Abstract
Purpose
Suboptimal outcomes for children with acute myeloid leukemia (AML) necessitate maximally intensive therapy. Consequently, serious adverse events, such as prolonged periods of profound myelosuppression, contribute to AML treatment–related mortality. Telomeres, the repetitive DNA–protein structures at chromosome ends, influence cellular replicative capacity in that critically short telomeres can induce cell senescence or apoptosis. Our objective was to evaluate the impact of telomere length on duration of post-therapy neutropenia in a pediatric AML cohort.
Patients and Methods
Patients were diagnosed with de novo AML, enrolled in Children’s Oncology Group study AAML0531, and included those with (n = 53) and without (n = 62) significantly delayed neutrophil recovery after chemotherapy. We used quantitative polymerase chain reaction to measure telomere content (TC), a validated proxy for telomere length, from remission bone marrow samples obtained after the second induction chemotherapy course.
Results
Less TC was significantly associated with prolonged neutropenia after the fourth (P < .001) and fifth chemotherapy courses (P = .002). Cox regression adjusting for age at diagnosis confirmed that TC remained independently predictive of time to recovery of absolute neutrophil count for both the fourth and fifth courses (P = .002 and .009, respectively). DNA from patients was analyzed for germline mutations in four telomere maintenance genes associated with telomere biology disorders. Sequence analysis revealed no enrichment of rare or novel variants in the delayed recovery group.
Conclusion
Our results suggest that TC at end of AML induction is associated with hematopoietic reconstitution capacity independently of age and may identify those at highest risk for markedly delayed bone marrow recovery after AML therapy.
INTRODUCTION
Acute myeloid leukemia (AML) represents 20% of childhood leukemias and is associated with 5-year event-free survival (EFS) of 47% to 53%.1 Intensified therapy has resulted in treatment-related mortality (TRM) in up to 19% of patients.2 Prolonged, profound neutropenia is a well-recognized risk factor for sepsis and invasive fungal infections,3 both major contributors to TRM.4 Patients with newly diagnosed AML enrolled in Children’s Oncology Group (COG) study AAML0531 (not qualifying for transplantation) received five courses of intensive chemotherapy. Prolonged myelosuppression of more than 60 days was noted during the final two chemotherapy courses in 5% to 10% of patients.1 Despite advances in supportive care measures and efforts to deliver intensive therapies with reduced overall toxicity, TRM in AAML0531 approached 5%.1 Host factors associated with impaired hematopoietic recovery in AML are largely unknown. Hematopoietic cell (nonleukemic) telomere length is a quantifiable host factor that may indicate potential risk for impaired bone marrow recovery after chemotherapy.
Telomeres are specialized DNA–protein structures at chromosome ends, protecting chromosome integrity by preventing end-to-end fusions, nucleolytic processing, and homologous recombination. Telomeric DNA is composed of tandem repeats of the hexanucleotide 5′TTAGGG, with length that is influenced by genetic determinants and environmental factors, and varying between individuals and cell populations. Telomeres shorten with aging in tissues lacking sufficient telomerase, a specialized reverse transcriptase that replenishes terminal telomeric repeats lost after genome duplication.5-7 Accelerated telomere attrition is induced by exposure to intensive chemotherapies.8-10 Once a critically short length is attained, telomeres lose their end-protection ability, triggering a DNA damage response that results in cellular senescence or apoptosis11; thus, telomere length predicts cellular replicative capacity.12
Hematopoiesis is particularly sensitive to shortened telomeres. Hematopoietic stem cells (HSCs) with short telomeres are unable to exit quiescence and produce progenitor cells, a checkpoint response that likely prevents the transmission of chromosomal aberrations.13 Clinically, this is evidenced as bone marrow failure (BMF), a highly penetrant phenotype of dyskeratosis congenita (DC) resulting from mutations in genes that regulate telomere maintenance. In individuals with DC, BMF is a consequence of premature cellular senescence within the hematopoietic compartment.14 Patients with DC have a 200-fold increased risk for developing AML,15,16 thought to result from genomic instability driven by the loss of telomere end protection. Telomerase variants associated with reduced telomerase activity occur as rare polymorphisms but are enriched in adults with hematologic malignancies.17 Deleterious variants in the TERT gene, which encodes the telomerase catalytic subunit, occurred in 6% of an adult sporadic AML cohort,18 although a smaller pediatric AML cohort showed no such enrichment.19 Whether telomere length and/or presence of pathogenic variants in DC-associated genes is predictive of bone marrow toxicity in patients undergoing AML treatment has not been studied.
Because significantly shortened telomeres limit hematopoiesis, we predicted a proportion of patients with AML with pronounced telomere shortening might experience marrow exhaustion after repeated courses of intensive chemotherapy. Such exhaustion may result in a delay in time to bone marrow recovery and may also suggest underlying defects in genes commonly mutated in DC.
PATIENTS AND METHODS
Study Population
All patients were enrolled in AAML0531, a COG treatment protocol for individuals between the ages of 1 month and 29.99 years diagnosed with primary, untreated AML and without clinical evidence of Down syndrome or a genetic predisposition for BMF or AML (Appendix Fig A1, online only).1 This protocol was approved by all participating institutional review boards. Patients and families provided informed consent for participation or assent as appropriate. The trial was conducted under the Declaration of Helsinki. AAML11B2 (Telomere Length and Telomerase Mutations in Pediatric Acute Myeloid Leukemia; principal investigator, M.M.G.) is a correlative biology study to AAML0531, approved by the National Institutes of Health National Cancer Institute Cancer Therapy Evaluation Program. For this study, we analyzed paired diagnostic and remission bone marrow samples from patients in AAML0531 with either expected or shorter time to absolute neutrophil count (ANC) recovery (less than one standard deviation [SD] above the mean for all chemotherapy courses in patients receiving all five courses; n = 62) or significantly delayed time to ANC recovery (greater than one SD above the mean for at least two chemotherapy courses; n = 53), for a total sample size of 115. Sample size was determined to enable detection of a minimal difference of 0.2 units in telomere content (TC) between the groups. Infection data were submitted prospectively by institutional clinical research associates and were monitored by an investigator (L.S.) in real time to optimize reporting accuracy.
