Abstract
The Parapharyngeal Space (PPS) tumors are rare tumors accounting for 0.5% of the tumors in head and neck region. A retrospective study conducted at Department of Surgical oncology, Vydehi institute of Medical Sciences & Research center, Bengaluru, between 2010 and 2015 identified nine cases treated for PPS tumors. Patients are diagnosed on the basis of clinical examination fine needle aspiration cytology and imaging and considered for excision by one of the varied surgical approaches {transoral(1), transcervical(4), transparotid(0), transcervical-transparotid(1), transmandibular(3) or intratemporal(0) approach}. The choice of approach is defined by the size of the tumor, suspicion of malignancy and the position of the tumor with regard to the superior extent and proximity to the skull base as well as its relation with neurovascular bundle.
Keywords: Parapharyngeal space tumors, Parapharyngeal space masses, Surgery, Transcervical, Transparotid, Mandibular swing
Introduction
The Paraphaeryngeal space(PPS) tumors are rare, accounting for 0.5% of tumors of the head and neck [1, 2]. Benign neoplasms account for 75–80% and malignant neoplasms account for 20–25% [3]. The diverse range and complexity of anatomy with varied surgical approaches as well as rarity of presentation makes these tumors interesting to study and report.
The purpose of the study is to review our experience at our Oncology center, addressing the morphologic type of tumors, their histology, clinical aspects and surgical approach.
Materials and Methods
A retrospective study was conducted on the patients presenting to and treated at the Oncology center of Vydehi Institute of medical sciences, Bengaluru between 2010 and 2015, focusing on tumors originating from the structures located in this anatomical area. Nine patients were included in the study. All the patients were considered for surgical resection after radiological and cytological evaluation.
Results
During this period we treated nine patients. Of them five were women (56%); with mean age of 29 years and range of 19–50; four patients (44%) were men, with an average age 44, range 27–57 years.
The most frequently observed symptom was a discrete intraoral pharyngeal mass lesion in four patients (44%); Three (33%) presented with cervical swelling, one presented with hoarseness and one with trismus. The median time from the onset of symptoms to diagnosis was 5.8 months with a range of 0–24 months.
On physical examination, the most common sign (three patients, 33%) was the existence of a swelling on the side wall of the oropharynx, with medial displacement of the tonsillar region and/or soft palate (Fig. 1). In two patients (22%) we noticed a protrusion of lateral pharyngeal wall along with neck swelling. Two patients (22%) had cervical masses without any other clinical manifestations.
Fig. 1.
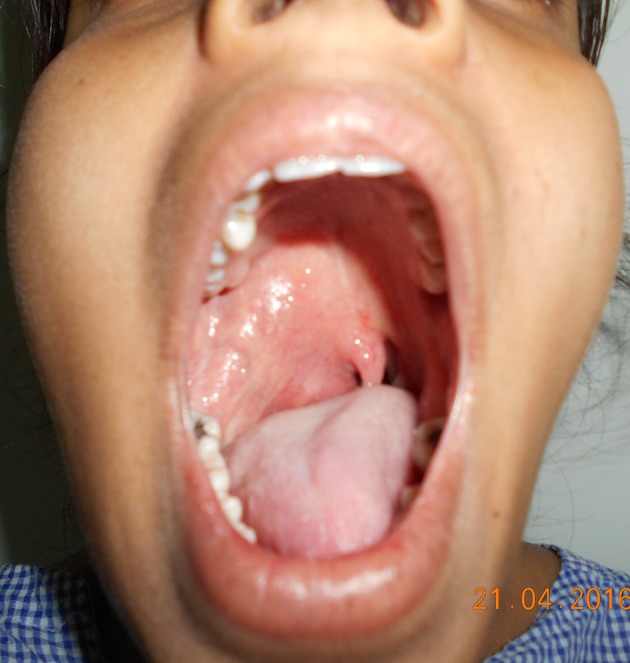
Clinical photograph showing a swelling in the right lateral side wall of oropharynx
Radiological techniques used for evaluation of these tumors were mainly computerized topography (CT) (5 patients) and Magnetic Resonance imaging (MRI) (3 patients). The imaging showed mass lesion in the PPS and their relation to vasculature. Neurofibroma on CT showed hypodense lesion with no or minimal contrast enhancement (Fig. 2). On MRI, Neurofibroma is hypointense on T1 image and on T2 has hyperintense rim with central area of low intensity. Pleomorphic adenoma on CT shows lobulated heterogenous mass with areas of necrosis.
