Abstract
Hearing loss is hidden disability and second most common congenital pathology. Prevention, early identification and early intervention of hearing loss can prevent further disability in development of speech, language, cognition and other developmental domains. The prevalence of congenital hearing loss has been estimated to be 1.2–5.7 per thousand in neonates. In these contexts, the aim of study was to determine outcomes of neonates hearing screening program in Hospital. It is a clinical cross-sectional study which was conducted in tertiary care centre from 8th July, 2015 to 31th May, 2016. Total no of 2254 cases were screened. Details case history including high risk register, Pediatric Audiometry, Otoacoustic Emission tests were performed followed brainstem evoked response audiometry. The Prevalence of hearing loss among high risk babies confirmation by BERA was 8.8% per 1000 babies and 16 cases were recommended for Cochlear Implant. The screening protocol with objective test i.e. Distortion Product Otoacoustic Emission and confirmation by Brainstem Evoked Response Audiometry is very useful tool in early identification of congenital hearing loss in neonates. Hence, the results of this study will be used to initiate universal newborn hearing screening in other hospitals. Moreover, this study highlights the relevance of neonatal hearing screening in other states of India and country where this screening is not performed routinely in all hospitals and creating awareness to identify neonatal risk factors associated with hearing loss and understand the importance of early identification and early intervention and among health care professionals.
Keywords: Hearing, Early identification, Otoacoustic emission, Auditory brainstem response, High risk, Neonate
Introduction
Hearing is very essential part of speech and language development in neonates. Hearing impairment is broad term that refers to hearing losses of varying degrees, ranging from hard-of-hearing to total deafness [1]. The prevalence of congenital hearing loss has been estimated to be 1.2–5.7 per thousand in neonates [2, 3] and more in high risk neonates. Hearing impairment is invisible disability which affects communication ability, social, education, personal achievement, economic independence and quality of life. Thus, prevents the impact of hearing loss on overall different developmental domains, this newborn hearing screening program were started for reducing the burden of congenital hearing loss in society. Newborn hearing screening program mainly focus on early identification and management of hearing loss as soon as possible. Studies reported that newborn screening should be within 1 months of age, diagnosis within 3 months of age and rehabilitation should be started at the age of 6 months [4, 5]. This Early identification and early intervention through aural habilitation and speech and language therapy utilize the critical development period (Birth to 5 years) for speech and language development [1]. Studies reported that neonatal risk factors can be associated with hearing loss [6]. Thus, another purpose of newborn hearing screening program is identify risk factors for hearing loss in high risk neonates and prevalence of hearing loss. In these contexts, the aim of study was Outcomes of Newborn Hearing Screening Program in Hospital. The major outcome measures were to determine the prevalence of hearing impairment, frequency of occurrence of neonatal risk indicators.
Material and Method
It is clinical cross-sectional study. The study was conducted in Audiology unit, in a tertiary care centre from 8th July, 2015 to 31th May, 2016.
Participates
Neonates who have following risk factors were included in this study i.e. Low birth weight (<1.5 kg), Premature delivery (<34 weeks of Gestation), Neonatal jaundice, Admitted NICU (more than 5 days), Birth asphyxia, Low APGAR score: 0–4 @1 min, 0–6 @5 min, Fetal distress, Aspiration of amniotic fluid, Craniofacial/Structural anomalies, Congenital anomalies, Convulsions, Viral/bacterial infections, Family history of deafness. Neonates without any risk factors and Parents refusing screening were excluded from study. For selection of participants, personal information and risk factors were recorded from medical records. They were not paid for participation and permission from a relevant institutional ethics committee was taken prior to the commencement of the study.
Stimulus and Procedure
Details Case History was taken for each baby. In this, included demographic details, Family history of deafness and Consanguity, Prenatal risk factors i.e. Excessive vomiting, Elderly pregnancy, High/low B.P, Blood sugar, History of abortions, Rh incompatibility and postnatal risk factors, natal factors i.e. Low birth weight, Premature delivery, Neonatal jaundice, Admitted NICU Birth asphyxia, APGAR score, Fetal distress, Aspiration of amniotic fluid and postnatal i.e. Craniofacial/Structural anomalies, Congenital anomalies, Convulsions, Poor vegetative skills, Viral/bacterial infections (only high fever related). Otoscopic examination was done to check the status of status of ear canal and tympanic membrane. Distortion product Otoacoustic emission (DPOAE) and Behavioral observation audiometry (BOA) were performed using Pediatric audiometer. DPOAE were performed after 24 h of birth for reduce the referral rate as presence of debris, vernix and amniotic fluid can affect OAE responses. The baby who failed in first hearing screening, second screening follow up was given after 1 week for retest DPOAE. The baby, who failed in second screening, underwent for BERA (Brainstem evoked response audiometry).
