Abstract
Objective
To determine whether systemic inflammation-modulating cytokine expression is related to heart rate variability (HRV) in newborns with hypoxic ischemic encephalopathy (HIE).
STUDY DESIGN
Data from 30 newborns with HIE were analyzed. Cytokine levels (IL-2, IL-4, IL-6, IL-8, IL-10, IL-13, IL-1β, TNF-α, IFN-λ) were measured either at 24 hours of cooling (n=5), 72 hours of cooling (n=4), or at both timepoints (n=21). The following HRV metrics were quantified in the time domain: alpha_S, alpha_L, root mean square (RMS) at short time scales (RMS_S), RMS at long time scales (RMS_L), while low frequency power (LF) and high frequency power (HF) were quantified in the frequency domain. The relationships between HRV metrics and cytokines were evaluated using mixed-models.
Results
IL-6, IL-8, IL-10, and IL-13 levels were inversely related to selected HRV metrics.
Conclusion
Inflammation-modulating cytokines may be important mediators in the autonomic dysfunction observed in newborns with HIE.
Introduction
Perinatal asphyxia can lead to multisystem organ dysfunction in the newborn, including possible effects on the pulmonary, renal, hematologic and cardiovascular systems. While these injuries are often reversible, damage to the central nervous system can lead to hypoxic-ischemic encephalopathy (HIE) and cause devastating long-term disability - and in severe cases - even death1, 2. Therapeutic hypothermia (TH) has been shown to improve outcomes in newborns with HIE. However, about half of all treated infants continue to suffer death or disability despite treatment with TH3, 4, 5, 6. In order to improve outcomes, additional treatment options need to be implemented for those infants failing to respond to TH alone. Therefore, it is crucial to be able to assess an individual infant’s response to treatment over time in order to gauge the need for escalation of care. Hence, research is directed towards understanding the pathophysiological mechanisms leading to irreversible brain injury after a perinatal hypoxic ischemic insult, and identifying real-time methods to assess these ongoing processes at the bedside.
In an earlier report, we described the use of heart rate variability (HRV) metrics as physiological biomarkers signifying the evolution of neonatal brain injury in patients with HIE7. The mechanism by which HRV is depressed after hypoxia-ischemia, however, remains unclear. Possible etiologies include autonomic dysfunction resulting from direct brainstem injury, hypoxia induced cardiac dysfunction affecting regulation of heart rate at the nodal level, or systemic inflammatory response triggering adrenergic and cholinergic desensitization8. While the association between decreased HRV and systemic inflammation has been described in animal models of sepsis9 and adult human patients with heart failure10, diabetes11, and sepsis12, only one study has evaluated the relationship between HRV and cytokines in newborns with sepsis13. No studies have evaluated the link between inflammatory cytokine response and reduced HRV in critically-ill newborns with HIE.
We recently demonstrated the presence of elevated plasma inflammation-modulating cytokine levels in newborns with HIE who died or had severe brain injury on MRI compared to survivors with minimal or no brain injury14. This study aims to examine the association between inflammation-modulating cytokine response and HRV metrics in HIE newborns. We hypothesized that increased inflammation-modulating cytokine levels would be associated with reduced HRV in newborns with HIE.
Subjects and Methods
Study Population and Data Collection
Newborns with HIE meeting the criteria for TH using the National Institute of Child Health and Development Protocol3 were enrolled in this prospective observational study evaluating biomarkers of brain injury. Cytokine and MRI data have been previously reported from this cohort14 and the current study includes patients with available HRV data. Demographic and clinical data were collected from the birth hospital and the NICU medical records. Serum samples were collected 24 hours after cooling initiation and at 72 hours of cooling (i.e. initiation of rewarming) and frozen at −80 °C for later bulk assay. Cytokine (IL-10, IL-13, IL-1β, IL-2, IL-4, IL-6, IL-8, TNF-α, IFN-λ) concentrations were measured using a commercially available multiplex electrochemiluminesce-based assay (Meso Scale Discovery; Rockville, MD, USA). Continuous recordings of electrocardiogram (EKG) from the NICU bedside cardiorespiratory monitor (Philips IntelliVue MP70, MA, USA) were collected prospectively at a rate of 500 Hz and up-sampled to 1 kHz using custom software developed in LabView (National Instruments, TX, USA). For newborns not enrolled within 24 hours of life, EKG data were collected if available from an institutional Research Data Export (RDE) archive (IntelliVue Information Center, Philips Healthcare, MA, USA), which allowed for adequate data resolution for calculation of all HRV metrics. The study was approved by the Children’s National Institutional Review Board and informed consent was obtained from the parent of each participant.