Fig. 2.
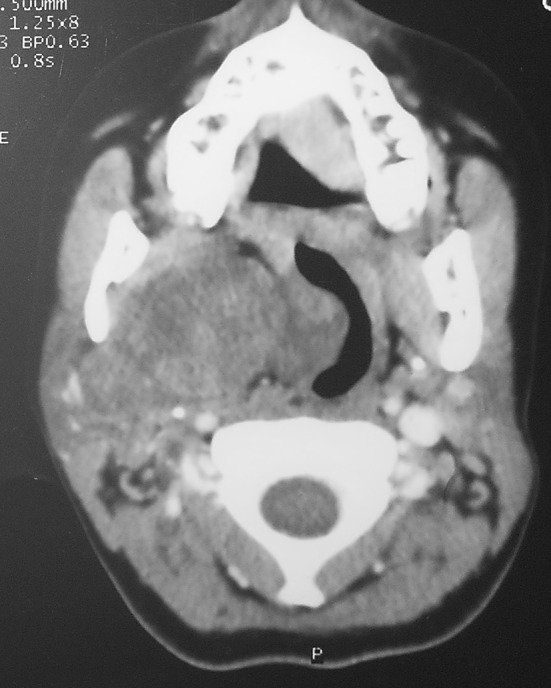
Axial CT showing mass lesion in the right parapharyngeal space
Fine needle aspiration was requested in 8 cases, except for the one with trismus. Fine needle aspiration cytology gave a clue about the benign or malignant pathological nature of the lesion.
All the 9 patients were operated. Of them, 8 tumors were completely excised while one was incomplete (fibromatous lesion).
The route of transcervical approach (Fig. 3) was the most commonly used (4 cases, 44%), for the resection 2 pleomorphic adenomas, 1 fibromatous lesion and 1 malignant lesion. The route transcervical-transparotid was practiced in 1 patient for brancial cyst-1st cleft and in 3 cases transmandibular route with mandibular swing is used- 2 neurogenic tumors and 1 malignant lesion. The transoral approach is used in 1 patient of pleomorphic adenoma of minor salivary gland.
Fig. 3.
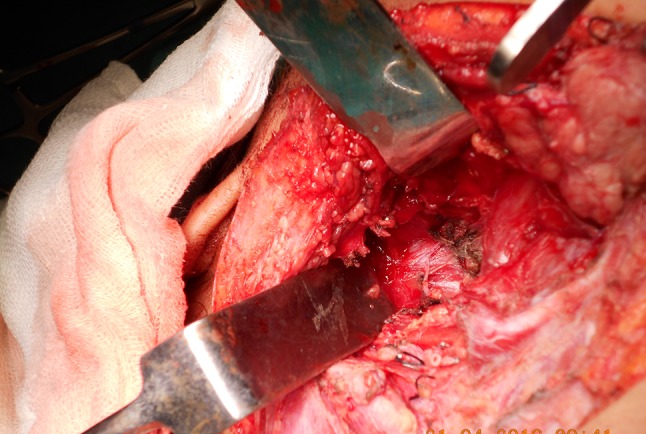
Post excision cavity after removal of the mass via transcervicall approach
Histopathological result of the 9 tumors diagnosed: 7 (78%) for a benign pathology and 2 (22%) for malignant. In the benign, the most common was the pleomorphic adenoma (3 cases, 33%), followed by tumors of neurogenic origin (2 cases, 22%), 1 fibromatous lesion (11%) and 1 branchial cleft cyst(11%).
All the three pleomorphic adenomas are noted to arise from the minor salivary glands. Of the two neurogenic tumors diagnosed, and one was a neurofibroma and the other corresponded to a schwannoma.
The histological type of malignancy was diagnosed in two patients as adenoid cystic carcinoma.
No major post-operative complications were noted in any patient.
The malignant cases were given adjuvant radiotherapy.
Discussion
PPS masses account for 0.5% of all head and neck tumours and a majority of them are histopathologically benign (78%) [13]. Literature [1, 4] suggests PPS as a virtual anatomic space, shaped like an inverted pyramid, extending from the base of the skull to the hyoid bone. Its boundaries are.
Superior boarder: small portion of the temporal bone. There is a fascia connecting the medial pterygoid plate to the spine of the sphenoid. This fascia lies medial to the foramen ovale and foramen spinosum. These foramina are not considered to be in the PPS.