Quantitative Polymerase Chain Reaction for TC
DNA extracted from remission bone marrow (isolated after second induction) was obtained from the COG AML Reference Laboratory. Quantitative polymerase chain reaction (PCR) was performed for all samples in triplicate, with patient cases and controls intermixed at random so that investigators were blinded to ANC recovery status. Each plate included a negative (water) control and serial dilutions of a control DNA sample for a standard curve. For each reaction, SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA) was combined with DNA and primers and run separately on a high-throughput real-time PCR system (7900HT; Applied Biosystems). TC was calculated for each sample, corresponding to the amount of telomeric DNA amplified relative to a single-copy gene (36B4, encoding acidic ribosomal phosphoprotein P0).20 Because telomere primers also amplify a minor population of intergenic telomere repeats, we refer to this value as TC rather than telomere length (details provided in the Appendix, online only).
Statistical Analysis
Data from AAML0531 were analyzed as of June 30, 2015. TC was measured as a continuous variable and divided into quartiles, with the first (shortest) quartile defined as TC less than 0.595. The Kaplan-Meier method21 was applied to determine if TC contributed to time to ANC recovery after each course of chemotherapy. Time to ANC recovery was defined as day of course start until day of ANC recovery to 500 cells/μL. Patients who did not recover were censored at course end. Both univariable and multivariable Cox regression analyses22 were then used to determine the impact of age on TC and time to ANC recovery. Poisson regression analysis was used to test whether the incidence rate ratio (IRR) of microbiologically documented sterile-site grade 3 to 4 infections was significant among TC quartile groups, using infection data obtained in the AAML0531 cohort.23 The Kaplan-Meier method was applied to determine contribution of TC to EFS and overall survival (OS). EFS was defined as time from study entry to induction failure, relapse, or death. OS was defined as time from study entry to death. Patients were censored at date of last known contact. Significance was assigned to P values less than .05. The significance of observed differences in proportions of study entry characteristics between the TC quartile groups was determined using Pearson’s χ2 test or Fisher’s exact test when data were sparse.
Sequencing of Select Telomere Maintenance Genes
Genes selected for Sanger sequencing included TERT, TERC, DKC1, and TINF2. Sequencing was first performed on DNA isolated from diagnostic bone marrow and then tested for germline status by directed sequencing of DNA from paired remission bone marrow samples (details provided in the Appendix).
RESULTS
Older Age and Less TC at End of Induction Predicts Time to Neutrophil Recovery in Later Courses of AML Chemotherapy
Quantitative PCR for TC was performed on DNA isolated from remission bone marrow samples obtained after the second induction. Eighteen samples (nine with expected recovery and nine with delayed recovery) were excluded from further analysis because of failure of the sample to amplify (n = 5) or divergence of threshold cycle values greater than 0.15 within a sample triplicate (n = 13), leaving 97 samples for analysis (53 expected and 44 delayed recovery). All unknown threshold cycle values were within range of the standard curve, with correlation coefficients of 0.98 or greater. The coefficient of variation was 0.40% within triplicates of both the telomere and single-gene assays.
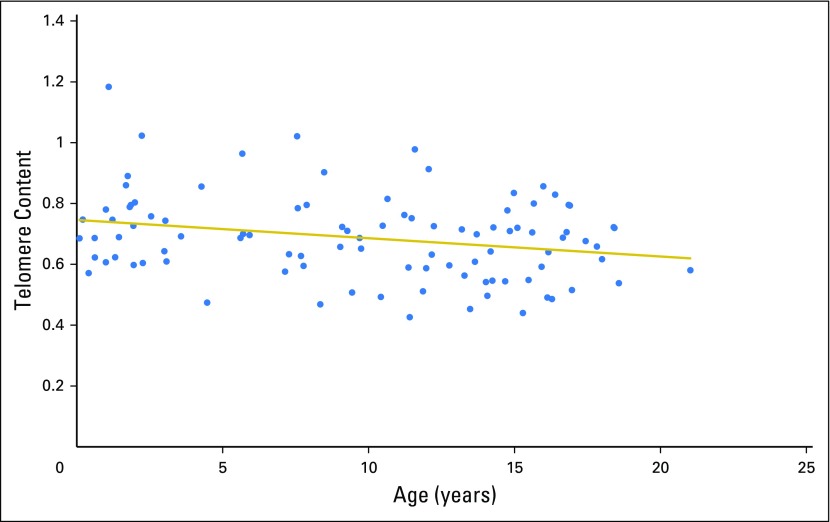
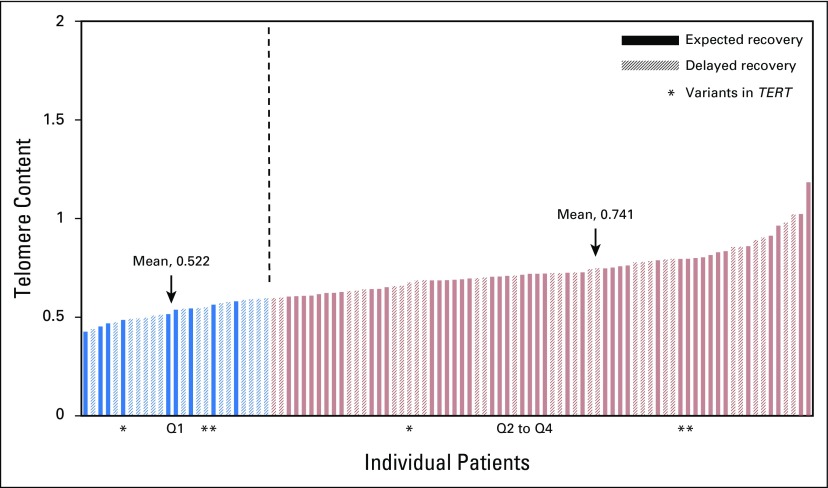
As expected, TC declined with increasing patient age (Fig 1). Comparison of mean (± SD) TC between those with and without delayed count recovery demonstrated a difference that was not significant (0.66 ± 0.13 v 0.71 ± SD 0.15, respectively; P = .06). To further examine a potential impact of TC on hematopoietic recovery, we combined and ranked TC from the 97 samples into quartiles, with the least TC in quartile one (Q1) and the most TC in Q4 (Fig 2). Patients with less TC (Q1: range, 0.43 to 0.59; mean, 0.52; SD, 0.05) were compared with the remaining patients (Q2 to Q4: range, 0.59 to 1.18; mean, 0.74; SD, 0.11) for differences in duration of neutropenia after chemotherapy.
