Abstract
Mesenchymal stem cells (MSCs) possess distinct immunomodulatory properties and have tremendous potential for use in therapeutic applications in various inflammatory diseases. MSCs have been shown to regulate pathogenic functions of mature myeloid inflammatory cells, such as macrophages and neutrophils. Intriguingly, the capacity of MSCs to modulate differentiation of myeloid progenitors to mature inflammatory cells remains unknown to date. Here, we report the novel finding that MSCs inhibit the expression of differentiation markers on myeloid progenitors under inflammatory conditions. We demonstrate that the inhibitory effect of MSCs is dependent on direct cell-cell contact and that this intercellular contact is mediated through interaction of CD200 expressed by MSCs and CD200R1 expressed by myeloid progenitors. Further, using an injury model of sterile inflammation, we show that MSCs promote myeloid progenitor frequencies and suppress infiltration of inflammatory cells in the inflamed tissue. We also find that downregulation of CD200 in MSCs correlates with abrogation of their immunoregulatory function. Collectively, our study provides unequivocal evidence that MSCs inhibit differentiation of myeloid progenitors in the inflammatory environment via CD200-CD200R1 interaction.
Keywords: mesenchymal stem cells, myeloid progenitor cells, immunoregulation, differentiation, inflammation
Introduction
During hematopoiesis, myeloid lineage-committed progenitors derived from hematopoietic stem cells (HSCs) in the bone marrow give rise to mature myeloid cells such as macrophages and neutrophils [1]. The bone marrow is also home to non-hematopoietic stromal cells such as mesenchymal stem cells (MSCs), which, in addition to providing a niche and trophic support for HSCs, maintain hematopoiesis by sustaining a part of the HSC population in an undifferentiated quiescent state through release of soluble factors and intercellular interactions [2, 3].
Acute inflammatory stresses lead to deviation of hematopoiesis toward preferential induction of committed myeloid progenitors and their subsequent differentiation into mature macrophages and neutrophils [4]. The highly proliferative capacity of myeloid progenitors plays a central role in inflammation-induced myelopoeisis, restoring consumed macrophages and neutrophils at the site of inflammation [5]. Despite the critical role of mature myeloid cells in host defense and resolution of inflammation, excessive innate immune response can have deleterious effects on tissue homeostasis and lead to undesired tissue damage.
In addition to supporting hematopoiesis, MSCs are characterized by their self-renewal and multilineage differentiation potential and unique immunoregulatory properties [6]. Studies on the interaction between MSCs and immune cells have shown that MSCs can regulate functions of mature innate immune cells, including polarization of inflammatory macrophages into an anti-inflammatory phenotype and enhancement of the phagocytic capacity of neutrophils [7, 8].
Although much is known about the regulatory role of MSCs on function of mature myeloid cells, information regarding potential regulatory interactions between MSCs and myeloid progenitor cells is lacking. Given the central role of myeloid progenitors in inflammation, regulating differentiation of these precursors into pathogenic myeloid cells could effectively inhibit inflammatory response at an earlier stage. In this study, we sought to determine whether MSCs can inhibit the differentiation of myeloid progenitors into mature inflammatory cells during inflammation. Specifically, we demonstrate that MSCs inhibit differentiation of myeloid progenitors and maintain these cells in an immature state. Using both in vitro co-culture assays and an in vivo model of injury-induced sterile inflammation, we show that MSCs exert immunoregulatory effects on myeloid progenitors in a cell-cell contact dependent manner – a process mediated through CD200-CD200R1 interaction.
Materials and Methods
Animals
Six- to eight-week-old male C57BL/6 mice (Charles River Laboratories, Wilmington, MA, USA) were used in the experiments. Mice were kept in a pathogen-free environment at the Schepens Eye Research Institute Animal Facility. The protocol was approved by the Schepens Eye Research Institute Animal Care and Use Committee, and all animals were treated in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Isolation, expansion, and characterization of mesenchymal stem cells
Bone marrow was harvested from femur bones of euthanized C57BL/6 mice. Using the previously described plastic adherence method of MSC cultivation [9, 10], bone marrow cells were cultured at 37°C in murine MesenCult basal medium and supplement (Stem Cell Technologies, Vancouver, BC, Canada). Cells were passaged at every three to five days intervals. Before using in experiments, MSCs from third passage were characterized phenotypically for the expression of MSC markers (CD45−CD34−SCA1+CD29+) using flow cytometry, and functionally by in vitro differentiation using adipocytes using MesenCult adipogenic stimulatory supplements (Stem Cell Technologies). Oil-red-O (Sigma-Aldrich, St. Louis, MO) staining was used to confirm the differentiation of MSCs into the adipocytes. MSCs from third passage were used in both in vitro and in vivo experiments.
Myeloid progenitor cell characterization and isolation
Single cell suspensions from spleen, bone marrow, and draining submandibular lymph nodes harvested from C57BL/6 mice were stained with fluorochrome-conjugated monoclonal antibodies to CD14 (#123308), CD11b (#101210), CD34 (#119307), c-kit (#105817), and FcγRII/III (#101327) (Biolegend, San Diego, CA, USA) for characterization of myeloid progenitors. Due to higher frequencies of myeloid progenitors in the spleen, CD14+CD11b− progenitors were then isolated from the spleen by flow sorting (MoFlo XDP, Beckman Coulter). Purity of isolated myeloid progenitors (>95%) was determined by flow cytometry. Isolated spleen-derived progenitors were characterized for the expression of progenitor and mature myeloid cell markers before being used in in vitro experiments, as described later in Flow cytometry method.
Co-culture and transwell assays
Isolated spleen-derived myeloid progenitors (2×105 cells) were cultured with or without in-vitro expanded MSCs (4×104 cells) for 72 hours in the presence of 100ng/mL IFNγ, 100 ng/mL IL-1β, or 10 ng/mL GMCSF (Biolegend, San Diego, CA, USA) as inflammatory or hematopoietic growth factor stimuli. For the indirect co-culture, MSCs were first cultured in a monolayer on 6.5 mm transwell inserts with 0.4 μm pore size (Corning, NY, USA) and then co-cultured with isolated myeloid progenitors at the ratio of 1:5 MSCs to myeloid progenitors in the presence of 100ng/mL IFNγ for 72 hours.
shRNA transfection
MSCs (1.5×106 cells) were plated in a 75 cm2 flask and incubated for 18–24 hours to reach to 60–70% confluency. The cells were then washed and transfected with CD200-specific or non-specific control shRNA using transfection reagent in shRNA transfection media according to the protocol suggested by the manufacturer (Santa Cruz Biotechnology, Dallas, TX). After overnight incubation, transfection media was replaced with normal MSC growth culture media and cells were cultured for additional 2 days. Knockdown efficiency of shRNA was validated by real-time PCR using CD200-specific primers 48 hours after transfection (Supplement Fig. S4).