Inferior boarder: junction of the posterior belly of the digastric muscle and the greater cornu of the hyoid bone.
Medial boarder: pharyngobasilar fascia and pharyngeal wall. Superiorly it is formed by the approximation of the fascia from the tensor veli palatine muscle to the medial pterygoid muscle. Inferiorly the medial boarder is contiguous with the fascia over the superior constrictor muscle.
Lateral boarder: medial pterygoid muscle fascia, the ramus of the mandible, retromandibular portion of the deep lobe of the parotid gland and posterior belly of the digastric muscle. Sphenomandibular ligament extends from the spine of the sphenoid to the lingula of the mandible. The medial pterygoid muscle fascia incorporates this ligament superiorly to the skull base as a dense fascia. This fascia separates the inferior alveola nerve, lateral pterygoid muscle and mandibular condyle from the PPS. Stylomandibular ligament extend from the styloid process to the angle of the mandible. This ligament is a part of the stylomandibular tunnel. The other two boundaries of the stylomandibular tunnel are ascending mandibular ramus and skull base. Primary tumors of the deep lobe of the parotid gland can grow into the PPS through this tunnel. The constriction of this tunnel on tumor growth gives a characteristic “dumbbell” shape on the CT scan.
Posterior boarder: vertebral fascia and paravertebral muscles.
Anterior boarder: pterygomandibular raphe and medial pterygoid fascia.
Clinically, the PPS should be considered in two spaces: pre-styloid space and post-styloid space. Fascia from the styloid process to the tensor veli palatine muscle divides the PPS into these two compartments. The pre-styloid space is anterolateral and contains retromandibular portion of the deep lobe of the parotid gland, minor or ectopic salivary gland, a small branch of the CN V to the tensor veli palatine muscle, ascending pharyngeal artery and pharyngeal venous plexus. The majority of the pre-styloid space is actually fat. The post-styloid compartment is posteromedial and contains internal carotid artery, internal jugular vein, CN IX to XII, cervical sympathetic chain, lymph nodes and glomus bodies.
It is therefore easy to understand the great diversity of histology in tumor originating from the structures outlined above. About 70 different histologic types of parapharyngeal pathologies are reported [5]. Of them about 80% account to benign histologies and the rest being malignant. Salivary gland neoplasms (45%) are the most common lesions followed by neurogenic tumors (41%) and lymph node lesions (2%). The rest are included in miscellaneous group. The same multeity of histopathology is also found in our series.
These tumors are usually asymptomatic, painless and often not diagnosed on a routine examination. The volume increase of the tumor tends to occur to areas with less resistance as the lateral pharyngeal wall, tonsillar fossa and submandibular triangle. As it extends caudally, it presents as a palpable mass near the angle of the mandible. Therefore, in the clinical examination, it is of great importance to conduct a thorough inspection of pharynx, as well as bimanual palpation of this region and systematized neurological examination to rule out any neurological deficits of cranial and cervical sympathetic chain [6, 7].
For the great anatomical complexity and difficult access for direct examination of this space, complementary radiological examinations (mainly CT and MRI) are essential in the topographic diagnosis of tumor. MRI with gadolinium contrast is the preferred investigation to delineate soft tissue, demonstrate intracranial extension and to outline vasculature in relation to the lesion [8]. CT is the practical first choice for demonstration of bony involvement and calcifications within the lesions [9].
Angiography would be interested in large vagal paragangliomas, carotid body tumors and other enhancing vascular tumors [10].
The goal of parapharyngeal surgery is to provide adequate tumour visualization to achieve complete tumor removal, while preserving the surrounding nerves and vessels and control of any hemorrhage. Many surgical approaches have been reported in the literature. The choice of procedure is dictated by the size of the tumor, suspicion of malignancy and the position of the tumor with regard to the superior extent and proximity to the skull base as well as its relation with neurovascular bundle.
There are disparate number of approaches associated with the excision of PPS tumors like transoral, transcervical, transparotid, transcervical-transparotid, transmandibular and infratemporal fossa approach.
