately in locked cabinets and data from these forms were entered into database by a separate data entry team. There was no contact between the Study Anesthesiologist/Anesthetist performing intervention and outcome assessment staff. For researchers interested in studying delirium as an outcome, the resource requirements should not be underestimated. Although similar delirium instruments and diagnostic criteria were applied in several other trials of hip fracture repair patients,24–32 having an independent delirium diagnosis panel distinguishes our approach from the existing approaches. We encourage other researchers to use a rigorous approach such as ours to establish delirium diagnosis and share their experiences of whether the approach is feasible in larger scale multi-center trials.
There are a few additional design considerations that may affect the interpretation of the findings from STRIDE. In terms of primary outcome, the question has been raised whether the study intervention itself may alter the length of stay and thereby the number of delirium assessments. We planned to examine this possibility by comparing the length of stay among eligible patients who enrolled and who did not enroll, and by study groups. We also planned to account for the differential length of stay between study groups in the analysis. Further, different discharge destinations, which were out of the control of the study team, may influence medical care, consequently affecting re-admission rates, death, and other outcomes. Finally, STRIDE is a single clinical center trial based at a tertiary care hospital. It would have been logistically impossible to conduct STRIDE at primary care facilities due to the nature of the condition and interventions under study. However, involving other tertiary centers would improve the generalizability of the trial results.
Conclusions
The STRIDE trial treatment has the potential to affect delirium risk postoperatively and to reduce delirium associated mobility and mortality. The trial was designed to generate critical information that will begin establishing the evidence base for managing sedation level during anesthesia where evidence-based clinical practice guidelines are currently lacking. Lessons learned from designing and implementing the STRIDE trial are likely to provide useful information to others designing trials in surgical setting and for those who are interested in assessing delirium as an outcome rigorously.
Supplementary Material
Acknowledgments
Financial Support:
This study is funded by Grant R01 AG033615 from the National Institute of Aging, National Institutes of Health, USA. Support is also received from the Institute of Clinical and Translation Research through Grants M01 RR02719, UL1 TR000424, and UL1 TR001079 from National Center for Research Resources (NCRR) and the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health, USA.
Footnotes
Trial Registration:
STRIDE is registered at ClinicalTrials.gov under registration number: NCT00590707.
References
- 1.Inouye SK. Delirium in older persons. N Engl J Med. 2006;354:1157–1165. doi: 10.1056/NEJMra052321. [DOI] [PubMed] [Google Scholar]
- 2.Witlox J, Eurelings LS, de Jonghe JF, et al. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta-analysis. JAMA. 2010;304:443–451. doi: 10.1001/jama.2010.1013. [DOI] [PubMed] [Google Scholar]
- 3.McCusker J, Cole MG, Dendukuri N, et al. Does delirium increase hospital stay? J Am Geriatr Soc. 2003;51:1539–1546. doi: 10.1046/j.1532-5415.2003.51509.x. [DOI] [PubMed] [Google Scholar]
- 4.Pandharipande P, Jackson J, Ely EW. Delirium: acute cognitive dysfunction in the critically ill. Curr Opin Crit Care. 2005;11:360–368. doi: 10.1097/01.ccx.0000170503.76528.4b. [DOI] [PubMed] [Google Scholar]
- 5.Marcantonio ER. In the clinic. Delirium. Ann Intern Med. 2011;154 doi: 10.7326/0003-4819-154-11-201106070-01006. ITC6-1-15; quiz ITC6-16. [DOI] [PubMed] [Google Scholar]
- 6.Marcantonio ER, Goldman L, Mangione CM, et al. A clinical prediction rule for delirium after elective noncardiac surgery. JAMA. 1994;271:134–139. [PubMed] [Google Scholar]
- 7.Rasmussen LS, Moller JT. Central nervous system dysfunction after anesthesia in the geriatric patient. Anesthesiol Clin North America. 2000;18:59–70. vi. doi: 10.1016/s0889-8537(05)70149-8. [DOI] [PubMed] [Google Scholar]
- 8.Marcantonio ER, Flacker JM, Wright RJ, et al. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc. 2001;49:516–522. doi: 10.1046/j.1532-5415.2001.49108.x. [DOI] [PubMed] [Google Scholar]