Corneal injury model
Corneal injury was induced in mice as described previously [11, 12]. Briefly, Mice were anesthetized by intraperitoneal injection of Ketamine and Xylazine. Central cornea of deeply anesthetized mice was marked by a 2mm trephine. Using the tip of a hand-held motor brush (AlgerBrush II, Alger Company Inc., Lago Vista, TX), total corneal epithelium and anterior stroma were removed mechanically to create corneal injury. Upon completion of the procedure, triple antibiotic ointment was applied to the injured eyes, and a subcutaneous injection of Buprenorphine was given to mice to minimize injury-induced pain. To study the therapeutic effect of MSCs on corneal inflammation, mice were randomly divided into injury only or MSC (wild-type or CD200 shRNA)-recipient groups, with n=5 in each group. In vitro expanded and characterized MSCs or CD200 shRNA-treated MSCs (0.5×106 cells suspended in 100μL sterile saline) were injected into the tail veins of mice 1-hour post injury. Mice were euthanized 48 hours post injury to collect corneas for flow cytometry, real-time PCR, and fluorescence microscopy analyses as described later.
Flow cytometry
Flow cytometry was performed to characterize the phenotype of in vitro expanded MSCs and myeloid progenitors, to evaluate in vitro differentiation of myeloid progenitors, and to quantify the frequencies of CD45+ and myeloid progenitors in the cornea. Cultured MSCs in single cell suspension were stained with conjugated monoclonal antibodies to CD45 (#103115), CD34 (#119307), Sca-1 (#108107), CD29 (#102207), CD11b (#101210), c-Kit (#105817), CD105 (#120407), CD31 (#102407), and CD200 (#123807). Single cell suspensions were prepared from bone marrow, spleen, and draining submandibular lymph nodes and were stained with conjugated monoclonal antibodies to CD14 (#123308), CD11b (#101235), CD34 (#119307), c-kit (#105817), FcγRII/III (#101327), Ly6G (#127627), Ly6C (#128007), and CD200R1 (#123907). Single cell suspensions of cultured myeloid progenitors were stained with conjugated monoclonal antibodies to CD11b (#101210), Ly6G (#127627) and CD11c (#117329). Corneas were harvested 48 hours post injury and were digested in RPMI media (Lonza, Walkersville, MD) containing 2 mg/mL collagenase type IV (Sigma-Aldrich, St. Louis, MO) and 2 mg/mL DNase I (Roche, Basel, Switzerland) for 45 minutes at 37 °C and then filtered through a 70-μm cell strainer. Corneal single cell suspensions were then stained with conjugated monoclonal antibodies to CD45 (#103133), CD34 (#119307), CD14 (#123308) and CD11b (#101210). All the antibodies with their matched isotype controls were purchased from Biolegend (San Diego, CA, USA). Stained cells were analyzed using an LSR II flow cytometer (BD Biosciences, San Jose, CA, USA).
RNA isolation, RT-PCR, and quantitative real-time PCR
Corneas were harvested at 48 hours post injury from each group, and mRNA was isolated using the RNeasy Micro Kit (Qiagen, Germantown, MD, USA). Isolated RNA was reverse transcribed into cDNA using oligo (dT) primer and Superscript TM III (Invitrogen, Grand Island, NY, USA). Real-time PCR was performed using Taqman Universal PCR Mastermix and preformulated primers for PDL-1 (Mm00452054_m1), VTCN-1 (Mm00628552_m1), Ceacam-1 (Mm04204476_m1), CD200 (Mm00487740_m1), IL-1β (Mm00434228_m1), and glyceraldehype-3-phosphate dehydrogenase (GAPDH, Mm99999915_g1) (Thermofisher Scientific, Waltham, MA, USA). The results were analyzed by the comparative threshold cycle method and normalized to GAPDH as an internal control.
Immunofluorescence and histopathology
Freshly excised corneas were washed in PBS and fixed with 4% paraformaldehyde for 20 minutes and permeabilized with 0.5% Triton X-100 for 10 minutes. Whole corneas were then immunostained with FITC-conjugated anti-CD14 (#123308) and PE-conjugated anti-CD11b (#101207) (Biolegend, San Diego, CA, USA) overnight at 4°C to detect myeloid progenitors and mounted onto slides with mounting medium (Vector Laboratories, Burlingame, CA, USA) and visualized using a confocal microscope (Leica TCS-SP5; Buffalo Grove, IL, USA) at ×20 magnification. Corneal sections fixed in 4% paraformaldehyde were stained with hematoxylin and eosin. Images were obtained using a bright field microscope (Nikon Eclipse E800; Melville, NY, USA) at ×20 magnification.
Statistical analysis
A two-tailed Student’s t-test was performed and P values <0.05 were regarded as statistically significant. Results are presented as the mean ± standard error of the mean (SEM) of at least three independent experiments. Samples sizes were estimated on the basis of previous experimental studies on corneal injury and inflammation [10, 13].
Results
Characterization of myeloid progenitor cells
As immune cells are primarily developed in lymphoid organs, single cell suspensions from bone marrow, spleen, and submandibular lymph nodes were immunostained for flow cytometry analysis as per the gating strategy shown in Supplement Figure S1. First, a population of CD14+CD11b− cells was identified (Fig. 1A) and gated for further characterization and for examining the expression of progenitor cell markers, including CD34, c-Kit, and FcγRII/III, as well as the mature myeloid cell markers, Ly6G granulocytic marker and Ly6C monocytic marker (Fig. 1B). Majority of CD14+CD11b− cells (~80%) were positive for the expression of CD34, c-Kit and FcγRII/III progenitor markers, and all (~99%) were negative for Ly6G and Ly6C mature myeloid cell markers (Fig. 1B). Based on our results, we estimate that myeloid progenitors (CD34+c-Kit+FcγRII/III+CD14+ CD11b−Ly6G−Ly6C−) constitute 4.8±1.09% of bone marrow cells, 8.7±0.29% of splenocytes, and 4.1±0.31% of lymph node cells (Fig. 1C).