Fig 1.
Telomere content (TC) declines with increasing patient age. First-remission TC distribution is shown relative to patient age in years.
Fig 2.
Telomere content (TC) in patients with expected and delayed recovery were distributed among all four TC quartiles. TC distribution is shown from least to greatest and divided into two groups by TC: quartile one (Q1) and Q2 to Q4. The mean TC for Q1 was 0.52 (range, 0.43 to 0.59; standard deviation [SD], 0.05); for Q2 to Q4, it was 0.74 (range, 0.59 to 1.18; SD, 0.11). Vertical dashed line divides Q1 from Q2 to Q4.
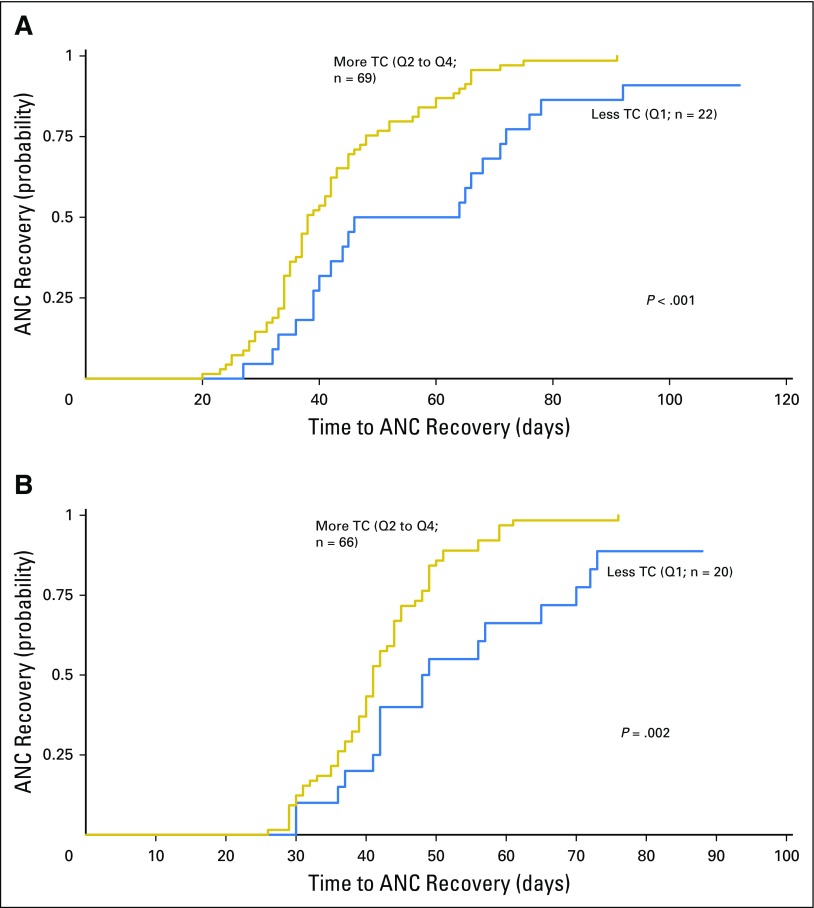
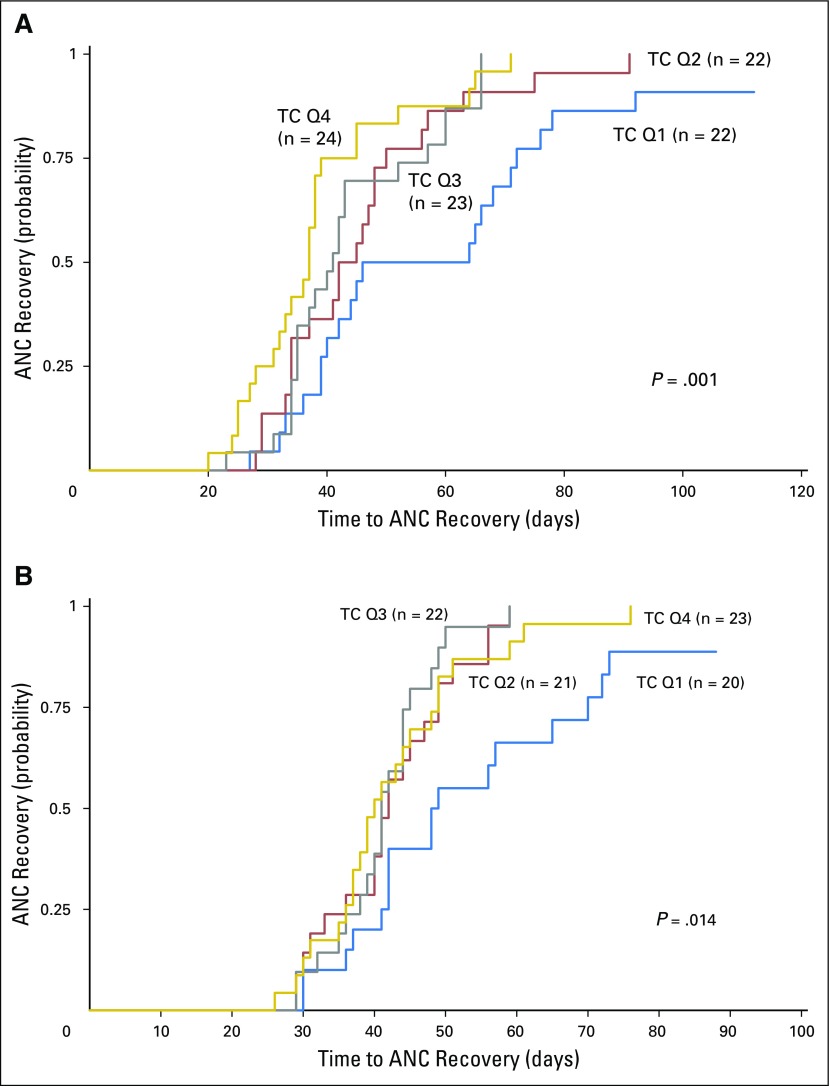
There was no difference between Q1 and Q2 to Q4 in number of days to ANC recovery after the first three chemotherapy courses, with recovery times for all patients comparable to those in the AAML0531 study population.1 After the fourth and fifth chemotherapy courses (intensifications two and three), patients in both TC groups demonstrated increased time to ANC recovery compared with the first three courses (mean, 45.2 days for intensification two and 43.7 days for intensification three). For Q2 to Q4, this observation reflected what was observed in the AAML0531 study population (mean, 40.9 days for intensification two and 40.5 days for intensification three).1 However, patients in Q1 had a significantly longer mean ANC recovery time than those in Q2 to Q4 (intensification two, P < .001; intensification three, P = .002; Figs 3A and 3B). Analysis of individual quartiles confirmed the association between less TC in Q1 and delays in ANC recovery (Appendix Fig A2, online only). This difference was especially notable for intensification two, with a mean of 53.8 days (range, 27 to 92 days) for those with less postinduction TC (Q1) compared with 42.7 days (range, 20 to 91 days) for those with more TC (Q2 to Q4; P = .021). Cox regression adjusting for age at diagnosis confirmed that both age and TC were predictive of time to ANC recovery by univariable analysis. After multivariable analysis, TC remained independently predictive of time to recovery for both intensifications two (P = .002) and three (P = .009), as did age (intensification two, P = .004; intensification three, P = .044; Table 1).
Fig 3.
Patients in telomere content (TC) quartile one (Q1) demonstrated a longer time to absolute neutrophil count (ANC) recovery after intensification (A) two and (B) three. First-remission TC was examined for all five chemotherapy courses, relative to time to ANC recovery. TC Q1 was compared with Q2 to Q4 relative to the number of days to ANC recovery (500 cells/μL). No difference in TC was noted for the first three courses. In the fourth and fifth courses, patients in Q1 demonstrated a longer mean ANC recovery time, which was significant for both intensifications (A) two (P < .001) and (B) three (P = .002). Analysis of individual quartiles confirmed the association between less TC in Q1 and delays in ANC recovery.
Table 1.
Cox Regression: ANC Recovery During Intensifications Two and Three