EKG Signal Processing
To attenuate the baseline wandering and high-frequency noise, the EKG data were bandpass-filtered between 1 – 60 Hz using a Butterworth filter with zero-phase distortion. The R-waves were identified using a combination of Hilbert transform and an adaptive threshold detection approach,15 and heart rate was defined. The artifacts in the heart rate data were cleaned using an automated approach16. Heart rate was converted into RR intervals and partitioned into 10-minute epochs for further processing. For frequency-domain spectral analysis, the RR intervals in each 10-minute epoch were converted into uniformly sampled data using a cubic spline interpolation at a rate of 4 Hz. We estimated power spectra of the RR intervals in each 10-minute epoch using the Welch-periodogram approach with a frequency resolution of 0.0167 Hz. Using the estimated spectrum, we calculated LF and HF powers as the sum of the spectral powers in 0.05–0.25 Hz and 0.3–1 Hz, respectively. For further analysis, we divided LF and HF powers by the total power (sum of powers in 0.05–2 Hz) and used the normalized representation of the spectral powers17, 18. The normalized LF and HF powers characterize the sympathetic and parasympathetic tones of the autonomic nervous system, respectively17, 19. For time domain, we used the detrended fluctuation analysis (DFA) to calculate scaling exponents αs(15–30 beats) and αL(35–150 beats) for short and long time scales, respectively. We also calculated root means square (RMSS, 15–50 beats) and (RMSL, 100–150 beats) at short and long time scales, respectively. The α exponent characterizes the auto-correlations within the specified number of beats whereas the RMS characterizes the variability within the specified number of beats20. All analyses were performed off-line using MATLAB (Mathworks Inc, MA, USA). Code availability can be accessed by contacting the authors.
Statistical Analysis
The median value of the HRV metric in the two hours preceding the cytokine measurement was used for analysis. Unadjusted analyses were performed by generating a local regression line using LOWESS (LOcally WEighted Scatter-plot Smoother) to fit the corresponding variables. LOESS performs nonparametric local regression smoothing for estimating regression surfaces and does not require any assumptions about the parametric relationship between variables. To account for multiple measurements per subject, the relationships between HRV metrics and cytokines were evaluated using mixed-models adjusting for gestational age, birth weight, gender and time of measurement. Both the HRV metrics and the cytokine levels were log-transformed to meet the required normality assumption. The t-statistic was calculated as the ratio of the regression model estimate divided by the standard error for a given variable, providing a measure of the strength and direction of the relationship between the cytokine and HRV metric. The significance level was set at p<0.01 given multiple comparisons. We used a convenience sample of 30 patients with available cytokine and HRV data for these analyses. This sample provided 80% statistical power with α=0.05 to detect a strong (r=0.5) correlation between cytokine and HRV metric, not accounting for repeated measures in subjects.
Results
A total of 30 newborns with moderate-to-severe HIE were included in this study. HRV was prospectively monitored in the majority of patients (n=23), while 7 patients were enrolled after 24 hours of life with data retrieved from the RDE archive. The median gestational age was 39 weeks, median birth weight was 2.96 kg and 33% were male. None had culture-positive sepsis during their NICU stay. Of the 22 patients for whom placental pathology information was available, 8 (36%) had chorioamnionitis. Other baseline and clinical characteristics are shown in Table 1.
Table 1.