Transoral approach is removal of tumor through mouth via a mucosal incision over the bulging tumor [11]. It has the advantage of avoiding any external skin incision, but the disadvantages include poor visualization with increased risk of damage to major vascular structures, uncontrolled bleeding, cranial nerve injury, and tumor spillage [12]. Despite many shortcomings this approach can be considered for well-selected cases (no hypervascularity, no expectation of malignancy, sharply demarcated from surrounding tissues, separated medially from major vessels, and non-dumbbell shaped) and good results have been reported [13]. Recently, there has been renewed interest in transoral approaches with robotic assistance and use of a Coblator to debulk the tumor internally [14].
Transcervical approach is the most accepted approach for PPS masses arising from minor salivary glands. The PPS is accessed by mobilization of the submandibular gland. A skin incision is made along the prominent cervical skin rhytid, extending from the mastoid tip to the greater cornu of the hyoid bone 2–3 finger-widths below the body of the mandible [15]. This incision can later be extended to include a lip-split incision should the submandibular approach be insufficient and when a mandibular osteotomy is also needed for better access and tumor visualization. Subplatysmal skin flaps are then elevated by sharp dissection to identify the marginal mandibular branch of the facial nerve. The submandibular gland is sharply dissected and mobilized. A few surgeons prefer to remove the submandibular gland altogether [16]; however, removal of the gland does not markedly improve access to the PPS and is not necessary in most cases [17]. The facial vessels are identified and mobilized with the gland. The hypoglossal and lingual nerves are then identified and preserved. The mandible is then retracted upward, and the tumor is dissected from the PPS with a sponge or the surgeon’s finger cautiously in the space between the mandible laterally and the constrictor muscles medially and the mobilized submandibular gland anteriorly [18, 19]. A better access to the PPS is expedited by the removal of the submandibular gland and division of the digastric muscle, as well as dissection and division of the stylomandibular ligament [18, 19]. This allows for greater anterior displacement of the mandible. The PPS tumors are mostly easy to dissect of the loose areolar fascia unless the mass is either an inflammatory lesion or a malignant infiltrative tumor. This part of the procedure must be performed with care and gentle pressure, so as to not push through the constrictor muscles and violate the mucosal lining.
The transparotid approach is used to extirpate most deep lobe parotid tumors [20, 21]. If mass in the prestyloid PPS is contiguous with the parotid gland, the transparotid approach needs to be used to ensure surgical control and protection of the facial nerve and all its branches for removal of all such tumors. Via a superficial parotidectomy incision or a face lift type incision, along posterior hair line for better cosmesis, the tragus pointer and the insertions of the digastric and sternocleidomastoid muscles into the mastoid tip are exposed. The main trunk of the facial nerve is identified and isolated in a standard fashion, and a superficial parotidectomy is performed. The branches are then dissected and mobilized free from the deep lobe of the parotid, using either a Crile dissector or fine scissors. The deep parotid lobe containing the remainder of the tumor is then dissected free between the now mobilized facial nerve branches and removed. In some cases, the styloid process may need to be cut and the mandible displaced anteriorly to gain access to the tumor within the deep lobe of the parotid gland. This requires careful attention to the facial nerve branches so that traction is avoided to them so as to minimize morbidity [15].
The transcervical-transparotid approach is advocated for large tumors arising from the deep parotid lobe that extend into and obliterate the prestyloid PPS. A better accessibility and surgical safety is achieved on combination of both the approaches reducing the morbidity [18]. The transparotid approach is performed first which offers the surgeon safe identification of the facial nerve and all its branches, followed by the transcervical approach, by the extension of the of the posterior hair line incision of transparotid approach, anteriorly on to the neck along the skin rhytid, which gives excellent direct visualization of the tumor and all critical neurovascular structures within the PPS facilitating adequate exposure and safe dissection to remove the tumor [15].
The mandibulotomy or mandibular swing approach is a time-tested approach [22, 23]. There are three options for osteotomy of the mandible: (1)through the body(lateral mandibulotomy), (2)midline, or (3)paramedian. Transcervical approach is mostly combined with mandibulotomy. The key is to try to limit injury to the inferior alveolar nerve while providing access to the PPS. The risk of mandibulotomy includes inferior alveola nerve anaesthesia, loss of dentition, malocclusion, mandibular malunion or nonunion, and possibly warrant a tracheostomy [24]. After the osteotomy, the mandibular segments are distracted and the stretched floor of the mouth mucosa is incised 1 cm medial to the gingiva along with the underlying soft tissue and musculature of the floor of the mouth, extending the incision to the anterior tonsillar pillar enabling unimpeded access to the PPS.