Figure 1. Frequencies and phenotypic characterization of myeloid progenitor cells.
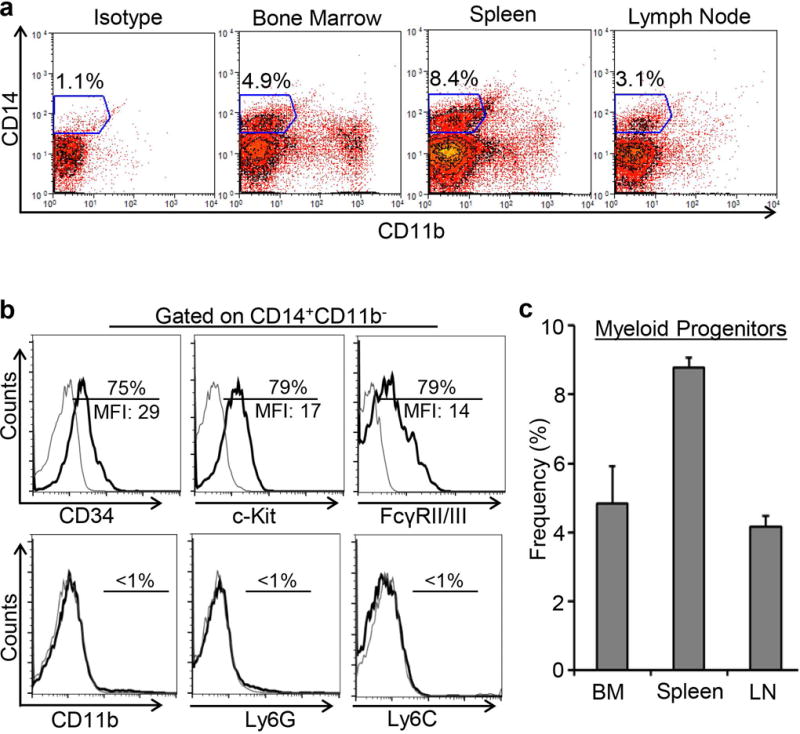
A. Representative flow cytometric dot plots showing gating strategy for selecting CD14+CD11b− cells in the bone marrow, spleen, and submandibular lymph nodes. B. Representative flow cytometric histograms demonstrating the expression of progenitor markers CD34, c-Kit and FcγRII/III, myeloid marker CD11b, monocytic marker Ly6C, and granulocytic marker Ly6G by CD14+CD11b− cells. C. Bar chart comparing the frequencies of myeloid progenitors in the bone marrow, spleen and lymph node as analyzed by flow cytometry. Representative data from 3 independent experiments are shown and each experiment consisted of 5 animals. Data is represented as mean ± SEM.
MSCs inhibit differentiation of myeloid progenitor cells in vitro
Bone marrow-derived MSCs were cultured and characterized as per criteria defined by The International Society for Cellular Therapy [9, 10]. MSCs were expanded using the plastic adherence method, and were characterized phenotypically for positive expression of SCA1 and CD29 and negative expression of CD45 and CD34 surface markers, and functionally by their ability to differentiate into adipocytes (Fig. 2A). Next, sorted myeloid progenitor cells were cultured with or without MSCs in the presence or absence of inflammatory or hematopoietic growth stimuli such as IFNγ, IL-1β or GMCSF, which have been implicated in myeloid cell differentiation[14–16]. After stimulation with IFNγ, IL-1β or GMCSF, expression of mature myeloid cell markers, including CD11b (marker for macrophages; also known as macrophage-1 antigen [Mac-1]) and Ly6G (marker for granulocytes) was investigated using flow cytometry to assess progenitor cell differentiation. Our data showed that upon stimulation with IFNγ, myeloid progenitors acquire high expression of both CD11b and Ly6G. Further analysis demonstrated a significant reduction (55%) in expression of CD11b by myeloid progenitor cells cultured with MSCs in contrast to those cultured without MSCs (MFI 4.15 ±1.04 vs. 9.2 ±1.6; p= 0.000065) (Fig. 2B), and a significant 58% suppression in expression of Ly6G in progenitors cultured with MSCs compared to progenitors cultured without MSCs (MFI 9.9 ± 0.75 vs. 23.02 ±1.14, p= 0.0000013) (Fig. 2C). Strikingly, our data that myeloid progenitors fail to express CD11c in the steady state or upon stimulation, suggest that these myeloid progenitors do not differentiate into dendritic cells (Supplement Fig. S2). Similar to effects of IFNγ on myeloid progenitors, stimulation with IL-1β and GMCSF also resulted in selective expression of CD11b by myeloid progenitors, which was significantly suppressed in myeloid progenitors co-cultured with MSCs (Supplement Fig. S3). Taken together, these data suggest that MSCs suppress acquisition of differentiation markers by myeloid progenitors and maintain these cells in an immature state in an inflammatory environment.
Figure 2. MSCs inhibit differentiation of myeloid progenitor cells in vitro.