Clinical characteristics of study population
| Gestational Age (weeks) | 39, [35, 40] |
|
| |
| Birth Weight (Kilograms) | 2.96, [1.98,6.3] |
|
| |
| Presenting pH | 6.9, [6.6,7.11] |
|
| |
| Gender (n, % male) | 16 (53) |
|
| |
| APGARS | |
| 1 Minute | 1, [0,4] |
| 5 Minutes | 3, [0,7] |
| 10 Minutes | 5, [0,8] |
|
| |
| Encephalopathy Grade | |
| % Moderate | 92.8% |
| % Severe | 7.2% |
|
| |
| Hour of life on cooling | 2:24 (4:04, 6:00) |
|
| |
| Electrographic Seizures (n, %) | 10 (33) |
|
| |
| Chorioamnionitis (n, % of patients with placental pathology available) | 8 (36)* |
|
| |
| Received hydrocortisone (n, %) | 8 (27) |
|
| |
| Brain Injury by MRI (n, %) | |
| None | 12 (40) |
| Mild | 6 (20) |
| Moderate/Severe | 7 (23) |
| Died before MRI | 5 (17) |
Data presented as median [range] unless otherwise indicated
Placental information available for 22/30 patients
Cytokine levels were measured at both timepoints of interest in 21 infants. Nine infants only had single measurements: this was due either to a blood sample that was insufficient for analysis (n=4) or because death occurred before 72 hours of cooling (n=5). Consequently, we entered into analyses with 51 observations.
IL-6, IL-8, IL-10, and IL-13 demonstrated statistically significant (p<0.01) negative associations with certain HRV metrics, indicating reduced HRV with an increasing cytokine level (Figure 1). These relationships remained significant after adjustment for baseline characteristics including gestational age, birth weight, gender, and time of measurement. The results of the regression models are presented in Table 2. Sensitivity analyses were also performed evaluating the subset of infants with available information regarding chorioamnionitis status and the results were similar. Of note, the infants who died had significantly lower RMS_S and RMS_L and higher IL-8 and IL-10 levels compared to those who survived.
Figure 1.
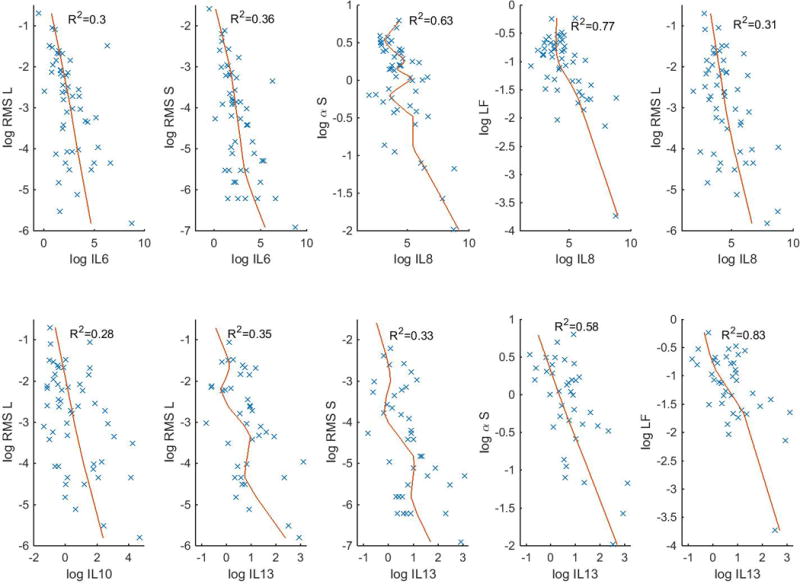
Relationship between HRV metrics and cytokine concentration shown by scatterplot and local regression line.
Table 2.
Association between HRV metrics and cytokine levels
| Cytokine | HRV Metric | t-value | P value* | Significant Covariates |
|---|---|---|---|---|
| Log IL-6 | Log RMS_S | −4.12 | 0.0006 | |
| Log IL-6 | Log RMS_L | −3.34 | 0.0034 | |
| Log IL-8 | Log alpha_S | −4.81 | 0.0001 | |
| Log IL-8 | Log LF | −4.53 | 0.0002 | |
| Log IL-8 | Log RMS_L | −3.42 | 0.0029 | |
| Log IL-10 | Log RMS_L | −3.12 | 0.0056 | |
| Log IL-13 | Log alpha_S | −6.73 | <.0001 | GA (t=2.6, p=0.016) |
| Log IL-13 | Log LF | −5.14 | <.0001 | GA (t=2.4, p=0.028) |
| Log IL-13 | Log RMS_L | −3.94 | 0.0009 | |
| Log IL-13 | Log RMS_S | −3.75 | 0.0014 |
P-values indicate significance of association between HRV metric and cytokine after adjusting for gestational age (GA), birth weight, gender and time of measurement
Discussion
To our knowledge, this is the first study to demonstrate the association of elevated inflammation-modulating cytokine levels with depressed heart rate variability in newborns with HIE undergoing TH. In earlier studies, we demonstrated that inflammation-modulating cytokines are able to differentiate newborns with adverse neurological outcomes14. We expand upon this work to elucidate a possible pathophysiological link between systemic inflammatory response and disease evolution in HIE. The relationship between inflammation-modulating cytokine release and reduced HRV supports the notion that ANS dysfunction in newborns with HIE is mediated by inflammatory processes triggered by hypoxia-ischemia. As one of the proposed mechanisms of TH includes reduction of inflammation21, 22, results of this study suggest that one of the downstream neuroprotective properties of TH may be the preservation of ANS function during the 72 hours following an asphyxial insult.