Although this study was not powered to detect differences in infectious complications, we compared the rates of microbiologically documented sterile-site infections between patients in Q1 and Q2 to Q4. In intensification two, 13 of 22 Q1 patients had grade 3 to 4 infections, compared with 50 of 69 patients in Q2 to Q4 (IRR, 0.65; P = .081). In intensification three, 15 of 20 Q1 patients had grade 3 to 4 infections, compared with 37 of 66 patients in Q2 to Q4 (IRR, 1.36; P = .225). There was no difference in 5-year EFS (± SD) between patients in Q1 and Q2 to Q4 (Q1: 52% ± 21% v Q2 to Q4: 67% ± 11%; P = .225), with the events predominantly resulting from relapse in both TC groups (Q1: nine of 11 v Q2 to Q4: 21 of 23). There was also no difference in 5-year OS (± SD; Q1: 87% ± 14% v Q2 to Q4: 90% ± 7%; P = .676). Of note, because transplantation was an exclusion criterion for our study, 33% of patients were stratified as low risk and only 3% as high risk, compared with the AAML0531 study cohort, in which 24.1% were considered low risk and 16.5% high risk.1 There were no statistically significant differences in risk assignment between Q1 and Q2 to Q4.
Relevant host factors (sex, age at diagnosis, race, and ethnicity), AML disease characteristics, and treatment with or without gemtuzumab ozogamicin (GO) were equivalent between TC Q1 and Q2 to Q4, except for median age and some AML cytogenetic characteristics (Table 2). The median age in years was higher in Q1 (P = .007), driven by a greater percentage of patients age 1 to 2 years in Q2 to Q4, with no difference in the remaining age categories (Appendix Table A1, online only).
Table 2.
AML Cohort Distribution of Relevant Host Factors and Disease and Treatment Characteristics
All patients with AML inversion 16 [inv(16)], a favorable prognostic indicator, fell into Q2 to Q4 (P = .019). There was an increased incidence of patients with FLT3 internal tandem duplication (ITD), an unfavorable prognostic indicator, in Q1 (P = .014). We observed no difference in age between patients with or without inv(16) (median age, 12.5 years; n = 14 v 14.2 years; n = 59; P = .127). For patients with FLT3-ITD, which increases in prevalence with increasing age, the median age in Q1 was 14.1 years, compared with 10.6 years for Q2 to Q4, similar to the median age of both TC groups overall. Therefore, age-related factors did not seem to play a role in inv(16) or FLT3-ITD distribution. Patients with FLT3-ITD had longer times to ANC recovery compared with those without FLT3-ITD, a difference that was significant for both induction one (P = .010) and intensification one (P = .015), but not significant for other courses. Those in Q1 with FLT3-ITD AML had the longest time to ANC recovery for all courses (data not shown). A subanalysis of TC including only FLT3 wild-type patients was performed to remove the potential impact of FLT3-ITD on recovery time. The difference in time to recovery between FLT3 wild-type patients in Q1 versus Q2 to Q4 was maintained for later chemotherapy courses (intensification two, P = .009; intensification three, P = .019), suggesting the associations we observed were not driven by the presence of FLT3-ITD.