- 9.Lee HB, Mears SC, Rosenberg PB, et al. Predisposing factors for postoperative delirium after hip fracture repair in individuals with and without dementia. J Am Geriatr Soc. 2011;59:2306–2313. doi: 10.1111/j.1532-5415.2011.03725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oh ES, Li M, Fafowora TM, et al. Preoperative risk factors for postoperative delirium following hip fracture repair: a systematic review. Int J Geriatr Psychiatry. 2015;30:900–910. doi: 10.1002/gps.4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pandharipande P, Shintani A, Peterson J, et al. Lorazepam is an independent risk factor for transitioning to delirium in intensive care unit patients. Anesthesiology. 2006;104:21–26. doi: 10.1097/00000542-200601000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Sieber FE, Zakriya KJ, Gottschalk A, et al. Sedation depth during spinal anesthesia and the development of postoperative delirium in elderly patients undergoing hip fracture repair. Mayo Clin Proc. 2010;85:18–26. doi: 10.4065/mcp.2009.0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan AW, Tetzlaff JM, Altman DG, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med. 2013;158:200–207. doi: 10.7326/0003-4819-158-3-201302050-00583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schulz KF, Altman DG, Moher D CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med. 2010;152:726–732. doi: 10.7326/0003-4819-152-11-201006010-00232. [DOI] [PubMed] [Google Scholar]
- 15.Chernik DA, Gillings D, Laine H, et al. Validity and reliability of the Observer’s Assessment of Alertness/Sedation Scale: a study with intravenous midazolam. J Clin Psychopharmacol. 1990;10:244–251. [PubMed] [Google Scholar]
- 16.Hernandez-Gancedo C, Pestana D, Pena N, et al. Monitoring sedation in critically ill patients: Bispectral index, ramsay and observer scales. Eur J Anaesthesiol. 2006;23:649–653. doi: 10.1017/S0265021506000056. [DOI] [PubMed] [Google Scholar]
- 17.Khasraghi FA, Christmas C, Lee EJ, et al. Effectiveness of a multidisciplinary team approach to hip fracture management. J Surg Orthop Adv. 2005;14:27–31. [PubMed] [Google Scholar]
- 18.MMSE copyright © 2001 by MiniMental, LLC. All rights reserved. Published 2001 by Psychological Assessment Resources, Inc. (PAR); [Google Scholar]
- 19.Strub R, Black F. The mental status examination in neurology. 4th. Vol. 4. FA Davis Company; 2000. ISBN: 0-8036-0427-0. [Google Scholar]
- 20.Jorm AF. A short form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): development and cross-validation. Psychol Med. 1994;24:145–153. doi: 10.1017/s003329170002691x. Erratum in: Psychol Med 1995; 25: 437. [DOI] [PubMed] [Google Scholar]
- 21.Trzepacz PT, Mittal D, Torres R, et al. Validation of the delirium rating scale – revised – 98: Comparison with the delirium rating scale and the cognitive test for delirium. J Neuropsychiatry Clin Neurosci. 2001;13:229–242. doi: 10.1176/jnp.13.2.229. [DOI] [PubMed] [Google Scholar]
- 22.Trzepacz PT, Maldonado JR, Kean J, et al. Delirium rating scale – revised – 98 (DRS-R98): Administration manual. 2010 Electronic edition. [Google Scholar]
- 23.Inouye SK. The Confusion Assessment Method (CAM): Training Manual and Coding Guide. Yale University School of Medicine; 2003. [Google Scholar]
- 24.Siddiqi N, Harrison JK, Clegg A, et al. Interventions for preventing delirium in hospitalised non-ICU patients. Cochrane Database Syst Rev. 2016;3:CD005563. doi: 10.1002/14651858.CD005563.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Jonghe A, van Munster BC, Goslings JC, et al. Effect of melatonin on incidence of delirium among patients with hip fracture: a multicentre, double-blind randomized controlled trial. CMAJ. 2014;186:E547–E556. doi: 10.1503/cmaj.140495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Jonghe A, van Munster BC, van Oosten HE, et al. The effects of melatonin versus placebo on delirium in hip fracture patients: study protocol of a randomised, placebo-controlled, double blind trial. BMC Geriatr. 2011;11:34. doi: 10.1186/1471-2318-11-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gruber-Baldini AL, Marcantonio E, Orwig D, et al. Delirium outcomes in a randomized trial of blood transfusion thresholds in hospitalized older adults with hip fracture. J Am Geriatr Soc. 2013;61:1286–1295. doi: 10.1111/jgs.12396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marcantonio ER, Flacker JM, Wright RJ, et al. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc. 2001;49:516–522. doi: 10.1046/j.1532-5415.2001.49108.x. [DOI] [PubMed] [Google Scholar]
- 29.Marcantonio ER, Palihnich K, Appleton P, et al. Pilot randomized trial of donepezil hydrochloride for delirium after hip fracture. J Am Geriatr Soc. 2011;59:S282–S288. doi: 10.1111/j.1532-5415.2011.03691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mouzopoulos G, Vasiliadis G, Lasanianos N, et al. Fascia iliaca block prophylaxis for hip fracture patients at risk for delirium: a randomized placebo-controlled study. J Orthop Traumatol. 2009;10:127–133. doi: 10.1007/s10195-009-0062-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Watne LO, Torbergsen AC, Conroy S, et al. The effect of a pre- and postoperative orthogeriatric service on cognitive function in patients with hip fracture: randomized controlled trial (Oslo Orthogeriatric Trial) BMC Med. 2014;12:63. doi: 10.1186/1741-7015-12-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wyller TB, Watne LO, Torbergsen A, et al. The effect of a pre- and post-operative orthogeriatric service on cognitive function in patients with hip fracture. The protocol of the Oslo Orthogeriatrics Trial. BMC Geriatr. 2012;12:36. doi: 10.1186/1471-2318-12-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.