Several studies have evaluated the relationship between inflammatory cytokines and brain injury in HIE. Elevated levels of IL-623, 24, 25, 26, 27, IL-825, 26, 27 IL-1014, 27 and IL-1314, specifically, have been related to brain injury based on severity of clinical encephalopathy grade, MRI abnormalities and/or later neurodevelopmental impairments. The fact that we did not find a relationship between HRV and IL-1β, TNF-α and IFN-γ is of interest, as early measurements of these cytokines have also been linked to brain injury in HIE24, 25, 27. Whether this lack of relationship relates to timing of measurement or represents a specificity of certain cytokines in the pathogenesis of ANS dysfunction warrants further study in a larger population of infants with HIE.
The timing of cytokine responses and HRV evolution also supports a mechanistic link between the peak of inflammation and reduced HRV. Based on animal28, 29 and human studies25, 27, cytokines have been shown to peak within 12–24 hours after a hypoxic-ischemic insult, with some having a biphasic response.27 Mirroring this time course, we demonstrated that HRV most differentiated HIE infants by outcome at 24 hours of life30 - which is the time when the peak of secondary injury is thought to happen31 - and again later after 72 hours post-rewarming. Based on this rationale, we evaluated the relationship between the HRV metrics and cytokines measured at these two timepoints with key clinical implications.
The biological plausibility of the association between cytokine levels and reduced HRV is based on observations from several animal studies and limited human data. Both parasympathetic and sympathetic inputs have been demonstrated to have immunomodulatory effects. Vagal activation has been shown to attenuate proinflammatory cytokine release32. Bernik et al showed that vagus nerve stimulation inhibited TNF synthesis33, while vagotomy was associated with worsening inflammation34. The sympathetic nervous system also plays a role in the regulation of the immune system, possessing both pro- and anti-inflammatory properties.35 It is possible that primary ANS dysfunction leads to loss of these immunomodulatory functions and an unregulated pro-inflammatory state. Other data, however, support the notion that elevated cytokine levels, whether triggered by sepsis or hypoxia-ischemia, lead to reduced HRV. Fairchild et al showed that HRV was depressed in mice in the setting of a pro-inflammatory cytokine response after injecting them with endotoxins9. These investigators proposed that while cytokines are expected to activate the efferent vagal nerve and thus increase HRV, repeated vagal nerve activations cause desensitization of the cholinergic receptors in the heart, which results in decreased HRV8. In fact, cytokine-mediated desensitization of cholinergic receptors has been shown in several organ systems36, 37, 38. The negative association between inflammatory mediators and HRV was recently demonstrated in a large nationally representative sample of adults, where HRV assessed by HF and LF was inversely associated with fibrinogen, CRP, and IL-639. Our findings are consistent with this inverse relationship between inflammation-modulating cytokines and HRV. Only one prior study has evaluated the relationship between cytokines and HRV in neonatal patients. Raynor et al showed an association of cytokines with HRV in newborns diagnosed with sepsis13. Our study confirms the association reported between IL-8 and HRV and revealed additional associations of IL-6, IL-10, and IL-13 with HRV.
Our study has limitations. We made assumptions regarding the temporal scale of the cytokine-HRV response. We evaluated the median of HRV metrics in the two hours preceding the cytokine measurement as we felt our measurement reflected circulating levels in the time period immediately preceding the blood sampling time. Given their dynamic nature and variable time-dependency, this 2-hour window may not be ideal for evaluating the relationship with all cytokines. The fact that not all subjects had cytokines measured at both timepoints of interest influenced our already relatively small sample size. Future studies with more frequent collection of samples during cooling and rewarming will be useful to further define the relationship between inflammation-modulating cytokine profiles, HRV, and brain injury in newborns with HIE. Despite these limitations, this study highlights the importance of inflammation in the evolution of brain injury and provides insights into the pathogenesis of autonomic dysfunction that is observed in HIE.