The infratemporal approach combined with the cervical–parotid or cervical approach is indicated for malignant PPS lesions involving the skull base or jugular foramen or with intracranial extension [25]. Three surgical approaches are described in this approach by Bilsky et al. [26].
Because primary parapharyngeal tumors are exceedingly rare, only few large scale studies are available. Shahab [27] recently published his review of 114 parapharyngeal tumors for over 27 years of experience. The 5-year and 10 year survival for benign parapharyngeal tumor is 100%. For malignancies the 5 year survival was 93%, but fall to 57% at 10 years. This study showed that a patient is highly unlikely to die of a benign PPS tumor, therefore careful consideration of surgical treatment and discussion with the patient are crucial. While surgery is the mainstay of the treatment for parapharyngeal tumor, radiation therapy can be considered in elderly patients with paragangliomas. Also isolated asymptomatic parapharyngeal schwannomas in elderly patients with no nerve deficit should probably be observed.
Conclusion
These are rare tumors generally with an asymptomatic behavior and which sometimes appear clinically suspected while performing a routine examination by what must be present as a diagnostic possibility in a patient with a tumor protuberance in the lateral pharyngeal wall, this being the most common form of presentation. The presence of pain or paralysis in any cranial nerve may indicate malignancy. Trismus may be present in a few cases.
The surgical approach will depend on the location and size of the tumor; one way transcervical or cervical-parotid be useful for removal of the vast majority of these tumors, both pre- and retroauricular. In cases of large tumors of deep lobe of the parotid, a transparotid approach will suffice. Mandibular swing is advised for deep seated and malignant lesions.
Compliance with Ethical Standards
Conflict of interest
This study was not funded and has no conflict of interest
Informed Consent
Informed consent was obtained from the patients.
References
- 1.Olsen KD. Tumors and surgery of the parapharyngeal space. Laryngoscope. 1994;104(Suppl 63):1–28. doi: 10.1002/lary.1994.104.s63.1. [DOI] [PubMed] [Google Scholar]
- 2.Stanley RE. Parapharyngeal space tumours. Ann Acad Med Singapore. 1991;20(5):89–96. [PubMed] [Google Scholar]
- 3.Singh Mangal. Our experiences with parapharyngeal space tumors and systematic review of the literature. Indian J Otolaryngol Head Neck Surg. 2009;61:112–119. doi: 10.1007/s12070-009-0047-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shields G, Bailey B (2002) Parapharyngeal space tumour. Dr. Quinn’s Online Textbook 2002. http://www.utmb.edu
- 5.Riffat F, Dwivedi RC, Palme C, Fish B, Jani P. A systematic review of 1143 parapharyngeal space tumors reported over 20 years. Oral Oncol. 2014;50(2014):421–430. doi: 10.1016/j.oraloncology.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 6.Maran AG, Mackenzie IJ, Murray JA. The parapharyngeal space. J Laryngol Otol. 1984;98(4):371–380. doi: 10.1017/S0022215100146766. [DOI] [PubMed] [Google Scholar]
- 7.Shoss SM, Donovan DT, Alford BR. Tumors of the parapharyngeal space. Arch Otolaryngol. 1985;111(11):753–757. doi: 10.1001/archotol.1985.00800130085011. [DOI] [PubMed] [Google Scholar]
- 8.Som PM, Curtin HD. Lesions of the parapharyngeal space, role of MR imaging. Otolaryngol Clin North Am. 1995;28(3):515–542. [PubMed] [Google Scholar]
- 9.Ajay Gupta J, Levi Chazen C, Phillips Douglas. Imaging of head and neck spaces for diagnosis and treatment, imaging evaluation of the parapharyngeal space. Otolaryngol Clin North Am. 2012;45(6):1223–1232. doi: 10.1016/j.otc.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 10.Som PM, Biller HF, Lawson W, Sacher M, Lanzieri CF. Parapharyngeal space masses: an updated protocol based upon 104 cases. Radiology. 1984;153(1):149–156. doi: 10.1148/radiology.153.1.6089262. [DOI] [PubMed] [Google Scholar]