Referral Criteria
High Risk Register (HRR) Baby refer if more than two high risk factors.
Behavioral Audiometry Response (BOA) in either of the ear.
Oto-Acoustic Emissions absent in each ear.
Pediatric Audiometry
Interacoustic Pediatric Audiometer (PA-5) were used for test the behavior responses of neonates at 500, 1000 and 4000 Hz at 75–90 dBHL.
Immittance Audiometry
Tympanometry was performed bilaterally using 1000-Hz probe tone. Acoustic reflex thresholds were measured at octave frequencies from 250, 500, 1000, 2000 and 4000 Hz ipsilateral and contralateral in both ears. The repeated testing was done for to test the reliability of results.
Distortion Product Otoacoustic Emission (DPOAE)
DPOAE were performed in setting of frequency band from 1000 to 6000 Hz, stimulus intensity L1 = 65 dBSPL and L2 = 55 dBSPL, frequency ratio F2:F1 = 1.2:1 and signal to noise ratio of at least 6 dB with a reproducibility score of at least 70%.
Brainstem Evoked Response Audiometry (BERA)
Click-evoked ABR were performed in both ears monaurally. The testing was performed after sedation with sleeping status of patient. Single channel recording were used in which non-inverting electrode was placed on the vertex; the inverting electrode was placed on mastoid of test ear and ground electrode was placed on mastoid of nontest ear. Electrode impedances were less than 5 kΩ, and inter-electrode impedances were less than 2 kΩ. Eartone-3A insert earphone was used to present the stimuli. At least 1500 click stimuli in rarefaction polarity presented at 11.1 click/sec repetition rate. Potentials were recorded in band-pass filter setting 30–3000 Hz with an amplification factor of 100,000. Recordings were started from 70 dBnHL to 10dB step lowing intensity and to ensure the replicability of the waveform at each intensity. ABR waveforms were analyzed at 15–20 ms time window setting.
All objective audiological testing were conducted in an acoustically treated room where the noise levels were within permissible limits [7].
Result
Prevalence of Hearing Impairment
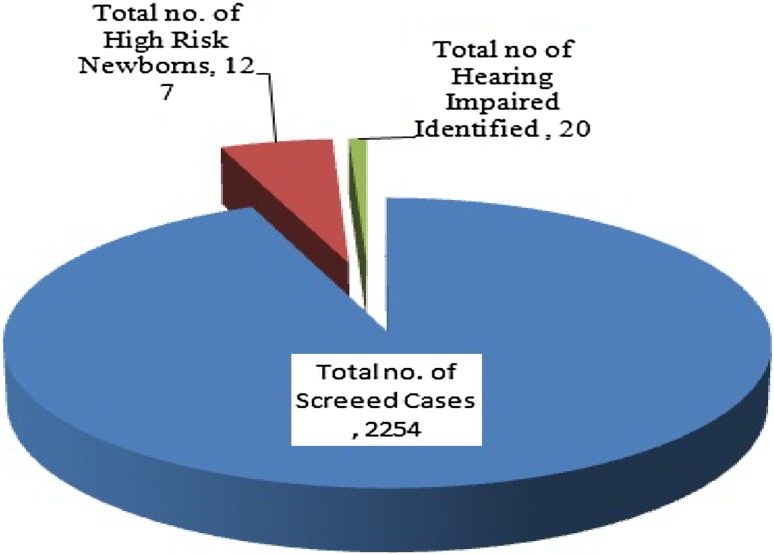
Total number of 2254 cases was screened, in which 127 cases have present of risk factors, 2127 cases was not at risk and 20 (0.88%) high risk cases were conformed diagnosed as hearing impaired by BERA test (Fig. 1).
Fig. 1.