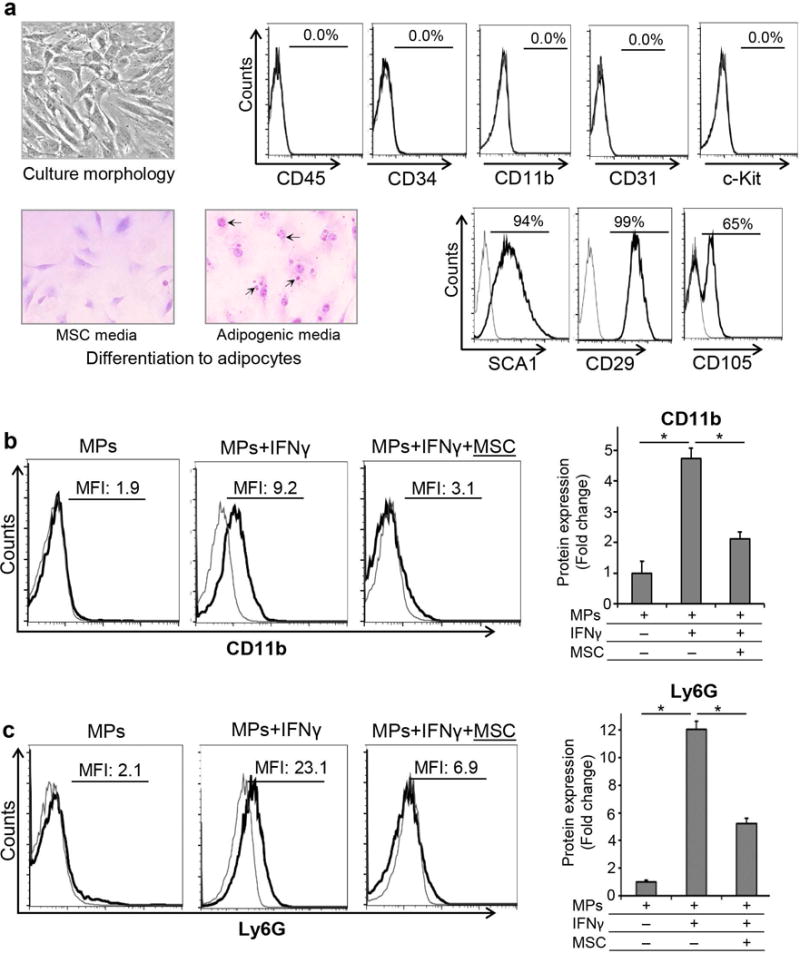
A. Left Panel: Expansion and characterization of MSCs. Microscopic images of MSCs cultured in MSC or adipogenic media (×40 magnification). Oil-Red-O staining after 2 weeks showed red colored fat vacuoles (black arrows) in the cytoplasm of MSCs cultured in adipogenic media, confirming their differentiation into adipocytes. Right Panel: Representative flow cytometry plots demonstrating the phenotype of bone marrow derived MSCs as CD45−CD34−CD11b−c-Kit−CD31−Sca-1+CD29+CD105+ cells. B. Representative flow cytometric histograms and bar chart demonstrating CD11b expression by myeloid progenitors (MPs) cultured with or without MSCs with IFNγ stimulation for 72 hours. C. Representative histograms of flow cytometric data and bar chart showing Ly6G expression by myeloid progenitors (MPs) cultured with or without MSCs with IFNγ stimulation for 72 hours. Results are representative of 3 independent experiments. Myeloid progenitors were isolated from a pool of 5–6 animals in each experiment. P values are calculated using student’s t-test and data is represented as mean ± SEM. *p< 0.0001.
MSCs interact with myeloid progenitors in a cell-cell contact-dependent manner
To delineate whether the inhibitory effect of MSCs on myeloid progenitor differentiation was through direct cell-cell contact or by MSC-secreted soluble factors, MSCs were either cultured in direct contact with isolated myeloid progenitors, or were first plated into transwell inserts and then cultured with myeloid progenitor cells with IFNγ stimulation. Expression of CD11b surface marker was assessed using flow cytometry. As shown in Figure 3A, MSCs that were cultured directly with myeloid progenitors significantly suppressed acquisition of CD11b differentiation marker by these cells. However, MSCs in the transwell chamber system failed to suppress CD11b expression by myeloid progenitors, suggesting that the suppressive function of MSCs is dependent on direct cell-cell contact rather than secretion of soluble factors by MSCs. To further explore the molecular mechanism underlying such contact-dependency, we investigated the expression of following cell membrane-bound inhibitory molecules by MSCs using real time PCR: programmed death-ligand 1 (PD-L1), a transmembrane protein, which delivers inhibitory signals to immune cells upon binding with PD-1 expressed by T cells and activated monocyte; v-set domain containing T cell activation inhibitor 1 (VTCN-1) or B7-H4, a transmembrane protein that negatively regulates the function of T cells and neutrophils [17]; CD200 (OX2), a transmembrane glycoprotein that inhibits function of myeloid immune cells [18]; and carcinoembryonic antigen-related cell adhesion molecule 1 (Ceacam-1), a cell adhesion molecule involved in contact-dependent regulation of the innate and adaptive immune responses [19] (Fig. 3B). Significantly higher mRNA expression of CD200 compared to other molecules prompted us to speculate that CD200 may be the critical ligand mediating the immunoregulatory function of MSCs. Our data further demonstrated that MSCs significantly upregulate their expression of CD200 in the inflammatory environment (Fig. 3C). Using flow cytometry, we also confirmed protein expressions of CD200 on MSCs and its receptor, CD200R1 on myeloid progenitor cells (Fig. 3D & E).
Figure 3. MSCs inhibit differentiation of myeloid progenitors in a contact-dependent manner.
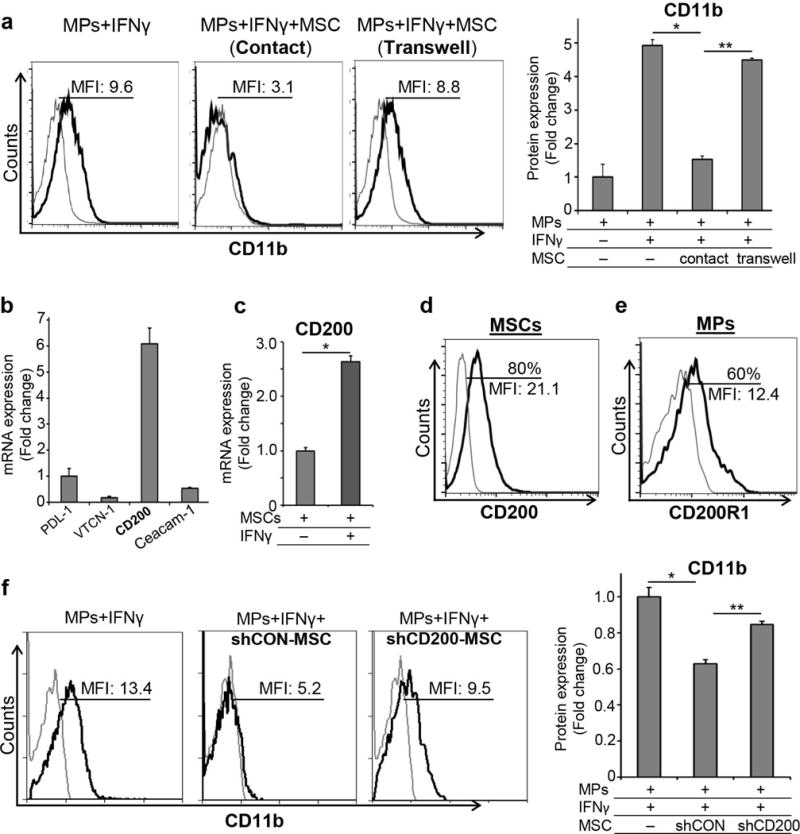
A. Representative flow cytometric histograms and bar chart demonstrating CD11b expression by myeloid progenitors (MPs) cultured with MSCs either in direct contact or using a transwell chamber system, which separated MSCs from MPs, in the presence of IFNγ for 72 hours. Results are representative of 3 independent experiments. Myeloid progenitors were isolated from a pool of 5–6 animals in each experiment. B. Real-time PCR analysis of PD-L1, VTCN-1, CD200 and Ceacam-1 mRNA expression levels by resting MSCs. C. Real-time PCR analysis of CD200 expression on resting and IFNγ-stimulated MSCs. Representative flow cytometric histograms demonstrating expression of D. CD200 on MSCs, and E. CD200R1 on myeloid progenitor cells. F. Representative flow cytometric histograms and bar chart showing CD11b expression levels in myeloid progenitors cultured with control-shRNA (shCON) or CD200-shRNA (shCD200) MSCs with IFNγ stimulation for 72 hours. Results are representative of 3 independent experiments. Myeloid progenitors were isolated from a pool of 5–6 animals in each experiment. P values are calculated using student’s t-test and data is represented as mean ± SEM. *p< 0.01, ** p< 0.001