Results from AAML0531 indicated a higher incidence of TRM among patients receiving GO, associated with delays in ANC recovery in later chemotherapy courses.1 We examined the impact of exposure to GO relative to TC quartile for each chemotherapy course. Although for patients in Q2 to Q4, significant differences in mean time to ANC recovery were observed in both intensifications one and two (intensification one: 31 (GO) v 27.8 days (no GO); P = .006; intensification two: 45.9 (GO) v 39.1 days (no GO); P = .023), no statistically significant differences for Q1 patients receiving GO versus no GO were noted for any course.
Rare Variants in Telomere Biology Genes Were Not Associated With TC or Delay in ANC Recovery
Given the enrichment of variants in the TERT gene described in adult populations and the association between telomere biology defects and risk for AML, we sequenced selected regions of the four genes most commonly mutated in DC. Results were obtained from 53 of 53 delayed recovery samples and 60 of 62 expected recovery samples. Three TERT variants with a minor allele frequency (MAF) of less than 2% within patients’ self-described racial group were identified. There were two white non-Hispanic patients with TERT p.H412Y variants (rs34094720; MAF, 1.5%), one with delayed recovery and one with expected recovery, and one white non-Hispanic patient with a TERT p.K1050N variant (rs373400596; MAF, 0.02%) in the expected recovery group (Fig 2). None of these patients had TC in Q1, and no rare variants in the other genes analyzed were identified. Variants at expected frequencies were also observed in our study population (Appendix Table A2, online only).
DISCUSSION
This study represents the first analysis to our knowledge of remission TC and telomere gene sequencing data in patients with leukemia relative to delays in ANC recovery. Our results suggest that less TC at the end of AML induction may predict delays in ANC recovery in later chemotherapy courses. This association was not related to differences in host factors, telomere maintenance gene variants, AML disease characteristics, or therapeutic exposures such as GO. Because of the inverse relationship between age and TC, and because patients age 1 to 2 years were less prevalent in Q1, we adjusted for age in our model and showed TC to be independently predictive of time to ANC recovery. Although prolonged neutropenia is strongly associated with TRM, in this small cohort we were unable to demonstrate differences in infectious complications or EFS or OS in patients with less or more TC.
As observed in both individuals with DC and in murine serial transplantation models,24 significant telomere shortening is associated with profound stress on the hematopoietic compartment. In patients with lymphoma, increasingly prolonged hematopoietic recovery after consecutive transplantations was accompanied by progressive telomere shortening.25 Serial courses of intensive AML chemotherapy may similarly deplete marrow reserve. In our cohort, five patients fell into the greater than 90th percentile for ANC recovery after intensification two (range, 71 to 92 days), all in Q1. Of these, one withdrew from the study and did not receive intensification three. Of the four remaining, three had recovery times well above the mean (57 to 73 days) after intensification three, and one never recovered. Similarly, telomere shortening in association with grade 2 to 4 hematologic toxicities was observed in those with chronic myeloid leukemia in remission,26 suggesting a relationship between telomere shortening and therapy-associated cytopenias.
Diagnoses of BMF syndromes were exclusion criteria for AAML0531 because of associated chemosensitivity.27,28 Because clinical features of these disorders may be subtle, we screened patients for mutations in four genes most commonly associated with DC and found three patients with rare variants, none in Q1. Of note, a rare TERT variant at residue p.K1050 has been described in association with familial pulmonary fibrosis,29 a telomere biology disorder; however, in that report, glutamic acid was substituted for lysine rather than asparagine. Even with comprehensive sequencing of telomere maintenance genes in individuals meeting DC criteria, mutations are found in only 60%.30,31 Because AML may be a presenting feature of DC,16 it is possible that a minor percentage of pediatric patients with AML fall on the DC spectrum. However, our data do not suggest that undiagnosed DC resulting from mutations in the currently identified DC genes is a significant contributor to delays in neutrophil count recovery.
Our results do not elucidate whether patients in Q1 had shorter germline telomeres at diagnosis or increased rates of telomere attrition after two courses of induction chemotherapy. Furthermore, because of limitations in sample availability, we were unable to determine telomere length within WBC subpopulations. FLT3-ITD was related to time to ANC recovery in earlier courses, but sorafenib, a multikinase inhibitor effective against FLT3-ITD that is associated with cytopenias, was not used in this study. As indicative of high-risk disease, FLT3-ITD may represent a proxy for residual disease contributing to prolonged cytopenias. However, the effect we observed was maintained after restricting our study cohort to FLT3 wild-type patients. Moreover, all patients in this study were in remission by morphology (M1) at end of induction two.
In conclusion, both TC measured after chemotherapy induction and age at diagnosis are independently associated with significant delays in ANC recovery later in pediatric AML therapy. Our results suggest that less TC reflects stress on the HSC pool induced by serial courses of intensive chemotherapy. The contribution of other recognized HSC stressors such as infection or proinflammatory cytokines32 may further explain the differences in recovery time among individuals diagnosed with AML. Future studies may consider measuring telomere length prospectively with telomere flow fluorescent in situ hybridization to assess the impact of telomere length in WBC subsets.33 If validated in a larger cohort, prospective ascertainment of telomere length at end of AML induction may permit individualized risk assessment for severe myelosuppression and toxicities with subsequent therapy, as well as clarify the influence of age and cytogenetic or molecular disease characteristics.
ACKNOWLEDGMENT
We thank Kayla Stratton for her assistance in curating Children’s Oncology Group study samples and the American Society of Hematology Clinical Research Training Institute (participant, M.M.G.).