- 11.Sheahan P. Surgical approaches to the parapharynx and nasopharynx, transcervical approach for removal of benign parapharyngeal space tumors. Op Tech Otolaryngol. 2014;25:227–233. doi: 10.1016/j.otot.2014.04.003. [DOI] [Google Scholar]
- 12.Bradley PJ, Bradley PT, Olsen KD. Update on the management of parapharyngeal tumours. Curr Opin Otolaryngol Head Neck Surg. 2011;19:92–98. doi: 10.1097/MOO.0b013e328342b9b4. [DOI] [PubMed] [Google Scholar]
- 13.Betka J, Chovanec M, Klozar J, et al. Transoral and combined transoral-transcervical approach in the surgery of parapharyngeal tumors. Eur Arch Otorhinolaryngol. 2010;267(765–772):2010. doi: 10.1007/s00405-009-1071-z. [DOI] [PubMed] [Google Scholar]
- 14.De Virgilio A, Park YM, Kim WS, et al. Transoral robotic surgery for the resection of parapharyngeal tumour: our experience in ten patients. Clin Otolaryngol. 2012;37:483–488. doi: 10.1111/j.1749-4486.2012.02525.x. [DOI] [PubMed] [Google Scholar]
- 15.Mydlarz WK, Agrawal N. Surgical approaches to the parapharynx and nasopharynx, transparotid and transcervical approaches for removal of deep lobe parotid gland and parapharyngeal space tumors. Op Tech Otolaryngol. 2014;25:234–239. doi: 10.1016/j.otot.2014.04.004. [DOI] [Google Scholar]
- 16.Malone JP, Agrawal A, Schuller DE. Safety and efficacy of transcervical resection of parapharyngeal space neoplasms. Ann Otol Rhinol Laryngol. 2001;110(12):1093–1098. doi: 10.1177/000348940111001202. [DOI] [PubMed] [Google Scholar]
- 17.Chang SS, Goldenberg D, Koch WM. Transcervical approach to benign parapharyngeal space tumors. Ann Otol Rhinol Laryngo. 2012;121(9):620–624. doi: 10.1177/000348941212100910. [DOI] [PubMed] [Google Scholar]
- 18.Bass RM. Approaches to the diagnosis and treatment of tumors of the parapharyngeal space. Head Neck Surg. 1982;4(4):281–289. doi: 10.1002/hed.2890040404. [DOI] [PubMed] [Google Scholar]
- 19.Olsen KD. Tumors and surgery of the parapharyngeal space. Laryngoscope. 1984;104(5Pt2suppl63):1–28. doi: 10.1288/00005537-199405000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Day TA, Deveikis J, Gillespie MB, et al. Salivary gland neoplasms. Curr Treat Options Oncol. 2004;5(1):11–26. doi: 10.1007/s11864-004-0002-x. [DOI] [PubMed] [Google Scholar]
- 21.Nigro MF, Jr, Spiro RH. Deep lobe parotid tumors. Am J Surg. 1977;134(4):523–527. doi: 10.1016/0002-9610(77)90391-9. [DOI] [PubMed] [Google Scholar]
- 22.Spiro RH, Gerold FP, Strong EW. Mandibular “swing” approach for oral and oropharyngeal tumors. Head Neck Surg. 1981;3(5):371–378. doi: 10.1002/hed.2890030505. [DOI] [PubMed] [Google Scholar]
- 23.Shah JP, Patel SG, Singh B. JatinShah’s head and neck surgery and oncology. 4. London: Elsevier Science; 2012. [Google Scholar]
- 24.Patel SG. Surgical approaches to the parapharynx and nasopharynx, Transmandibular surgical approach for malignant parapharyngeal space tumors. Op Techn Otolaryngol. 2014;25:240–247. doi: 10.1016/j.otot.2014.04.005. [DOI] [Google Scholar]
- 25.Banuchi V, Dennis MPH, Kraus H. Surgical approaches to the parapharynx and nasopharynx, The infratemporal fossa approach to the lateral skull base and parapharynx. Op Techn Otolaryngol. 2014;25:254–258. doi: 10.1016/j.otot.2014.04.006. [DOI] [Google Scholar]
- 26.Bilsky MH, Bentz B, Vitaz T, et al. Craniofacial resection for cranial base malignancies involving the infratemporal fossa. Neurosurgery. 2005;57(suppl 4):339–347. doi: 10.1227/01.NEU.0000176648.06547.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shahab R, Heliwell T, Jones AS. How we do it: a series of 114 primary parapharyngeal space neoplasms. Clin Otolaryngol. 2005;30:364–383. doi: 10.1111/j.1365-2273.2005.00993.x. [DOI] [PubMed] [Google Scholar]