Indicate total of identified high risk neonates and hearing impairment
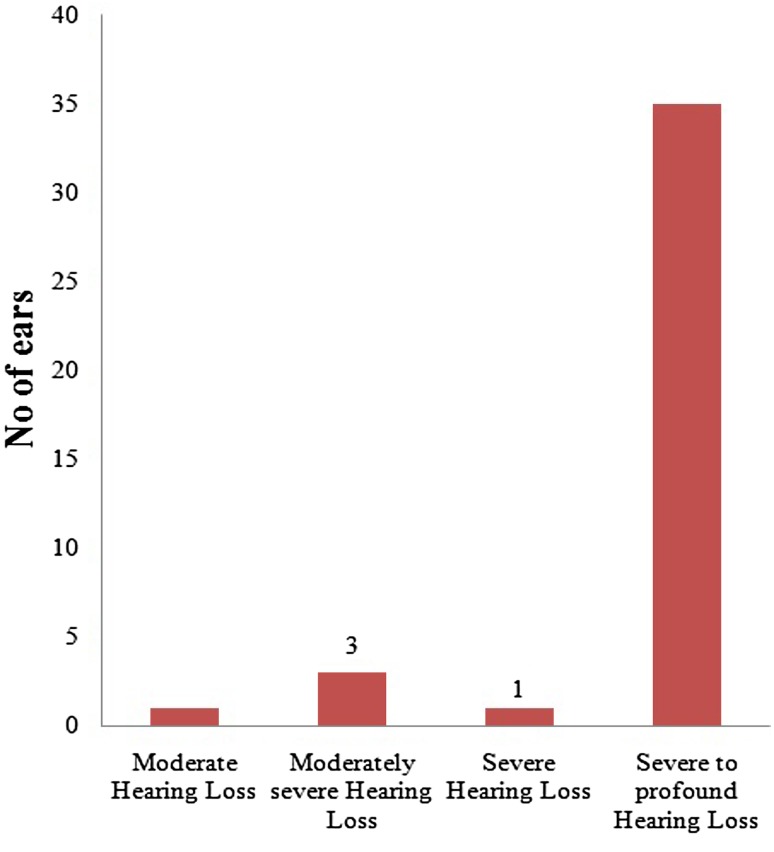
Degree of Identified Hearing Loss
Fig. 2
Fig. 2.
Indicate degree of hearing loss in total no of 20 referred babies (40 ears) based on BERA test
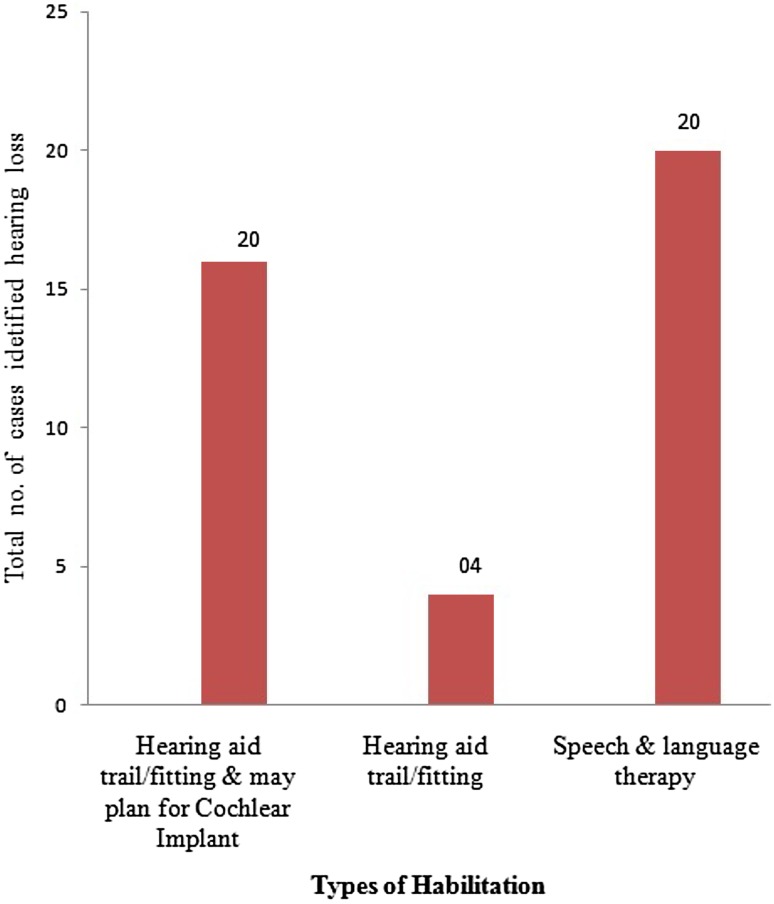
Habilitation of Identified Hearing Impairment
All infants with bilateral severe to profound hearing has recommended for cochlear implants followed by 3 months auditory training with hearing aid. Infants with moderate to severe hearing loss recommended hearing aid fitting along with speech and language therapy (Fig. 3).
Fig. 3.