MSCs inhibit differentiation of myeloid progenitors via CD200-CD200R1 interaction
Next, to investigate the role of CD200 in mediating the immunoregulatory function of MSCs in vitro, functional expression of CD200 on MSCs was silenced using CD200-shRNA (Supplement Fig. S4). Control-shRNA or CD200-shRNA treated MSCs were then cultured with myeloid progenitors in the presence of IFNγ. Our data regarding expression of CD11b demonstrated that CD200-shRNA-treated MSCs had 22% less ability in suppressing myeloid progenitor differentiation compared to control-shRNA-treated MSCs (p=0.008) (Fig. 3F). Compromised ability of CD200-shRNA MSCs to suppress myeloid progenitor acquisition of CD11b suggests that expression of CD200 by MSCs is critical for their inhibitory function on myeloid progenitor differentiation.
CD200 expression in MSCs is indispensable for suppression of inflammation and accumulation of undifferentiated myeloid progenitors in the inflamed tissue
Lastly, we chose a sterile inflammation in vivo model of mouse eye injury – a well-established system to study inflammation [13, 20] – to confirm the immunoregulatory effect of MSCs on myeloid progenitors. This well-characterized model provides an excellent system to study inflammation. Simple anatomy of the eye and its paucity of resident immune cells facilitate study of recruited immune cells and their contribution to the inflammatory response [21]. As demonstrated previously [10, 22], we show that MSCs administered systemically home specifically to the injured cornea (Supplement Fig. S5). Interestingly, similar to the bone marrow, spleen and lymph node, we identified a population of CD34+CD14+CD11b− myeloid progenitors in the cornea (Fig. 4A). Our immunofluorescence microscopy results also confirmed the presence of CD14+CD11b− progenitors primarily in the stromal layer of cornea (Fig. 4B). Similar to lymphoid tissue-derived progenitors, upon stimulation with IFNγ, sorted corneal myeloid progenitors expressed CD11b, and MSCs suppressed their acquisition of CD11b in vitro (Supplement Fig. S6). To determine the effect of systemic administration of MSCs on myeloid progenitor cell frequencies and tissue inflammation, mice were intravenously injected with control-shRNA or CD200-shRNA-treated MSCs 1 hour after corneal injury induction, followed by harvesting of corneas 48 hours post-injury (Fig. 4C). Our flow cytometry data demonstrated that normal (control-shRNA-treated) MSCs led to a 5-fold increase in the frequencies of corneal myeloid progenitors, while CD200-shRNA-treated MSCs failed to do so, suggesting that MSC expression of CD200 is important for expansion of myeloid progenitor cell frequencies in the inflamed tissue (Fig. 4E). Similar to our previous findings on the anti-inflammatory effect of MSCs in the inflamed tissue, our data demonstrated that normal (control-shRNA-treated) MSCs, but not CD200-silenced MSCs, have a significant suppressive effect on tissue inflammation as evidenced by reduced frequencies of CD45+ cells (Fig. 4D), decreased expression of inflammatory cytokine IL-1β (Fig. 4G) and less inflammatory cell infiltration in the corneal stroma (Fig. 4F) compared to untreated mice with corneal injury. These findings strongly suggest that MSCs suppress tissue inflammation by reducing inflammatory cell infiltration and by expanding frequencies of myeloid progenitor cells through a CD200-dependent mechanism.
Figure 4. MSCs suppress ocular inflammation through expansion of myeloid progenitor cells in a CD200-dependent manner.
