Abstract
Background
Implantable cardioverter defibrillators (ICD) provide a significant mortality benefit for appropriately selected patients with advanced heart failure. ICDs are associated with a mortality benefit when used in patients with a pulsatile left ventricular assist device (LVAD). However it is unclear whether patients with a continuous flow LVAD (CF-LVAD) derive the same benefit. This study sought to determine if the presence of an ICD provided a mortality benefit during CFLVAD support as a bridge to transplantation.
Methods
Patients were identified in the United Network for Organ Sharing (UNOS) registry that underwent LVAD implantation as bridge to transplantation from May 2004 and April 2014, with follow-up through June 2014. The primary outcome was freedom from death while on CF-LVAD support with adjustment for complications requiring UNOS listing status upgrade. Secondary endpoints included freedom from delisting while on CF-LVAD support and incidence of transplantation.
Results
2,990 patients composed the study cohort and propensity score matching identified 1,012 patients with similar propensity scores. There was no difference in survival during device support between patients with and without an ICD (Hazard Ratio [HR] 1.20, 95% Confidence Interval [CI] 0.66-2.17, p=0.55). Adjusting for device complications requiring a UNOS listing status upgrade had minimal influence (HR 1.11, 95% CI 0.60-2.05, p=0.74). There was no increased risk of delisting due to being too sick for those with an ICD (HR 1.08, 95% CI 0.63-1.86, p=0.78). Likewise, the probability of transplantation was similar (HR 1.05, 95% CI 0.87-1.27, p=0.62).
Conclusions
Among patients bridged to transplantation with a CF-LVAD, the presence of an ICD did not reduce mortality.
Introduction
Stage D heart failure (HF) impacts over 250,000 Americans, decreasing their quality of life and carrying 5-year mortality greater than many cancers. (1) This population has an increased risk of ventricular arrhythmias (VA) and accordingly implantable cardioverter defibrillators (ICD) are recommended for many patients. The HRS/ACC/AHA guidelines provide a Class I recommendation for ICD therapy for NYHA Class II and III patients with a left ventricular ejection fraction less than 35%, however ICD therapy is not indicated (Class III recommendation) for NYHA Class IV patients with drug-refractory congestive heart failure who are not candidates for heart transplantation or patients with less than one year of life expectancy. (2) Left ventricular assist devices (LVAD) have become the most common form of durable heart replacement therapy in the United States (1), with one year survival now 80% in the INTERMACS registry. (3) Furthermore, more than 40% of patients who undergo heart transplantation have been bridged to transplant with an LVAD. (4) As many as half of patients with LVADs have VA following implantation (5), which are associated with increased right ventricular (RV) failure and mortality.
Major societal guidelines do not address ICD use in LVAD patients and data is inconsistent, limited to predominantly single center analyses. Studies with primarily pulsatile LVADs have demonstrated a mortality benefit associated with ICDs during LVAD support (6,7); however this finding has not been replicated with continuous-flow LVADs (CF-LVAD). (8-10) Two recent meta-analyses using similar data (292 patients shared) have shown a non-statistically significant trend towards decreased mortality associated with ICD use in CF-LVAD patients: one with 361 patients with a CF-LVAD (68% with an ICD, RR: 0.76; 95% CI: 0.51-1.12) (11) and the other with 292 patients (70% with an ICD, OR 0.63, 95% CI 0.33–1.18).(12) This study sought to determine if the presence of an ICD provided a mortality benefit during CFLVAD support as a bridge to transplantation.
Methods
Patient Selection
The United Network for Organ Sharing (UNOS) registry and mechanical circulatory support device dataset were analyzed for all patients bridged to transplantation with a CF-LVAD between May 2004 and April 2014. Follow-up data were collected through June 2014. Adult candidates (age ≥18 years) registered for a single organ primary heart transplant who received a Food and Drug Administration approved CF-LVAD were identified (Supplemental Figure 1) in the UNOS registry. Devices were limited to the Heartmate II (Thoratec/St. Jude, Pleasanton, CA) and Heartware HVAD (Heartware, Framingham, MA). Patients who required temporary left sided mechanical circulatory support, BiVAD, or total artificial heart were excluded from the analysis. Patients with incomplete data were excluded from the analysis (imputation was not used). Similarly, patients who received an ICD after LVAD implantation were excluded as the date of ICD implant was not available and this would have introduced bias due to crossover. Patients were analyzed from the date of LVAD implantation to transplant, death, or delisting (due to being too sick). The primary outcome was freedom from death while on LVAD support. The primary outcome was also analyzed adjusting for complications requiring UNOS listing status upgrade. Secondary endpoints included freedom from delisting due to being too sick while on LVAD support and incidence of transplantation. Studies involving this dataset have been determined to be exempt from review by the Institutional Review Board of Columbia University Medical Center.