Appendix
Study Population: Therapeutic Regimen
From August 2006 to June 2010, the Children’s Oncology Group study AAML0531 (ClinicalTrials.gov identifier NCT01407757) enrolled 1,022 eligible patients with primary, untreated acute myeloid leukemia between the ages of 1 month and 29.99 years. Patients with an underlying inherited predisposition to bone marrow failure or acute myeloid leukemia (such as those with Fanconi anemia) or with Down syndrome were excluded. All patients were randomly assigned on enrollment to receive standard therapy or standard therapy plus gemtuzumab ozogamicin. The therapeutic regimen for all AAML0531 patients (not eligible for hematopoietic stem-cell transplantation) included five courses of intensive chemotherapy, composed of two induction courses of cytarabine, daunomycin, and etoposide and three intensification courses. Intensification one included cytarabine and etoposide; intensification two included cytarabine and mitoxantrone; intensification three included cytarabine and Escherichia coli L-asparaginase. Patients randomly assigned to GO received the drug in induction one and intensification two. An absolute neutrophil count of 1,000 cells/μL was recommended, but not required, before moving forward with a subsequent course. The study met targeted accrual goals in 2010 for detecting significant differences in event-free and overall survival between the two study arms.1
Telomere Content Determination From Quantitative Polymerase Chain Reaction
Samples were quantified using a NanoDrop spectrophotometer (Thermo Scientific, Waltham, MA). After polymerase chain reaction amplification, the threshold cycle (Ct) values were averaged for the telomere content (TC) calculation. A threshold of deviation of 0.15 from the middle value of each triplicate was selected, so for wells with Ct values greater than 0.15 from the middle value, or for wells failing to amplify, the Ct values of the remaining two wells were averaged for the TC calculation. If this rule applied to two or more wells in the triplicate, the sample was excluded from the analysis. Given that the efficiencies of the target and reference amplifications were not approximately equal and that the comparison was being made across multiple plates, TC was then calculated for each sample using the standard curve method. The average quantity of telomere DNA was divided by the average quantity of 36B4 DNA in each sample, based on the known quantity of DNA in the standardized dilutions.
Sequencing of Select Telomere Maintenance Genes
Sequencing of polymerase chain reaction–amplified segments of genomic DNA included all exons and intron/exon boundaries of TERT and TERC, which encode the telomerase RNA subunit. Also sequenced were exons harboring the vast majority of DC-associated mutations in DKC1 (exons 3, 4, 10, 11, and 12) and all of the DC-associated mutations in TINF2 (exon 6).22 Sanger sequencing and single-nucleotide variant or short insertion/deletion detection were performed by Beckman Coulter Agencourt (Danvers, MA). Sequence alterations were manually reviewed and verified using Seqman Pro software (Lasergene; DNASTAR, Madison, WI). All minor allele frequencies within specific racial and ethnic subgroups were obtained from the Exome Aggregation Consortium (Cambridge, MA; http://exac.broadinstitute.org).
Table A1.
Age Distribution of Patients in Two TC Quartile Groups
Table A2.
Polymorphisms Observed in Study Population
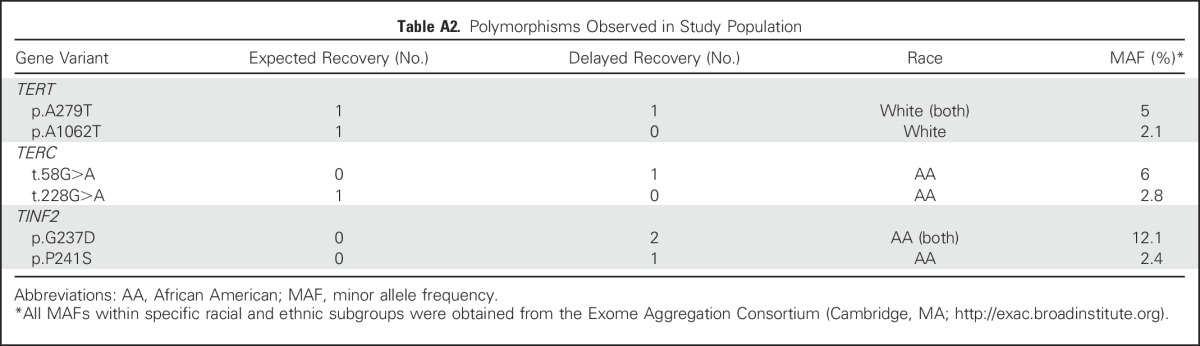
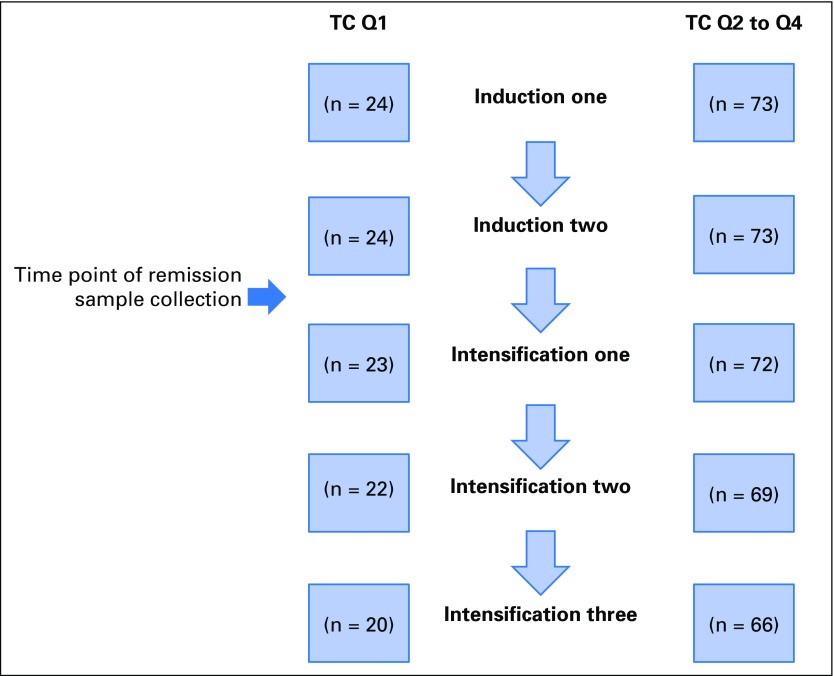
Fig A1.