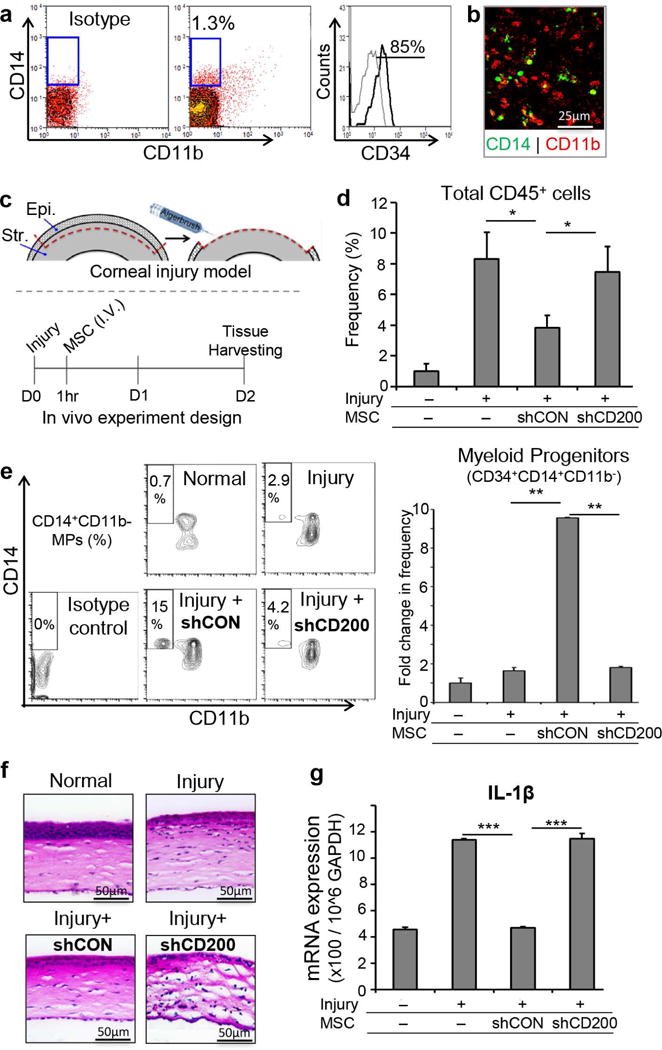
A. Representative flow cytometric plots demonstrating CD34+CD14+CD11b− myeloid progenitor in the cornea. B. Confocal microscopy image (×20 magnification) of corneal whole mount confirming the presence of CD14+ CD11b− cells in the peripheral corneal stroma (Green: CD14, Red: CD11b). C. Schematic representation of sterile injury induction in mouse and experiment timeline. Corneal epithelium and anterior stroma are mechanically removed using Algerbrush II. D. Bar chart demonstrating the frequencies of infiltrating corneal CD45+ cells in naïve mouse, injured mice without systemic MSC treatment, control-shRNA (shCON) MSC-treated and CD200-shRNA (shCD200) MSC-treated mice. E. Representative flow cytometric plots and bar chart demonstrating the frequencies of myeloid progenitors in naïve cornea, injured cornea, injured cornea with IV administration of control-shRNA-treated MSCs, and injured cornea with IV administration of CD200-shRNA-treated MSCs 48 hours after injury induction. F. H &E staining of corneal cross-sections (×20) from naïve, untreated, control-shRNA MSC-treated and CD200-shRNA MSC-treated mice demonstrating epithelial and stromal layers and inflammatory cell infiltration. G. Real-time PCR analysis of relative expression of IL-1β mRNA in naïve mice, injured mice without systemic MSC treatment, control-shRNA MSC-treated and CD200-shRNA MSC-treated mice. Results are representative of 2 independent experiments. Each group consisted of 4–5 animals in each experiment. P values are calculated using student’s t-test and data is represented as mean ± SEM. * p< 0.05, ** p< 0.01, *** p< 0.001
Discussion
The current study ascribes a novel immunoregulatory function for MSCs on myeloid progenitor cell differentiation. Our data indicate that MSCs inhibit differentiation of myeloid progenitor cells in an inflammatory environment through direct cell-cell contact. Furthermore, we demonstrate that this intercellular contact is mediated by CD200-CD200R1 interaction, and that CD200 expression by MSCs is indispensable for inhibition of myeloid progenitor differentiation and suppression of tissue inflammation.
Myeloid progenitors are precursors of mature myeloid cells, critical effector cells in innate immune response. Upregulation of pro-inflammatory cytokines such as IFNγ, IL1β and TNFα during inflammation activates steady state progenitors in the bone marrow to differentiate into myeloid effector cells [4, 23]. Myeloid progenitors are primarily found in the bone marrow and cord blood [24]. Some studies have demonstrated the presence of undifferentiated monocytes and DC precursors in non-bone marrow tissues such as spleen [25, 26]. Our findings demonstrate a population of myeloid progenitors, which in addition to the bone marrow are also present in peripheral lymphoid tissues, including spleen and lymph nodes. These progenitors express high levels of CD34, CD14, c-Kit and FcγRII/III progenitor markers, which makes them phenotypically similar to early myeloid progenitors such as common myeloid progenitors (CMPs) and granulocyte/macrophage progenitors (GMPs) [27, 28]. CMPs are thought to be precursors of common DC progenitors, which eventually give rise to DCs [27]. Our data, however, demonstrate that the myeloid progenitors identified do not express the DC marker CD11c in the steady or activated states, suggesting that these cells are not DC precursors. Rather, these progenitors acquire high levels of CD11b and Ly6G myeloid markers in the inflammatory milieu, suggesting these myeloid progenitors are phenotypically closer to GMPs that give rise to macrophages and granulocytes [1, 27, 29].
Interestingly, we find that MSCs inhibit acquisition of CD11b and Ly6G differentiation markers on myeloid progenitors. MSCs have been shown to interact with cells of both innate and adaptive immunity [30]. Recent reports on the interaction of MSCs with DC precursors have demonstrated that MSCs inhibit differentiation of peripheral blood-derived CD14+ monocytes to mature DCs [31, 32]. Here, our data show that MSCs negatively regulate both bone marrow- and peripheral lymphoid tissue-resident myeloid progenitors. MSCs maintain these cells in an undifferentiated quiescent state and further prevent their differentiation into inflammatory cells. MSCs primarily exert their immunoregulatory effects through secretion of paracrine factors such as IDO, IL-10, TGF-β and TSG6 [33, 34]. In contrast, we find that MSCs inhibit differentiation of myeloid progenitors mainly through direct cell-cell contact. The results of our study demonstrate that silencing of CD200 expression in MSCs abrogates their ability to suppress myeloid progenitor cell differentiation, suggesting that CD200-CD200R1 interaction is critical for MSCs to exert their immunoregulatory effect. CD200 or Ox-2 is a transmembrane glycoprotein, which binds to its receptor CD200R1 [35]. The CD200R family of receptors consists of 4 isoforms [36], among which CD200R1 is mainly expressed by myeloid cells and T cells [35, 37]. CD200-CD200R1 pathway plays a central role in regulation of innate immune system by inhibiting myeloid cell activation [38, 39]. We show that bone marrow-derived MSCs constitutively express CD200, and significantly upregulate CD200 expression in response to inflammatory stimuli. These results are consistent with previous studies which demonstrated that IFNγ in particular induces CD200 expression in bone marrow-derived stromal cells [40].