Propensity Score Matching
The ICD and non-ICD cohorts differed in baseline characteristics (Table 1). In an effort to create comparable groups of patients, propensity score matching was performed based on covariates (selected a priori) that were available in the UNOS registry. The propensity score was calculated using a non-parsimonious multivariable logistic regression model, including clinical (etiology of heart failure, body mass index, diabetes, renal function, cardiac index, pulmonary vascular resistance, panel reactive antibodies >10%, UNOS listing status at the time of LVAD implantation, ventilator use at listing, functional status at listing, LVAD type, history of cerebrovascular disease, and history of cigarette use) and demographic characteristics (age, sex, race). Notable baseline covariates that were not available in the UNOS registry for inclusion in the propensity score were history of ventricular arrhythmia, INTERMACS profile, right ventricular function, serum albumin, antiarrhythmic medications, and right atrial pressure. Patients were matched 1:1 using a greedy matching algorithm (nearest match without replacement) based on the propensity score of each patient. A caliper width of 20% of the standard deviation of the logit of the propensity score was used, which eliminates 99% of the bias due to measured confounding variables. (13) An absolute standardized difference of less than 10% was considered to represent relative balance. (14)
Table 1.
Study cohort baseline characteristics
| No ICD | ICD | ASD (%) | |
|---|---|---|---|
| n | 515 | 2,475 | |
| Age | 52 (39-60) | 56 (47-63) | 39.5 |
| Male | 370 (71.8) | 1,946 (78.6) | 12.0 |
| ICM | 216 (41.9) | 1,034 (41.7) | 0.1 |
| Race | 12.2 | ||
| White | 343 (66.6) | 1,624 (65.6) | |
| Black | 116 (22.5) | 629 (25.4) | |
| Other | 56 (10.9) | 222 (9.0) | |
| Device Type | 10.3 | ||
| Heartmate II | 441 (85.6) | 2,084 (84.2) | |
| Heartware HVAD | 74 (14.4) | 391 (15.8) | |
| Functional Status at Listing | 20.2 | ||
| Disabled & Hospitalized | 166 (32.2) | 642 (25.9) | |
| Needs Assistance | 205 (39.8) | 918 (37.1) | |
| Cares for Self | 144 (28.0) | 915 (37.0) | |
| Ventilator Use at Listing | 13 (2.5) | 36 (1.5) | 8.0 |
| Renal Function | 18.7 | ||
| GFR>60 | 319 (61.9) | 1,384 (55.9) | |
| CKD Stage III | 156 (30.3) | 949 (38.3) | |
| CKD Stage IV | 17 (3.3) | 74 (3.0) | |
| CKD Stage V, Not on HD | 1 (0.2) | 6 (0.2) | |
| Dialysis | 22 (4.3) | 62 (2.5) | |
| BMI at Listing | 27.4 (24.1-31.5) | 28.3 (25.0-32.1) | 16.8 |
| Diabetes | 150 (29.1) | 839 (33.9) | 10.3 |
| Prior Smoker | 238 (46.2) | 1,383 (55.9) | 12.0 |
| Cerebrovascular Disease | 24 (4.7) | 155 (6.3) | 7.1 |
| UNOS Status at LVAD Implant | 13.1 | ||
| Status 1A | 185 (35.9) | 873 (35.3) | |
| Status 1B | 277 (53.8) | 1,256 (50.8) | |
| Status 2 | 33 (6.4) | 246 (9.9) | |
| Temporarily Inactive* | 20 (3.9) | 100 (4.0) | |
| PRA >10% | 18 (3.5) | 57 (2.3) | 7.0 |
| PVR (Wood units) | 14.3 | ||
| PVR < 1.5 | 139 (27.0) | 548 (22.1) | |
| 1.5 ≤ PVR < 3.0 | 229 (44.5) | 1,091 (44.1) | |
| 3.0 ≤ PVR < 4.5 | 97 (18.8) | 519 (21.0) | |
| PVR > 4.5 | 50 (9.7) | 317 (12.8) | |
| CI (L/min/m2) | 2.16 (1.74-2.59) | 2.08 (1.72-2.47) | 12.0 |
Temporarily Inactive Patients limited to those too sick or with an LVAD complication.