Both telomere content (TC) comparison groups (quartile one [Q1] and Q2 to Q4) show some degree of patient attrition over the five chemotherapy courses. Shown is a comparison of patient attrition in each TC quartile group over five chemotherapy courses. After induction two, two patients were excluded for not achieving remission. After intensification one, four patients were excluded for proceeding to hematopoietic stem-cell transplantation. After intensification two, one patient experienced relapse and was removed from the study, three withdrew because of unacceptable toxicities, and one withdrew by physician decision. Not all patients were permitted complete recovery before chemotherapy was initiated. For Q1, recovery rates to absolute neutrophil count of 500 cells/μL before receiving subsequent chemotherapy were 24 of 24 for induction one, 23 of 24 for induction two, 21 of 23 for intensification one, 20 of 22 for intensification two, and 17 of 20 for intensification three. For Q2 to Q4, recovery rates before receiving subsequent chemotherapy were 70 of 73 for induction one, 73 of 73 for induction two, 70 of 72 for intensification one, 69 of 69 for intensification two, and 64 of 66 for intensification three.
Fig A2.
Analysis of individual telomere content (TC) quartiles confirmed the association between less TC in quartile one (Q1) and delays in absolute neutrophil count (ANC) recovery. TC Q1 was compared with Q2, Q3, and Q4 relative to the number of days to ANC recovery (500 cells/μL). No difference in TC was noted for the first three courses. In the fourth and fifth courses, patients in Q1 demonstrated a longer mean ANC recovery time, which was significant for both intensifications (A) two (P = .001) and (B) three (P = .014).
Footnotes
Supported by an Alex’s Lemonade Stand Young Investigator Award (M.M.G.), St Baldrick’s Foundation Scholar Award (M.M.G.), and National Institutes of Health K23 Award No. CA158148 (M.M.G.) and by Grants No. CA180899 and CA98413 (Statistics and Data Center), CA98543 (Chair’s Grant), and CA180886 (National Clinical Trials Network Operation Center Grant) to the Children’s Oncology Group.
Presented as an abstract in poster form at the 56th Annual Meeting of the American Society of Hematology, San Francisco, CA, December 6-9, 2014, and as an oral presentation at the Children’s Oncology Group Fall Meeting, Dallas, TX, September 16-19, 2014.
Authors’ disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHOR CONTRIBUTIONS
Conception and design: Sharon E. Plon, Alison A. Bertuch, Maria M. Gramatges
Provision of study materials or patients: Alan S. Gamis, Soheil Meshinchi
Collection and assembly of data: Robert B. Gerbing, Lillian Sung, Alan S. Gamis, Soheil Meshinchi, Maria M. Gramatges
Data analysis and interpretation: Robert B. Gerbing, Todd A. Alonzo, Sharon E. Plon, Alison A. Bertuch, Maria M. Gramatges
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Shorter Remission Telomere Length Predicts Delayed Neutrophil Recovery After Acute Myeloid Leukemia Therapy: A Report From the Children’s Oncology Group
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Robert B. Gerbing
No relationship to disclose
Todd A. Alonzo
No relationship to disclose
Lillian Sung
No relationship to disclose
Alan S. Gamis
Consulting or Advisory Role: Pfizer, Novartis
Soheil Meshinchi
No relationship to disclose
Sharon E. Plon
Stock or Other Ownership: Dexcom, Johnson & Johnson, Insulet, Tandemdiabetes, Lexicon
Consulting or Advisory Role: Baylor Miraca Genetic Laboratory
Alison A. Bertuch
No relationship to disclose
Maria M. Gramatges
No relationship to disclose
REFERENCES
- 1.Gamis AS, Alonzo TA, Meshinchi S, et al. Gemtuzumab ozogamicin in children and adolescents with de novo acute myeloid leukemia improves event-free survival by reducing relapse risk: Results from the randomized phase III Children’s Oncology Group trial AAML0531. J Clin Oncol. 2014;32:3021–3032. doi: 10.1200/JCO.2014.55.3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lange BJ, Smith FO, Feusner J, et al. Outcomes in CCG-2961, a Children’s Oncology Group phase 3 trial for untreated pediatric acute myeloid leukemia: A report from the Children’s Oncology Group. Blood. 2008;111:1044–1053. doi: 10.1182/blood-2007-04-084293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bodey GP, Buckley M, Sathe YS, et al. Quantitative relationships between circulating leukocytes and infection in patients with acute leukemia. Ann Intern Med. 1966;64:328–340. doi: 10.7326/0003-4819-64-2-328. [DOI] [PubMed] [Google Scholar]
- 4.Sung L, Lange BJ, Gerbing RB, et al. Microbiologically documented infections and infection-related mortality in children with acute myeloid leukemia. Blood. 2007;110:3532–3539. doi: 10.1182/blood-2007-05-091942. [DOI] [PubMed] [Google Scholar]
- 5.Iwama H, Ohyashiki K, Ohyashiki JH, et al. Telomeric length and telomerase activity vary with age in peripheral blood cells obtained from normal individuals. Hum Genet. 1998;102:397–402. doi: 10.1007/s004390050711. [DOI] [PubMed] [Google Scholar]
- 6.Vaziri H, Dragowska W, Allsopp RC, et al. Evidence for a mitotic clock in human hematopoietic stem cells: Loss of telomeric DNA with age. Proc Natl Acad Sci USA. 1994;91:9857–9860. doi: 10.1073/pnas.91.21.9857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiang YJ, Calado RT, Hathcock KS, et al. Telomere length is inherited with resetting of the telomere set-point. Proc Natl Acad Sci USA. 2010;107:10148–10153. doi: 10.1073/pnas.0913125107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diker-Cohen T, Uziel O, Szyper-Kravitz M, et al. The effect of chemotherapy on telomere dynamics: Clinical results and possible mechanisms. Leuk Lymphoma. 2013;54:2023–2029. doi: 10.3109/10428194.2012.757765. [DOI] [PubMed] [Google Scholar]