Conclusions
Elevated cytokines IL-6, IL-8, IL-10, and IL-13 showed an association with quantitative measures of reduced HRV. These data suggest a possible role of inflammation-modulating cytokines in mediating ANS dysfunction in infants with HIE.
Acknowledgments
We would like to thank Ms. Sophie Wohlers for her editorial assistance. We also thank Karuna Panchapakesan and Susan Knobloch for their assistance with cytokine determinations.
This work was supported by the Clinical and Translational Science Institute at Children’s National (UL1TR000075 and 1KL2RR031987-01), the Intellectual and Developmental Disabilities Research Consortium (NIH P30HD040677), and the NCMRR-DC Molecular and Functional Outcome Measures in Rehabilitation Medicine Core (NICHD/NINDS 5R24HD050846-08).
Footnotes
Conflict of interest
The authors have no financial relationships relevant to this article to disclose.
The authors declare no conflict of interest.
References
- 1.Shankaran S, Woldt E, Koepke T, Bedard MP, Nandyal R. Acute neonatal morbidity and long-term central nervous system sequelae of perinatal asphyxia in term infants. Early human development. 1991;25(2):135–148. doi: 10.1016/0378-3782(91)90191-5. [DOI] [PubMed] [Google Scholar]
- 2.Dilenge ME, Majnemer A, Shevell MI. Long-term developmental outcome of asphyxiated term neonates. Journal of child neurology. 2001;16(11):781–792. doi: 10.1177/08830738010160110201. [DOI] [PubMed] [Google Scholar]
- 3.Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. The New England journal of medicine. 2005;353(15):1574–1584. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- 4.Azzopardi DV, Strohm B, Edwards AD, Dyet L, Halliday HL, Juszczak E, et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med. 2009;361(14):1349–1358. doi: 10.1056/NEJMoa0900854. [DOI] [PubMed] [Google Scholar]
- 5.Gluckman PD, Wyatt JS, Azzopardi D, Ballard R, Edwards AD, Ferriero DM, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet. 2005;365(9460):663–670. doi: 10.1016/S0140-6736(05)17946-X. [DOI] [PubMed] [Google Scholar]
- 6.Jacobs SE, Morley CJ, Inder TE, Stewart MJ, Smith KR, McNamara PJ, et al. Whole-body hypothermia for term and near-term newborns with hypoxic-ischemic encephalopathy: a randomized controlled trial. Archives of pediatrics & adolescent medicine. 2011;165(8):692–700. doi: 10.1001/archpediatrics.2011.43. [DOI] [PubMed] [Google Scholar]
- 7.Massaro AN, Govindan RB, Al-Shargabi T, Andescavage NN, Metzler M, Chang T, et al. Heart rate variability in encephalopathic newborns during and after therapeutic hypothermia. J Perinatol. 2014;34(11):836–841. doi: 10.1038/jp.2014.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fairchild KD, Srinivasan V, Moorman JR, Gaykema RP, Goehler LE. Pathogen-induced heart rate changes associated with cholinergic nervous system activation. American journal of physiology Regulatory, integrative and comparative physiology. 2011;300(2):R330–339. doi: 10.1152/ajpregu.00487.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fairchild KD, Saucerman JJ, Raynor LL, Sivak JA, Xiao Y, Lake DE, et al. Endotoxin depresses heart rate variability in mice: cytokine and steroid effects. American journal of physiology Regulatory, integrative and comparative physiology. 2009;297(4):R1019–1027. doi: 10.1152/ajpregu.00132.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malave HA, Taylor AA, Nattama J, Deswal A, Mann DL. Circulating levels of tumor necrosis factor correlate with indexes of depressed heart rate variability: a study in patients with mild-to-moderate heart failure. Chest. 2003;123(3):716–724. doi: 10.1378/chest.123.3.716. [DOI] [PubMed] [Google Scholar]