Data presented as Count (%) or Median (interquartile range). ASD=Absolute Standardized Difference; BMI=Body Mass Index; CI=Cardiac Index; CKD=Chronic Kidney Disease; ICD=Implantable Cardioverter Defibrillator; ICM=Ischemic Cardiomyopathy; PRA=Panel Reactive Antibodies; PVR=Pulmonary Vascular Resistance
Statistical Analysis
Demographic and clinical variables were expressed as mean (± standard deviation) for continuous variables and count (with percentage) for categorical variables. Absolute standardized differences were estimated for all the baseline covariates (between those with and without an ICD) before and after matching to assess group balance. An absolute standardized covariable difference of less than 10% between baseline ICD and no ICD groups was considered to be balanced. (15) Group comparisons were made with McNemar's test and the Wilcoxon rank-sum test where appropriate. Kaplan-Meier survival analysis, unadjusted, and adjusted Cox proportional-hazards regression (stratifying on the matched pairs) were performed to determine if survival differed by ICD status. Cumulative incidence functions of transplantation and delisting were estimated using competing risks regression, with transplantation or delisting and death serving as competing events. A sensitivity analysis was performed using the entire cohort through stratification into quintiles by propensity score (16) and adjusted Cox proportional-hazards regression (using the propensity score and ICD status only to avoid collinearity). This has been suggested to be superior to matching alone for estimating the treatment effect. (17) A two-tailed p-value of less than 0.05 was considered significant. Analyses were performed using SAS version 9.4 (SAS Institute, Inc., Cary, North Carolina). Figures were created using SAS version 9.4 (SAS Institute, Inc., Cary, North Carolina) and R version 3.3.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
A total of 3,821 patients were identified of which 3,025 (79.2%) had an ICD and 796 (20.8%) did not. Of the 2,990 patients that met the study entry criteria, 2,475 (82.8%) had an ICD and 515 (17.2%) did not have an ICD. The baseline characteristics for the entire cohort are displayed in Table 1, with differences in most baseline characteristics as evidenced by an absolute standardized difference (ASD) of greater than 10%. Propensity score matching created a total cohort of 1,012 patients, 506 patients with and 506 patients without an ICD. The ASD was less than 10% for all baseline characteristics, indicating suitable matching (Table 2).
Table 2.
Propensity score matched cohort baseline characteristics
| No ICD | ICD | ASD (%) | |
|---|---|---|---|
| n | 506 | 506 | |
| Age | 52 (40-60) | 51 (38-59) | 3.0 |
| Male | 366 (72.3) | 360 (71.2) | 1.6 |
| ICM | 213 (42.1) | 215 (42.5) | 0.8 |
| Race | 6.8 | ||
| White | 338 (66.8) | 328 (64.8) | |
| Black | 113 (22.3) | 112 (22.1) | |
| Other | 55 (10.9) | 66 (13.0) | |
| Device Type | 3.0 | ||
| Heartmate II | 433 (85.6) | 441 (87.1) | |
| Heartware HVAD | 73 (14.4) | 65 (12.9) | |
| Functional Status at Listing | 3.7 | ||
| Disabled & Hospitalized | 159 (31.4) | 158 (31.2) | |
| Needs Assistance | 204 (40.3) | 197 (38.9) | |
| Cares for Self | 143 (28.3) | 151 (29.9) | |
| Ventilator Use at Listing | 11 (2.2) | 10 (2.0) | 1.0 |
| Renal Function | 6.6 | ||
| GFR>60 | 314 (62.0) | 311 (61.5) | |
| CKD Stage III | 155 (30.6) | 150 (29.6) | |
| CKD Stage IV | 16 (3.2) | 18 (3.6) | |
| CKD Stage V, Not on HD | 1 (0.2) | 2 (0.4) | |
| Dialysis | 20 (4.0) | 25 (4.9) | |
| BMI at Listing | 27.4 (24.3-31.6) | 27.5 (24.1-31.0) | 3.0 |
| Diabetes | 147 (29.1) | 141 (27.9) | 3.0 |
| Prior Smoker | 238 (47.0) | 238 (47.0) | <0.1 |
| Cerebrovascular Disease | 24 (4.7) | 20 (4.0) | 4.0 |
| UNOS Status at LVAD Implant | 8.4 | ||
| Status 1A | 182 (36.0) | 184 (36.4) | |
| Status 1B | 272 (53.8) | 282 (55.7) | |
| Status 2 | 32 (6.3) | 24 (4.7) | |
| Temporarily Inactive* | 20 (3.9) | 16 (3.2) | |
| PRA >10% | 18 (3.6) | 23 (4.6) | 5.0 |
| PVR (Wood units) | 4.6 | ||
| PVR < 1.5 | 135 (26.7) | 141 (27.9) | |
| 1.5 ≤ PVR < 3.0 | 224 (44.3) | 228 (45.1) | |
| 3.0 ≤ PVR < 4.5 | 97 (19.2) | 91 (18.0) | |
| PVR > 4.5 | 50 (9.9) | 46 (9.1) | |
| CI (L/min/m2) | 2.16 (1.74-2.59) | 2.12 (1.74-2.61) | 1.6 |
Temporarily Inactive Patients limited to those too sick or with an LVAD complication.