Indicate types of habilitation provided to newborns
Frequency of Occurrence of Natal Risk Factors
Discussion
The present study outlines the outcomes of neonatal hearing screening program in hospital. The result of this study indicates 20 high risk babies (0.88%) in total no. of 2254 screened babies were diagnosed as hearing impairment. Thus, The Prevalence of hearing loss among high risk babies confirmation by BERA was 8.8% per 1000 babies. Thus, newborn hearing screening is crucial, and universal newborn hearing screening (UNHS) is more valuable rather than screening just those who have been admitted to neonatal intensive care unit (NICU) [8] as high risk baby can be missed 50% of babies which is better identify UNHS [9]. Previous study reported that congenital sensorineural hearing loss occurs in approximately 1–5 per 1000 babies and this rate may be higher in high risk neonates [10] but it is 10.2% reported in India [11]. The prevalence rate of hearing loss in neonates varies 1 and 8 per 1000 babies screened in India which is mainly due to methodological differences among studies [9, 12, 13]. In present study slight higher prevalence of hearing loss obtained which may be due to rural population, lack of awareness, screened both normal and high risk factors, lack of manpower resulting larger coverage area for screening and referral rate. In support of these findings, previous studies reported that prevalence of hearing loss higher in rural than urban population [11] and high risk neonates. [9].
The management option for hearing impaired included hearing aid, cochlear implant and auditory training along with speech and language therapy [14]. Cochlear implant mainly recommended for bilateral severe to profound hearing loss with normal auditory nerve functioning [15]. Thus, in the current study, 16 cases diagnosed as bilateral severe to profound hearing loss were recommended to use hearing aid with further plan for cochlear implant and 04 cases identified as moderate to severe degree of hearing loss either of ears were advice to regular use of hearing aid only. Regular follow up of 3, 6 then 12 months has been given for all high risk babies who had passed in hearing screening as it may cause late onset or progressive hearing loss. Similar follow up procedure suggested in previous study [16]. Follow up is major concern in newborn hearing screening program as a change of address, thus difficult to contact, rural population, poor parental response, illiterate and lower economic status, resulting late onset/progressive hearing loss and delayed speech and language development may be missed out. Thus, speech and language milestone development lists were provided for improving follow up rate.
In the present study, Table 1 indicate the % of identified risk factors in high risk babies which may cause hearing loss i.e. delayed birth cry (20%), neonatal jaundice (15%), convulsion (15%), Birth asphyxia (15%), Family history of deafness (5%). It was also reported that high risk factors may cause hearing loss [17, 18].
Table 1.
Indicate frequency of occurrence of natal risk factor in neonates
| S. No. | Risk factors | Total screened cases | Hearing impaired | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| 1. | Low birth weight | 33 | 1.46 | 02 | 10 |
| 2. | Neonatal Jaundice | 81 | 3.59 | 03 | 15 |
| 3. | Delayed birth cry | 139 | 5.94 | 04 | 20 |
| 4. | Premature delivery | 71 | 6.16 | 01 | 0.0 |
| 5. | Birth asphyxia | 128 | 5.67 | 03 | 15 |
| 6. | Fetal distress | 40 | 1.77 | 00 | 00 |
| 7. | Aspiration of amniotic fluids | 47 | 2.08 | 00 | 00 |
| 8. | NICU (>5 days) | 140 | 6.21 | 05 | 25 |
| 9. | Convulsion | 32 | 1.41 | 03 | 15 |
| 10. | Craniofacial abnormalities | 01 | 0.05 | 00 | 00 |
| 11. | Congenital abnormalities | 02 | 0.10 | 00 | 00 |
| 12. | Family history of deafness | 01 | 0.05 | 01 | 05 |
| 13. | Viral/bacterial infection | 05 | 0.22 | 00 | 00 |
OAE test were recommended for first step of hearing screening as it is objective, easy, less expensive and lesser time consuming [19]. In current study, DPOAE was used for neonatal hearing screening. Thus, furthermore TEOAE should be used for identify mild degree of hearing loss.
Conclusion
The screening protocol with objective test i.e. Distortion Product Otoacoustic Emission and confirmation by Brainstem Evoked Response Audiometry is very useful tool in early identification and early intervention of congenital hearing loss in newborn which prevents further disability of speech, language and auditory memory. Hence, the results of this study will be used to initiate universal newborn hearing screening in other hospitals. Moreover, this study highlights the relevance of neonatal hearing screening in other states of India and country where this screening is not performed routinely in all hospitals and creating awareness to identify neonatal risk factors associated with hearing loss and understand the importance of early identification and early intervention and among health care professionals.
Authors Contribution
The manuscript has been read and approved by all the authors, that the requirements for authorship as stated earlier in this document have been met, and that each author believes that the manuscript represents honest work.
Compliance with Ethical Standards
Conflicts of interest
None.