Finally, the functional relevance of MSC regulation of myeloid progenitor cell differentiation during inflammation was tested using a standardized mouse cornea model of sterile injury [10, 41]. Similar to the bone marrow, spleen and lymph nodes, we have identified myeloid progenitors residing in the cornea. MSCs have been shown to migrate to the sites of inflammation and promote wound repair [42, 43]. Previously, we showed that systemically administered MSCs home to the inflamed eye, and accelerate wound healing [10, 22]. Here, we show that MSCs suppress infiltration of inflammatory cells and increase the frequencies of corneal myeloid progenitors. Consistent with our in vitro findings, systemically administered CD200-shRNA-treated MSCs lose their ability to suppress differentiation of myeloid progenitors and tissue inflammation. The increase in myeloid progenitor frequencies at the inflamed site could be the result of MSC-mediated expansion of corneal resident myeloid progenitors, or due to MSCs inhibiting differentiation of recruited myeloid progenitors from the bone marrow. Early myeloid progenitors have recently been identified as immunosuppressive cells that are capable of inhibiting T cell proliferation [44]. If MSCs promote recruitment of myeloid progenitors to the inflamed tissue, suppression of inflammation could be the cumulative result of MSC-and myeloid progenitor-mediated regulation of the immune response. However, we acknowledge that further experiments will be needed to elucidate the exact mechanism by which MSCs promote myeloid progenitor frequencies at the site of inflammation.
Conclusion
In conclusion, our findings provide new insight into the immunoregulatory effect of MSCs on myeloid progenitor cell differentiation. Herein, we show that MSCs suppress inflammation not only by regulating inflammatory cell infiltration, but also by preventing differentiation of early myeloid precursors into inflammatory cells. Our data further supports a critical role for CD200 expressed by MSCs in regulating function of myeloid progenitors and thus inhibiting inflammatory response. These observations could provide a framework for the development of potential CD200-based therapeutics that could effectively modulate the generation of innate immune cells and inhibit inflammation at early stage.
Supplementary Material
Acknowledgments
The authors thank Balaraj B. Menon, PhD for helpful scientific discussions and editorial contribution in the preparation of this manuscript.
This work was supported in part by the National Institutes of Health (EY024602 to S.K.C. and core grant P30EY003790), and the Department of defense (W81XWH-15-1-0024 to S.K.C.).
Footnotes
Afsaneh Amouzegar: collection and assembly of data, data analysis and interpretation, manuscript writing
Sharad K. Mittal: collection and assembly of data, data analysis and interpretation
Anuradha Sahu: data analysis and interpretation
Srikant K. Sahu: data analysis and interpretation
Sunil K. Chauhan: conception and design, data analysis and interpretation, manuscript writing and final approval of manuscript
Disclosure of Potential Conflicts of Interest: The authors declare no potential conflicts of interest.
References
- 1.Kondo M. Lymphoid and myeloid lineage commitment in multipotent hematopoietic progenitors. Immunol Rev. 2010;238:37–46. doi: 10.1111/j.1600-065X.2010.00963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li T, Wu Y. Paracrine molecules of mesenchymal stem cells for hematopoietic stem cell niche. Bone Marrow Res. 2011;2011:353878. doi: 10.1155/2011/353878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.da Silva CL, Goncalves R, dos Santos F, et al. Dynamic cell-cell interactions between cord blood haematopoietic progenitors and the cellular niche are essential for the expansion of CD34+, CD34+CD38- and early lymphoid CD7+ cells. J Tissue Eng Regen Med. 2010;4:149–158. doi: 10.1002/term.226. [DOI] [PubMed] [Google Scholar]
- 4.Maltby S, Hansbro NG, Tay HL, et al. Production and differentiation of myeloid cells driven by proinflammatory cytokines in response to acute pneumovirus infection in mice. J Immunol. 2014;193:4072–4082. doi: 10.4049/jimmunol.1400669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.King KY, Goodell MA. Inflammatory modulation of HSCs: viewing the HSC as a foundation for the immune response. Nat Rev Immunol. 2011;11:685–692. doi: 10.1038/nri3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 7.Hall SR, Tsoyi K, Ith B, et al. Mesenchymal stromal cells improve survival during sepsis in the absence of heme oxygenase-1: the importance of neutrophils. Stem Cells. 2013;31:397–407. doi: 10.1002/stem.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bernardo ME, Fibbe WE. Mesenchymal stromal cells: sensors and switchers of inflammation. Cell Stem Cell. 2013;13:392–402. doi: 10.1016/j.stem.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 9.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 10.Lan Y, Kodati S, Lee HS, et al. Kinetics and function of mesenchymal stem cells in corneal injury. Invest Ophthalmol Vis Sci. 2012;53:3638–3644. doi: 10.1167/iovs.11-9311. [DOI] [PubMed] [Google Scholar]
- 11.Basu S, Hertsenberg AJ, Funderburgh ML, et al. Human limbal biopsy-derived stromal stem cells prevent corneal scarring. Sci Transl Med. 2014;6:266ra172. doi: 10.1126/scitranslmed.3009644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mittal SK, Omoto M, Amouzegar A, et al. Restoration of Corneal Transparency by Mesenchymal Stem Cells. Stem Cell Reports. 2016;7:583–590. doi: 10.1016/j.stemcr.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee RH, Yu JM, Foskett AM, et al. TSG-6 as a biomarker to predict efficacy of human mesenchymal stem/progenitor cells (hMSCs) in modulating sterile inflammation in vivo. Proc Natl Acad Sci U S A. 2014;111:16766–16771. doi: 10.1073/pnas.1416121111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Bruin AM, Voermans C, Nolte MA. Impact of interferon-gamma on hematopoiesis. Blood. 2014;124:2479–2486. doi: 10.1182/blood-2014-04-568451. [DOI] [PubMed] [Google Scholar]