Data presented as Count (%) or Median (interquartile range). ASD=Absolute Standardized Difference; BMI=Body Mass Index; CI=Cardiac Index; CKD=Chronic Kidney Disease; ICD=Implantable Cardioverter Defibrillator; ICM=Ischemic Cardiomyopathy; PRA=Panel Reactive Antibodies; PVR=Pulmonary Vascular Resistance
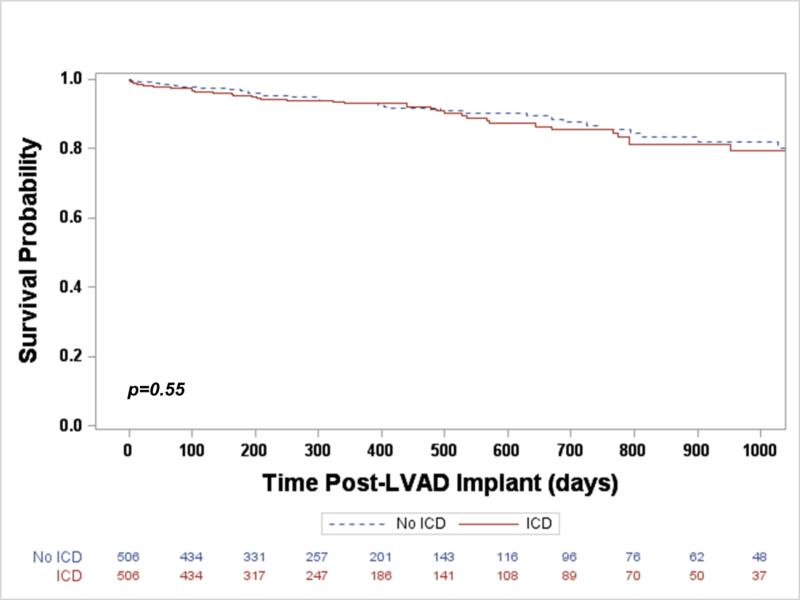
The median duration of CF-LVAD support was similar for both the ICD and non-ICD groups (287.5 days vs. 305.5 days, p=0.34). Patients with an ICD had a similar risk of death during LVAD support when compared to those without an ICD (Hazard Ratio [HR] 1.20, 95% Confidence Interval [CI] 0.66-2.17, p=0.55, Figure 1). Adjusting for post-implant device complications requiring UNOS listing status upgrade (including ventricular arrhythmia), the risk of death remained similar between groups (HR 1.11, 95% CI 0.60-2.05, p=0.74).
Figure 1.
Freedom from death during LVAD support
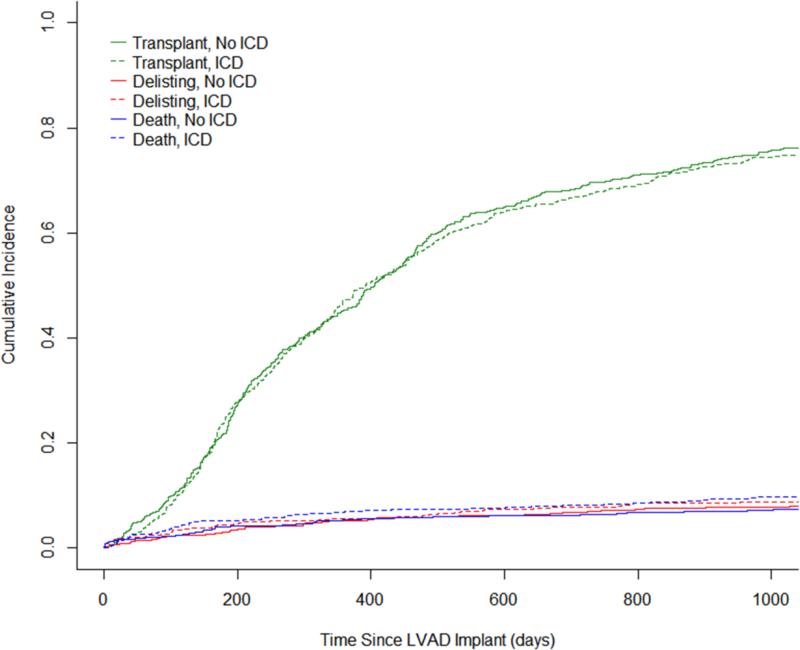
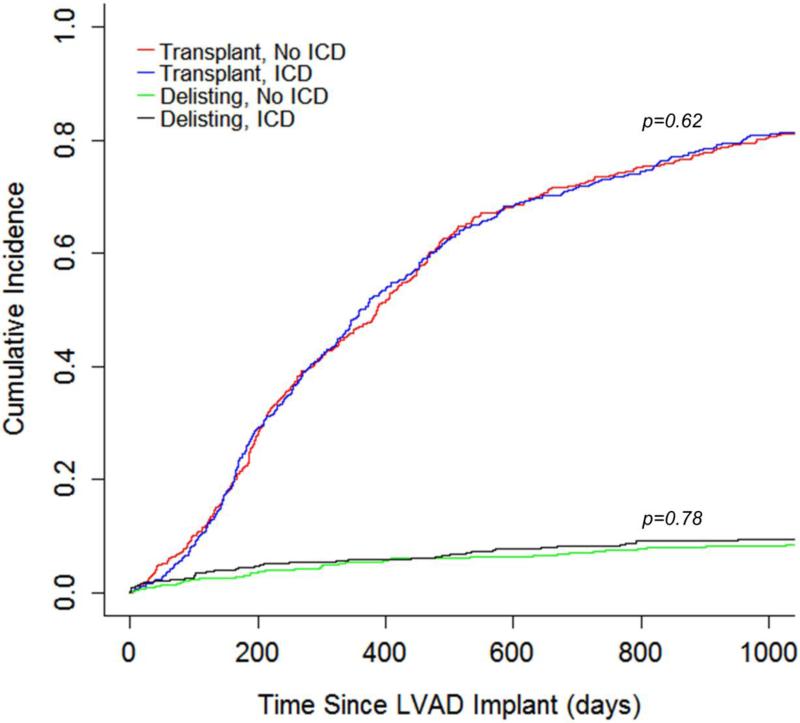
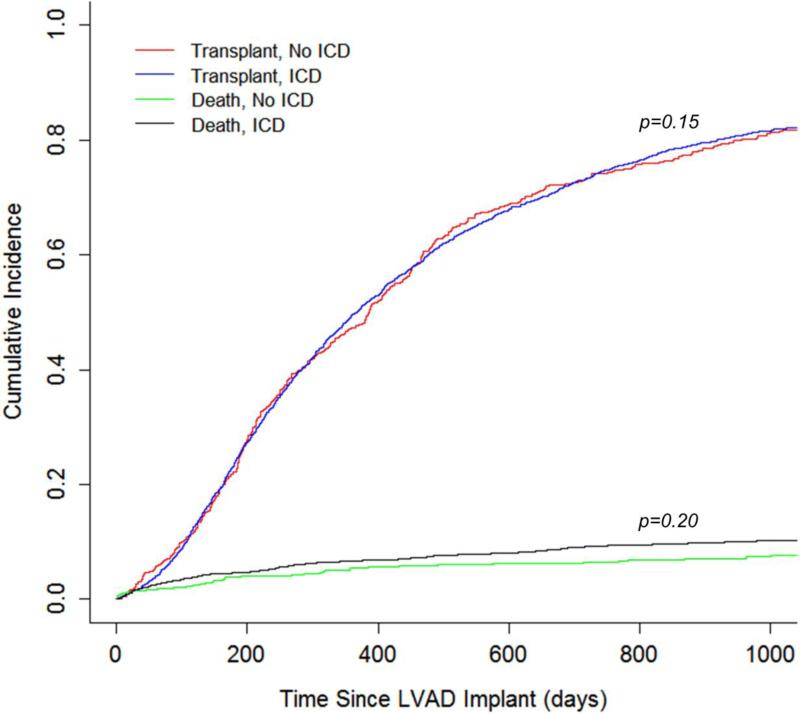
Recognizing that there may be unmeasured covariates contributing to the outcome, the cohort was analyzed for the risk of being delisted due to being too sick for transplant in an attempt to account for unmeasured covariates. A cause-specific hazard model was created treating death and transplantation as competing events (Figure 2) and did not demonstrate an increased risk of delisting for patients with an ICD (HR 1.08, 95% CI 0.63-1.86, p=0.78, Figure 3). Similarly, the likelihood of transplantation did not differ between patients with an ICD and those without an ICD (HR 1.05, 95% CI 0.87-1.27, p=0.62, Figure 3).
Figure 2.
Competing risks plot for events during LVAD support
Figure 3.
Incidence of Transplant and Delisting During LVAD Support