Ethical Standards
All the author have seen and approved the final version of manuscript being submitted there is no conflict of interest or financial interest of authors. Consent of parents is taken to use the clinical material for publishing. No animals were harmed during the publications. Publication is done in accordance with the Ethical Committee guidelines.
References
- 1.Shemesh R (2010) Hearing impairment: definitions, assessment and management. Int Encycl Rehabil
- 2.Parving A. Congenital hearing disability: epidemiology and identification—a comparison between two health authority districts. Int J Pediatr Otorhinolaryngol. 1993;27:29–46. doi: 10.1016/0165-5876(93)90034-Z. [DOI] [PubMed] [Google Scholar]
- 3.Kim SY, Bothwell NE, Backousn DD. The expanding role of the otolaryngologist in managing infants and children with hearing loss. Otolaryngol Clin N Am. 2002;35:699. doi: 10.1016/S0030-6665(02)00049-X. [DOI] [PubMed] [Google Scholar]
- 4.Hayes D. State programs for universal newborn hearing screening. Pediatr Clin N Am. 1999;46:89–94. doi: 10.1016/S0031-3955(05)70083-3. [DOI] [PubMed] [Google Scholar]
- 5.American Speech language Hearing Association (2008) Loss to follow up in early hearing detection and Intervention
- 6.Joint Committee on Infant Hearing Year 2007 position statement: principles and guidelines for early hearing detection and intervention programs. Pediatrics. 2007;120(4):898–921. doi: 10.1542/peds.2007-2333. [DOI] [PubMed] [Google Scholar]
- 7.American National Standards Institute (ANSI) (1991) Maximum permissible ambient noise levels for audiometric test rooms. ANSI S3.1-1991. ANSI, New York
- 8.Grill E, Hessel F, Siebert U, Schnell-inderst P, Kunze S, Nickisch A, Wasem J. Comparing the clinical effectiveness of different newborn hearing screening strategies. A decision analysis. BMC Public Health. 2005;5(1):12. doi: 10.1186/1471-2458-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagapoornima P, Ramesh A, Rao S, Patricia PL, Gore M, et al. Universal hearing screening. Indian J Pediatr. 2007;74:545–549. doi: 10.1007/s12098-007-0105-z. [DOI] [PubMed] [Google Scholar]
- 10.Mehra S, Eavey RD, Keamy DG., Jr The epidemiology of hearing impairment in the United States: newborns, children, and adolescents. Otolaryngol Head Neck Surg. 2009;140:461–472. doi: 10.1016/j.otohns.2008.12.022. [DOI] [PubMed] [Google Scholar]
- 11.Indian Council of Medical Research Report . Collaborative study on prevention and etiology of hearing impairment. New Delhi: Indian Council of Medical Research; 1983. [Google Scholar]
- 12.Paul AK. Early identification of hearing loss and centralized newborn hearing screening facility: the Cochin experience. Indian Pediatr. 2011;48:355–359. doi: 10.1007/s13312-011-0067-0. [DOI] [PubMed] [Google Scholar]
- 13.Rai N, Thakur N. Universal screening of newborns to detect hearing impairment: Is it necessary? Int J Pediatr Otorhinolaryngol. 2013;77:1036–1041. doi: 10.1016/j.ijporl.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 14.Jerger S, Roeser RJ, Tobey EA. Management of Hearing Loss in Infants: The UTD/Callier Center Position Statement. J Am Acad Audiol. 2001;12:329–336. [PubMed] [Google Scholar]
- 15.Papsin BC, Gordon KA. Cochlear implants for children with severe-to-profound hearing loss. N Engl J Med. 2007;357:2380–2387. doi: 10.1056/NEJMct0706268. [DOI] [PubMed] [Google Scholar]
- 16.Colella-Santos MF, Hein TAD, Souza GLD, Amaral MIRD. Newborn hearing screening and early diagnostic in the NICU. Biomed Res Int . 2014;2014:1–11. doi: 10.1155/2014/845308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salamy A, Eldredge L, Tooley WH. Neonatal status and hearing loss in high-risk infants. J Pediatr. 1989;114(5):847–852. doi: 10.1016/S0022-3476(89)80151-9. [DOI] [PubMed] [Google Scholar]
- 18.Cristobal R, Oghalai JS. Hearing loss in children with very low birth weight: current review of epidemiology and pathophysiology. Arch Dis Child Fetal Neonatal Ed. 2008;93(6):F462–F468. doi: 10.1136/adc.2007.124214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garg S, Singh R, Khurana D. Infant hearing screening in India: current status and way forward. Int J Prev Med. 2015;6(1):113. doi: 10.4103/2008-7802.170027. [DOI] [PMC free article] [PubMed] [Google Scholar]