- 11.Griffin MP, Lake DE, Bissonette EA, Harrell FE, Jr, O’Shea TM, Moorman JR. Heart rate characteristics: novel physiomarkers to predict neonatal infection and death. Pediatrics. 2005;116(5):1070–1074. doi: 10.1542/peds.2004-2461. [DOI] [PubMed] [Google Scholar]
- 12.Tateishi Y, Oda S, Nakamura M, Watanabe K, Kuwaki T, Moriguchi T, et al. Depressed heart rate variability is associated with high IL-6 blood level and decline in the blood pressure in septic patients. Shock. 2007;28(5):549–553. doi: 10.1097/shk.0b013e3180638d1. [DOI] [PubMed] [Google Scholar]
- 13.Raynor LL, Saucerman JJ, Akinola MO, Lake DE, Moorman JR, Fairchild KD. Cytokine screening identifies NICU patients with Gram-negative bacteremia. Pediatr Res. 2012;71(3):261–266. doi: 10.1038/pr.2011.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Orrock JE, Panchapakesan K, Vezina G, Chang T, Harris K, Wang Y, et al. Association of brain injury and neonatal cytokine response during therapeutic hypothermia in newborns with hypoxic-ischemic encephalopathy. Pediatric research. 2016;79(5):742–747. doi: 10.1038/pr.2015.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ulusar UD, Govindan RB, Wilson JD, Lowery CL, Preissl H, Eswaran H. Adaptive rule based fetal QRS complex detection using hilbert transform. Conf Proc IEEE Eng Med Biol Soc. 2009;1:4666–4669. doi: 10.1109/IEMBS.2009.5334180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Govindan RB, Al-Shargabi T, Metzler M, Andescavage NN, Joshi R, Du Plessis A. A spike correction approach for variability analysis of heart rate in sick infants. Physica A. 2016;444:35–42. [Google Scholar]
- 17.Govindan RB, Massaro AN, Niforatos N, du Plessis A. Mitigating the effect of non-stationarity in spectral analysis-an application to neonate heart rate analysis. Computers in biology and medicine. 2013;43(12):2001–2006. doi: 10.1016/j.compbiomed.2013.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Massaro AN, Govindan RB, Al-Shargabi T, Andescavage NN, Metzler M, Chang T, et al. Heart rate variability in encephalopathic newborns during and after therapeutic hypothermia. J Perinatol. 2013;34(11):836–841. doi: 10.1038/jp.2014.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cerutti S, Baselli G, Civardi S, Ferrazzi E, Marconi AM, Pagani M, et al. Variability analysis of fetal heart rate signals as obtained from abdominal electrocardiographic recordings. J Perinat Med. 1986;14(6):445–452. doi: 10.1515/jpme.1986.14.6.445. [DOI] [PubMed] [Google Scholar]
- 20.Govindan RB, Massaro AN, Al-Shargabi T, Andescavage NN, Chang T, Glass P, et al. Detrended fluctuation analysis of non-stationary cardiac beat-to-beat interval of sick infants. EPL (Europhysics Letters) 2014;108:40005–40001. [Google Scholar]
- 21.Yanagawa Y, Kawakami M, Okada Y. Moderate hypothermia alters interleukin-6 and interleukin-1alpha reactions in ischemic brain in mice. Resuscitation. 2002;53(1):93–99. doi: 10.1016/s0300-9572(01)00499-3. [DOI] [PubMed] [Google Scholar]
- 22.Diestel A, Roessler J, Berger F, Schmitt KR. Hypothermia downregulates inflammation but enhances IL-6 secretion by stimulated endothelial cells. Cryobiology. 2008;57(3):216–222. doi: 10.1016/j.cryobiol.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 23.Tekgul H, Yalaz M, Kutukculer N, Ozbek S, Kose T, Akisu M, et al. Value of biochemical markers for outcome in term infants with asphyxia. Pediatric neurology. 2004;31(5):326–332. doi: 10.1016/j.pediatrneurol.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 24.Bartha AI, Foster-Barber A, Miller SP, Vigneron DB, Glidden DV, Barkovich AJ, et al. Neonatal encephalopathy: association of cytokines with MR spectroscopy and outcome. Pediatric research. 2004;56(6):960–966. doi: 10.1203/01.PDR.0000144819.45689.BB. [DOI] [PubMed] [Google Scholar]