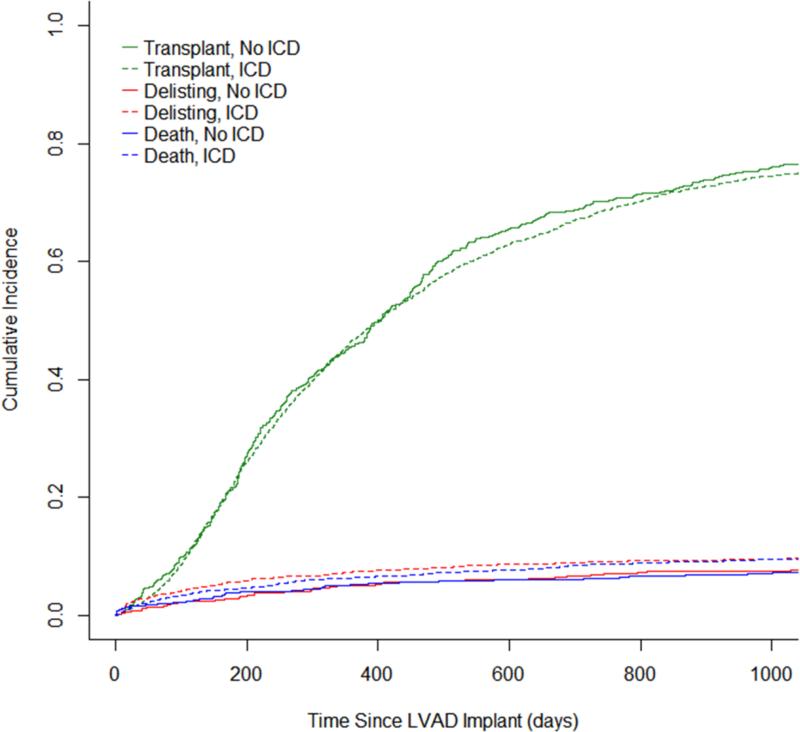
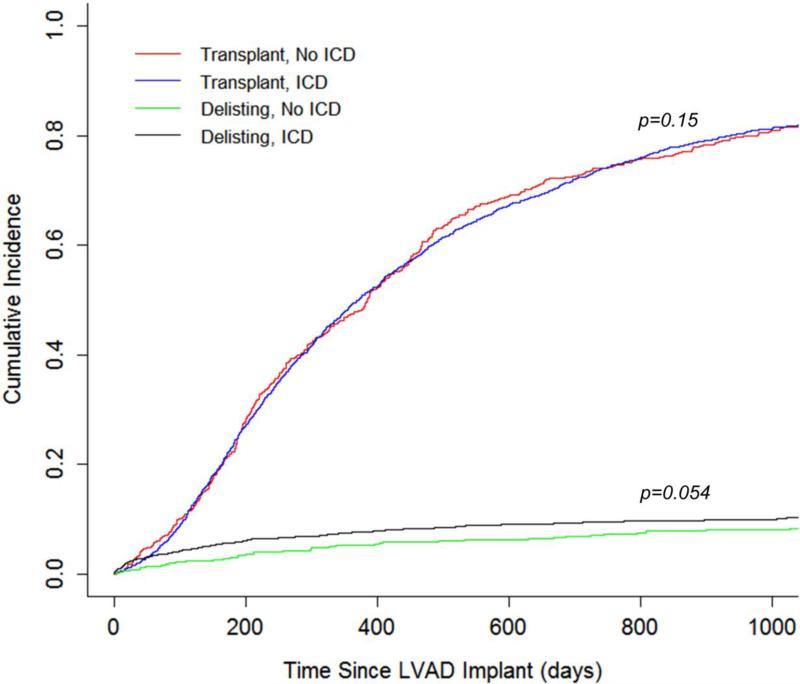
A sensitivity analysis was performed for the full study cohort of 2,990 using the propensity score to stratify and adjust a Cox proportional hazard model (Figure 4A). This analysis similarly demonstrated that patients with an ICD did not have a decreased risk of mortality during LVAD support (HR 1.24 [No ICD reference], 95% CI 0.89-1.71, p=0.20, Figure 4B). There was a trend towards an increased risk of delisting due to being too sick for patients with an ICD compared to those without an ICD (HR 1.38, 95% CI 0.99-1.91, p=0.054, Figure 4C).
Figure 4.
Sensitivity Analysis (A) Competing Risks Plot for Post-LVAD Implant Outcomes (B) Cumulative Incidence of Transplantation & Death (C) Cumulative Incidence of Transplantation & Delisting
Discussion
ICDs are frequently present at the time of LVAD implantation (79.2% in this study); however clinicians are left with a decision regarding ICD implantation for the remaining subset of patients. Ventricular arrhythmias are common in patients with advanced HF and as many as half of patients with an LVAD will experience a VA during device support. (18) While not all VA require treatment, nor do all VA result in death, there is a significant mortality benefit from ICDs in appropriately selected patients with heart failure (29% risk reduction). (19) Current societal guidelines do not address LVADs, but do provide a Class IIa recommendation for ICD implantation in non-hospitalized patients awaiting transplantation. (2) As time on the transplant waitlist continues to increase (20), the following question frequently arises: do patients with CF-LVAD derive the same mortality benefit while on device support by having an ICD? This study demonstrated that among patients bridged to transplantation with a CF-LVAD the presence of an ICD was not associated with a decreased risk of morality during device support.