- 9.Lee JJ, Nam CE, Cho SH, et al. Telomere length shortening in non-Hodgkin’s lymphoma patients undergoing chemotherapy. Ann Hematol. 2003;82:492–495. doi: 10.1007/s00277-003-0691-4. [DOI] [PubMed] [Google Scholar]
- 10.Schröder CP, Wisman GB, de Jong S, et al. Telomere length in breast cancer patients before and after chemotherapy with or without stem cell transplantation. Br J Cancer. 2001;84:1348–1353. doi: 10.1054/bjoc.2001.1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Artandi SE, Attardi LD. Pathways connecting telomeres and p53 in senescence, apoptosis, and cancer. Biochem Biophys Res Commun. 2005;331:881–890. doi: 10.1016/j.bbrc.2005.03.211. [DOI] [PubMed] [Google Scholar]
- 12.Allsopp RC, Vaziri H, Patterson C, et al. Telomere length predicts replicative capacity of human fibroblasts. Proc Natl Acad Sci USA. 1992;89:10114–10118. doi: 10.1073/pnas.89.21.10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang J, Lu X, Sakk V, et al. Senescence and apoptosis block hematopoietic activation of quiescent hematopoietic stem cells with short telomeres. Blood. 2014;124:3237–3240. doi: 10.1182/blood-2014-04-568055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balakumaran A, Mishra PJ, Pawelczyk E, et al. Bone marrow skeletal stem/progenitor cell defects in dyskeratosis congenita and telomere biology disorders. Blood. 2015;125:793–802. doi: 10.1182/blood-2014-06-566810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alter BP, Rosenberg PS, Giri N, et al. Telomere length is associated with disease severity and declines with age in dyskeratosis congenita. Haematologica. 2012;97:353–359. doi: 10.3324/haematol.2011.055269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alter BP, Giri N, Savage SA, et al. Cancer in dyskeratosis congenita. Blood. 2009;113:6549–6557. doi: 10.1182/blood-2008-12-192880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hills M, Lansdorp PM. Short telomeres resulting from heritable mutations in the telomerase reverse transcriptase gene predispose for a variety of malignancies. Ann N Y Acad Sci. 2009;1176:178–190. doi: 10.1111/j.1749-6632.2009.04565.x. [DOI] [PubMed] [Google Scholar]
- 18.Calado RT, Regal JA, Hills M, et al. Constitutional hypomorphic telomerase mutations in patients with acute myeloid leukemia. Proc Natl Acad Sci USA. 2009;106:1187–1192. doi: 10.1073/pnas.0807057106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aalbers AM, Calado RT, Young NS, et al. Telomere length and telomerase complex mutations in pediatric acute myeloid leukemia. Leukemia. 2013;27:1786–1789. doi: 10.1038/leu.2013.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gramatges MM, Liu Q, Yasui Y, et al. Telomere content and risk of second malignant neoplasm in survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Clin Cancer Res. 2014;20:904–911. doi: 10.1158/1078-0432.CCR-13-2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 22.Cox DR. Regression models and life tables. J R Stat Soc [Ser A] 1972;34:187–220. [Google Scholar]
- 23.Sung L, Aplenc R, Alonzo TA, et al. Effectiveness of supportive care measures to reduce infections in pediatric AML: A report from the Children’s Oncology Group. Blood. 2013;121:3573–3577. doi: 10.1182/blood-2013-01-476614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allsopp RC, Cheshier S, Weissman IL. Telomere shortening accompanies increased cell cycle activity during serial transplantation of hematopoietic stem cells. J Exp Med. 2001;193:917–924. doi: 10.1084/jem.193.8.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Widmann T, Kneer H, König J, et al. Sustained telomere erosion due to increased stem cell turnover during triple autologous hematopoietic stem cell transplantation. Exp Hematol. 2008;36:104–110. doi: 10.1016/j.exphem.2007.08.028. [DOI] [PubMed] [Google Scholar]
- 26.Lobetti-Bodoni C, Ferrero D, Genuardi E, et al. Telomere loss in Philadelphia-negative hematopoiesis after successful treatment of chronic myeloid leukemia: Evidence for premature aging of the myeloid compartment. Mech Ageing Dev. 2012;133:479–488. doi: 10.1016/j.mad.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 27.Dietz AC, Orchard PJ, Baker KS, et al. Disease-specific hematopoietic cell transplantation: Nonmyeloablative conditioning regimen for dyskeratosis congenita. Bone Marrow Transplant. 2011;46:98–104. doi: 10.1038/bmt.2010.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gluckman E, Devergie A, Schaison G, et al. Bone marrow transplantation in Fanconi anaemia. Br J Haematol. 1980;45:557–564. doi: 10.1111/j.1365-2141.1980.tb07178.x. [DOI] [PubMed] [Google Scholar]
- 29.Cronkhite JT, Xing C, Raghu G, et al. Telomere shortening in familial and sporadic pulmonary fibrosis. Am J Respir Crit Care Med. 2008;178:729–737. doi: 10.1164/rccm.200804-550OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ballew BJ, Savage SA. Updates on the biology and management of dyskeratosis congenita and related telomere biology disorders. Expert Rev Hematol. 2013;6:327–337. doi: 10.1586/ehm.13.23. [DOI] [PubMed] [Google Scholar]
- 31.Kocak H, Ballew BJ, Bisht K, et al. Hoyeraal-Hreidarsson syndrome caused by a germline mutation in the TEL patch of the telomere protein TPP1. Genes Dev. 2014;28:2090–2102. doi: 10.1101/gad.248567.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walter D, Lier A, Geiselhart A, et al. Exit from dormancy provokes DNA-damage-induced attrition in haematopoietic stem cells. Nature. 2015;520:549–552. doi: 10.1038/nature14131. [DOI] [PubMed] [Google Scholar]
- 33.Baerlocher GM, Vulto I, de Jong G, et al. Flow cytometry and FISH to measure the average length of telomeres (flow FISH) Nat Protoc. 2006;1:2365–2376. doi: 10.1038/nprot.2006.263. [DOI] [PubMed] [Google Scholar]