- 25.Chalak LF, Sanchez PJ, Adams-Huet B, Laptook AR, Heyne RJ, Rosenfeld CR. Biomarkers for severity of neonatal hypoxic-ischemic encephalopathy and outcomes in newborns receiving hypothermia therapy. The Journal of pediatrics. 2014;164(3):468–474 e461. doi: 10.1016/j.jpeds.2013.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramaswamy V, Horton J, Vandermeer B, Buscemi N, Miller S, Yager J. Systematic review of biomarkers of brain injury in term neonatal encephalopathy. Pediatric neurology. 2009;40(3):215–226. doi: 10.1016/j.pediatrneurol.2008.09.026. [DOI] [PubMed] [Google Scholar]
- 27.Jenkins DD, Rollins LG, Perkel JK, Wagner CL, Katikaneni LP, Bass WT, et al. Serum cytokines in a clinical trial of hypothermia for neonatal hypoxic-ischemic encephalopathy. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2012;32(10):1888–1896. doi: 10.1038/jcbfm.2012.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arvin B, Neville LF, Barone FC, Feuerstein GZ. The role of inflammation and cytokines in brain injury. Neurosci Biobehav Rev. 1996;20(3):445–452. doi: 10.1016/0149-7634(95)00026-7. [DOI] [PubMed] [Google Scholar]
- 29.Wang X, Feuerstein GZ. Role of immune and inflammatory mediators in CNS injury. Drug News Perspect. 2000;13(3):133–140. doi: 10.1358/dnp.2000.13.3.657283. [DOI] [PubMed] [Google Scholar]
- 30.Massaro AN, Govindan RB, Al-Shargabi T, Andescavage NN, Metzler M, Chang T, et al. Heart rate variability in encephalopathic newborns during and after therapeutic hypothermia. Journal of perinatology: official journal of the California Perinatal Association. 2014 doi: 10.1038/jp.2014.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnston MV, Fatemi A, Wilson MA, Northington F. Treatment advances in neonatal neuroprotection and neurointensive care. Lancet Neurol. 2011;10(4):372–382. doi: 10.1016/S1474-4422(11)70016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405(6785):458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 33.Bernik TR, Friedman SG, Ochani M, DiRaimo R, Susarla S, Czura CJ, et al. Cholinergic antiinflammatory pathway inhibition of tumor necrosis factor during ischemia reperfusion. J Vasc Surg. 2002;36(6):1231–1236. doi: 10.1067/mva.2002.129643. [DOI] [PubMed] [Google Scholar]
- 34.Peterson CY, Krzyzaniak M, Coimbra R, Chang DC. Vagus nerve and postinjury inflammatory response. Arch Surg. 2012;147(1):76–80. doi: 10.1001/archsurg.2011.237. [DOI] [PubMed] [Google Scholar]
- 35.Bellinger DL, Millar BA, Perez S, Carter J, Wood C, ThyagaRajan S, et al. Sympathetic modulation of immunity: relevance to disease. Cellular immunology. 2008;252(1–2):27–56. doi: 10.1016/j.cellimm.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Akiho H, Khan WI, Al-Kaabi A, Blennerhassett P, Deng Y, Collins SM. Cytokine modulation of muscarinic receptors in the murine intestine. American journal of physiology Gastrointestinal and liver physiology. 2007;293(1):12. doi: 10.1152/ajpgi.00545.2006. [DOI] [PubMed] [Google Scholar]
- 37.Barnes PJ, Haddad EB, Rousell J. Regulation of muscarinic M2 receptors. Life sciences. 1997;60(13–14):1015–1021. doi: 10.1016/s0024-3205(97)00042-8. [DOI] [PubMed] [Google Scholar]
- 38.Moreno L, Jacoby DB, Fryer AD. Dexamethasone prevents virus-induced hyperresponsiveness via multiple mechanisms. American journal of physiology Lung cellular and molecular physiology. 2003;285(2):25. doi: 10.1152/ajplung.00046.2003. [DOI] [PubMed] [Google Scholar]
- 39.Cooper TM, McKinley PS, Seeman TE, Choo TH, Lee S, Sloan RP. Heart rate variability predicts levels of inflammatory markers: Evidence for the vagal anti-inflammatory pathway. Brain, behavior, and immunity. 2015;49:94–100. doi: 10.1016/j.bbi.2014.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]