There are several potential explanations for this finding. The lack of mortality benefit from an ICD may simply be artifact due to a factor not measured in the UNOS registry. The presence of an ICD may be a marker of long-standing HF and identify a sicker patient population; while patients in this study were suitably propensity score matched based on measured covariates, the increased risk of delisting due to being too sick in the sensitivity analysis (but not in the propensity score matched analysis) suggested differences in unmeasured variables. Future analysis including unmeasured variables (such as history of VA, right ventricular function, and INTERMACS profile) may help identify patients who derive a survival benefit from an ICD during CF-LVAD support. Unlike medically-treated HF patients, those with a CF-LVAD do not typically develop acute hemodynamic compromise from a VA. While VAs in LVAD recipients are not tolerated for prolonged periods due to the development of RV failure, a continuous-flow LVAD often provides sufficient hemodynamic support even in the absence of native cardiac activity to allow a patient without successful cardioversion (with or without ICD) to present for care. (21,22) Lastly, it is possible that ICD firing to terminate VA that might have terminated spontaneously is detrimental in patients with a CF-LVAD. ICD discharge has been linked to echocardiographic RV dysfunction (23) and when recurrent, may precipitate RV failure. (5) Unfortunately due to the limitations of the UNOS registry this could not be examined in the present study and is speculative. A prospective study of permissive ICD programming will help to answer this question.