- 15.Orelio C, Haak E, Peeters M, et al. Interleukin-1-mediated hematopoietic cell regulation in the aorta-gonad-mesonephros region of the mouse embryo. Blood. 2008;112:4895–4904. doi: 10.1182/blood-2007-12-123836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Metcalf D. Hematopoietic cytokines. Blood. 2008;111:485–491. doi: 10.1182/blood-2007-03-079681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ceeraz S, Nowak EC, Noelle RJ. B7 family checkpoint regulators in immune regulation and disease. Trends Immunol. 2013;34:556–563. doi: 10.1016/j.it.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wright GJ, Cherwinski H, Foster-Cuevas M, et al. Characterization of the CD200 receptor family in mice and humans and their interactions with CD200. J Immunol. 2003;171:3034–3046. doi: 10.4049/jimmunol.171.6.3034. [DOI] [PubMed] [Google Scholar]
- 19.Gray-Owen SD, Blumberg RS. CEACAM1: contact-dependent control of immunity. Nat Rev Immunol. 2006;6:433–446. doi: 10.1038/nri1864. [DOI] [PubMed] [Google Scholar]
- 20.Oh JY, Roddy GW, Choi H, et al. Anti-inflammatory protein TSG-6 reduces inflammatory damage to the cornea following chemical and mechanical injury. Proc Natl Acad Sci U S A. 2010;107:16875–16880. doi: 10.1073/pnas.1012451107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gronert K. Ocular Inflammation Models. In: Serhan CNWP, Gilroy DW, editors. Fundamentals of Inflammation. Cambridge University Press; 2010. pp. 413–426. [Google Scholar]
- 22.Omoto M, Katikireddy KR, Rezazadeh A, et al. Mesenchymal stem cells home to inflamed ocular surface and suppress allosensitization in corneal transplantation. Invest Ophthalmol Vis Sci. 2014;55:6631–6638. doi: 10.1167/iovs.14-15413. [DOI] [PubMed] [Google Scholar]
- 23.Takizawa H, Boettcher S, Manz MG. Demand-adapted regulation of early hematopoiesis in infection and inflammation. Blood. 2012;119:2991–3002. doi: 10.1182/blood-2011-12-380113. [DOI] [PubMed] [Google Scholar]
- 24.Lee J, Breton G, Oliveira TY, et al. Restricted dendritic cell and monocyte progenitors in human cord blood and bone marrow. J Exp Med. 2015;212:385–399. doi: 10.1084/jem.20141442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hey YY, O’Neill HC. Murine spleen contains a diversity of myeloid and dendritic cells distinct in antigen presenting function. J Cell Mol Med. 2012;16:2611–2619. doi: 10.1111/j.1582-4934.2012.01608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Swirski FK, Nahrendorf M, Etzrodt M, et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612–616. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akashi K, Traver D, Miyamoto T, et al. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature. 2000;404:193–197. doi: 10.1038/35004599. [DOI] [PubMed] [Google Scholar]
- 28.Manz MG, Miyamoto T, Akashi K, et al. Prospective isolation of human clonogenic common myeloid progenitors. Proc Natl Acad Sci U S A. 2002;99:11872–11877. doi: 10.1073/pnas.172384399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hettinger J, Richards DM, Hansson J, et al. Origin of monocytes and macrophages in a committed progenitor. Nat Immunol. 2013;14:821–830. doi: 10.1038/ni.2638. [DOI] [PubMed] [Google Scholar]
- 30.Chen X, Armstrong MA, Li G. Mesenchymal stem cells in immunoregulation. Immunol Cell Biol. 2006;84:413–421. doi: 10.1111/j.1440-1711.2006.01458.x. [DOI] [PubMed] [Google Scholar]
- 31.Jiang XX, Zhang Y, Liu B, et al. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood. 2005;105:4120–4126. doi: 10.1182/blood-2004-02-0586. [DOI] [PubMed] [Google Scholar]
- 32.Nauta AJ, Kruisselbrink AB, Lurvink E, et al. Mesenchymal stem cells inhibit generation and function of both CD34+-derived and monocyte-derived dendritic cells. J Immunol. 2006;177:2080–2087. doi: 10.4049/jimmunol.177.4.2080. [DOI] [PubMed] [Google Scholar]
- 33.Choi H, Lee RH, Bazhanov N, et al. Anti-inflammatory protein TSG-6 secreted by activated MSCs attenuates zymosan-induced mouse peritonitis by decreasing TLR2/NF-kappaB signaling in resident macrophages. Blood. 2011;118:330–338. doi: 10.1182/blood-2010-12-327353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kyurkchiev D, Bochev I, Ivanova-Todorova E, et al. Secretion of immunoregulatory cytokines by mesenchymal stem cells. World J Stem Cells. 2014;6:552–570. doi: 10.4252/wjsc.v6.i5.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wright GJ, Cherwinski H, Foster-Cuevas M, et al. Characterization of the CD200 receptor family in mice and humans and their interactions with CD200. J Immunol. 2003;171:3034–3046. doi: 10.4049/jimmunol.171.6.3034. [DOI] [PubMed] [Google Scholar]
- 36.Gorczynski R, Chen Z, Kai Y, et al. CD200 is a ligand for all members of the CD200R family of immunoregulatory molecules. J Immunol. 2004;172:7744–7749. doi: 10.4049/jimmunol.172.12.7744. [DOI] [PubMed] [Google Scholar]
- 37.Wright GJ, Puklavec MJ, Willis AC, et al. Lymphoid/neuronal cell surface OX2 glycoprotein recognizes a novel receptor on macrophages implicated in the control of their function. Immunity. 2000;13:233–242. doi: 10.1016/s1074-7613(00)00023-6. [DOI] [PubMed] [Google Scholar]
- 38.Jenmalm MC, Cherwinski H, Bowman EP, et al. Regulation of myeloid cell function through the CD200 receptor. J Immunol. 2006;176:191–199. doi: 10.4049/jimmunol.176.1.191. [DOI] [PubMed] [Google Scholar]
- 39.Nathan C, Muller WA. Putting the brakes on innate immunity: a regulatory role for CD200? Nat Immunol. 2001;2:17–19. doi: 10.1038/83124. [DOI] [PubMed] [Google Scholar]
- 40.Najar M, Raicevic G, Jebbawi F, et al. Characterization and functionality of the CD200-CD200R system during mesenchymal stromal cell interactions with T-lymphocytes. Immunol Lett. 2012;146:50–56. doi: 10.1016/j.imlet.2012.04.017. [DOI] [PubMed] [Google Scholar]
- 41.Basu S, Hertsenberg AJ, Funderburgh ML, et al. Human limbal biopsy-derived stromal stem cells prevent corneal scarring. Sci Transl Med. 2014;6:266ra172. doi: 10.1126/scitranslmed.3009644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karp JM, Leng Teo GS. Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell. 2009;4:206–216. doi: 10.1016/j.stem.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 43.Hong HS, Lee J, Lee E, et al. A new role of substance P as an injury-inducible messenger for mobilization of CD29(+) stromal-like cells. Nat Med. 2009;15:425–435. doi: 10.1038/nm.1909. [DOI] [PubMed] [Google Scholar]
- 44.Pu S, Qin B, He H, et al. Identification of early myeloid progenitors as immunosuppressive cells. Sci Rep. 2016;6:23115. doi: 10.1038/srep23115. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