Our findings differ from prior studies reporting an associated survival benefit for patients with an ICD after LVAD. (6,7,11) Importantly, we restricted our analysis to those patients in the UNOS registry with contemporary continuous-flow LVADs as opposed to these studies that included mostly patients with pulsatile-flow pumps, where the LVAD function may be more dependent of native cardiac activity. It is important to note that our data do not support deactivation of ICD therapies after LVAD implant. Furthermore, for patients who have received appropriate shocks whether before or after LVAD-implant, we believe maintenance of ICD therapy is important to prevent the morbidities associated with prolonged VA. As such, we advocate for generator changes in patients with prior VA if required after LVAD implantation. However, as ours is a relatively large analysis of exclusively continuous-flow LVAD patients we believe the findings presented here are sufficient to question the utility of primary prevention ICDs in patients with a continuous-flow LVAD awaiting transplant. Importantly, bleeding and infectious complications related to ICD implantation may carry higher risk in patients supported by durable LVAD. To definitively answer these questions multi-center prospective analyses should be performed.
This study was limited by the retrospective nature of the data. The UNOS dataset that was used is high-quality in that for all U.S. transplant centers data submission is mandatory by law; however our analysis was limited to the data collected. The data available in the UNOS registry precluded inclusion of baseline covariates such as history of VA, INTERMACS profile, serum albumin, right ventricular function, antiarrhythmic medications, and right atrial pressure in the propensity score. Furthermore, post-LVAD implantation covariates of interest including post-implant VA, frequency of ICD therapy, frequency of inappropriate shocks, bleeding, concomitant cardiac resynchronization therapy, and rehospitalization were not available for this analysis. While propensity score matching with a caliper width of 20% of standard deviation of the logit of the propensity score eliminates 99% of the bias due to measured confounding variables (13), this technique is unable to account for unmeasured confounders.
Our analysis was limited by missing data, which resulted in patient exclusion and may limit the ability to apply these findings to all LVAD recipients awaiting transplant. Moreover, only 63 patients received an ICD after LVAD implant and the date was unknown, so we were unable to address the utility of an ICD implanted after LVAD implant. Whether these data can be applied to patients not listed for transplant who might have longer time on LVAD support is unknown. This question could be answered with an analysis of the INTERMACS registry. Lastly, absent a randomized trial of primary prevention ICD implantation in LVAD recipients, a prospective trial comparing standard with highly conservative tachytherapy settings would be highly informative on the clinical significance of VA in the presence of an LVAD and provide further insight into the utility of ICDs in this patient population.
In conclusion the presence of an ICD was not associated with a decrease in mortality among patients who were bridged to transplantation with a CF-LVAD.
Supplementary Material
Acknowledgements
This study was supported by Lisa and Mark Schwartz and the Program to Reverse Heart Failure at NewYork-Presbyterian Hospital/Columbia University Medical Center. Dr. Clerkin is supported by National Institutes of Health Grant T32 HL007854-16.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
Dr. Naka has received consulting fees from Thoratec and Heartware. Dr. Garan has received honoraria from Abiomed. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
References
- 1.Mancini D, Colombo PC. Left Ventricular Assist DevicesA Rapidly Evolving Alternative to Transplant. Journal of the American College of Cardiology. 2015;65:2542–2555. doi: 10.1016/j.jacc.2015.04.039. [DOI] [PubMed] [Google Scholar]
- 2.Epstein AE, DiMarco JP, Ellenbogen KA, et al. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm AbnormalitiesA Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices) Developed in Collaboration With the American Association for Thoracic Surgery and Society of Thoracic Surgeons. Journal of the American College of Cardiology. 2008;51:e1–e62. doi: 10.1016/j.jacc.2008.02.032. [DOI] [PubMed] [Google Scholar]
- 3.Kirklin JK, Naftel DC, Pagani FD, et al. Seventh INTERMACS annual report: 15,000 patients and counting. The Journal of Heart and Lung Transplantation. 2015;34:1495–1504. doi: 10.1016/j.healun.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 4.Lund LH, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirty-second Official Adult Heart Transplantation Report--2015; Focus Theme: Early Graft Failure. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2015;34:1244–54. doi: 10.1016/j.healun.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 5.Garan AR, Levin AP, Topkara V, et al. Early post-operative ventricular arrhythmias in patients with continuous-flow left ventricular assist devices. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2015;34:1611–6. doi: 10.1016/j.healun.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 6.Cantillon DJ, Tarakji KG, Kumbhani DJ, Smedira NG, Starling RC, Wilkoff BL. Improved survival among ventricular assist device recipients with a concomitant implantable cardioverter-defibrillator. Heart Rhythm. 2010;7:466–471. doi: 10.1016/j.hrthm.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 7.Refaat MM, Tanaka T, Kormos RL, et al. Survival Benefit of Implantable Cardioverter- Defibrillators in Left Ventricular Assist Device-Supported Heart Failure Patients. Journal of Cardiac Failure. 2012;18:140–145. doi: 10.1016/j.cardfail.2011.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Enriquez AD, Calenda B, Miller MA, Anyanwu AC, Pinney SP. The Role of Implantable Cardioverter-Defibrillators in Patients With Continuous Flow Left Ventricular Assist Devices. Circulation: Arrhythmia and Electrophysiology. 2013;6:668–674. doi: 10.1161/CIRCEP.113.000457. [DOI] [PubMed] [Google Scholar]
- 9.Garan AR, Yuzefpolskaya M, Colombo PC, et al. Ventricular Arrhythmias and Implantable Cardioverter-Defibrillator Therapy in Patients With Continuous-Flow Left Ventricular Assist DevicesNeed for Primary Prevention? Journal of the American College of Cardiology. 2013;61:2542–2550. doi: 10.1016/j.jacc.2013.04.020. [DOI] [PubMed] [Google Scholar]
- 10.Lee W, Tay A, Subbiah RN, et al. Impact of Implantable Cardioverter Defibrillators on Survival of Patients with Centrifugal Left Ventricular Assist Devices. Pacing and clinical electrophysiology : PACE. 2015;38:925–33. doi: 10.1111/pace.12654. [DOI] [PubMed] [Google Scholar]
- 11.Vakil K, Kazmirczak F, Sathnur N, et al. Implantable Cardioverter-Defibrillator Use in Patients With Left Ventricular Assist Devices: A Systematic Review and Meta-Analysis. JACC Heart failure. 2016 doi: 10.1016/j.jchf.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 12.Agrawal S, Garg L, Nanda S, et al. The role of implantable cardioverter-defibrillators in patients with continuous flow left ventricular assist devices — A meta-analysis. International Journal of Cardiology. 2016;222:379–384. doi: 10.1016/j.ijcard.2016.07.257. [DOI] [PubMed] [Google Scholar]
- 13.Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharmaceutical Statistics. 2011;10:150–61. doi: 10.1002/pst.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Austin PC. Using the Standardized Difference to Compare the Prevalence of a Binary Variable Between Two Groups in Observational Research. Communications in Statistics - Simulation and Computation. 2009;38:1228–1234. [Google Scholar]
- 15.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Statistics in medicine. 2009;28:3083–107. doi: 10.1002/sim.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenbaum PR, Rubin DB. Reducing Bias in Observational Studies Using Subclassification on the Propensity Score. Journal of the American Statistical Association. 1984;79:516–524. [Google Scholar]
- 17.D'Agostino RB. Propensity Scores in Cardiovascular Research. Circulation. 2007;115:2340–2343. doi: 10.1161/CIRCULATIONAHA.105.594952. [DOI] [PubMed] [Google Scholar]
- 18.Nakahara S, Chien C, Gelow J, et al. Ventricular Arrhythmias After Left Ventricular Assist Device. Circulation: Arrhythmia and Electrophysiology. 2013;6:648–654. doi: 10.1161/CIRCEP.113.000113. [DOI] [PubMed] [Google Scholar]
- 19.Woods B, Hawkins N, Mealing S, et al. Individual patient data network meta-analysis of mortality effects of implantable cardiac devices. Heart. 2015;101:1800–6. doi: 10.1136/heartjnl-2015-307634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schulze PC, Kitada S, Clerkin K, Jin Z, Mancini DM. Regional differences in recipient waitlist time and pre- and post-transplant mortality after the 2006 United Network for Organ Sharing policy changes in the donor heart allocation algorithm. JACC Heart failure. 2014;2:166–77. doi: 10.1016/j.jchf.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oz MC, Rose EA, Slater J, Kuiper JJ, Catanese KA, Levin HR. Malignant ventricular arrhythmias are well tolerated in patients receiving long-term left ventricular assist devices. Journal of the American College of Cardiology. 1994;24:1688–1691. doi: 10.1016/0735-1097(94)90175-9. [DOI] [PubMed] [Google Scholar]
- 22.Sims DB, Rosner G, Uriel NIR, GonzÁLez-Costello J, Ehlert FA, Jorde UP. Twelve Hours of Sustained Ventricular Fibrillation Supported by a Continuous-Flow Left Ventricular Assist Device. Pacing and Clinical Electrophysiology. 2012;35:e144–e148. doi: 10.1111/j.1540-8159.2011.03159.x. [DOI] [PubMed] [Google Scholar]
- 23.Malasana G, Daccarett M, Kuppahally S, Wasmund SL, Litwin SE, Hamdan MH. High prevalence of right ventricular dysfunction in ICD patients with shocks: a potential new predictor in risk stratification. Journal of interventional cardiac electrophysiology : an international journal of arrhythmias and pacing. 2011;31:165–9. doi: 10.1007/s10840-010-9536-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.