Abstract
A study was executed in a direction to attenuate Sclerotinia stalk rot (SSR) disease through biocontrol agent and also to enhance crop productivity. Culture filtrate of bacterial strain YSPMK11 inhibited growth of Sclerotinia sclerotiorum in vitro which also exhibited higher plant growth promoting attributes. Interaction studies revealed maximum (81.50%) growth inhibition at 35 °C and pH 7.0 after 72 h incubation period with 15% culture filtrate. Based upon 16S rRNA gene sequence strain, YSPMK11 was identified as Bacillus pumilus. Furthermore, the genome of this isolate was searched for antimicrobial lipopeptide, i.e., ItuD and SrfC genes. The PCR amplification results showed the presence of both these lipopeptide genes in isolate YSPMK11. Iturin A as antifungal compound was identified as major components of fraction through GC/MS. In field experiments, the application of strain YSPMK11 cell suspension (108 CFU/ml) suppressed disease severity by 93% and increased curd yield by 36% which was more that of commercially used fungicide in farmer practices under mid-hills of Himachal Pradesh. Conclusively, our study is first to demonstrate the effect of B. pumilus strain YSPMK11 in suppression of SSR under field conditions and would be employed as an efficient biocontrol agent to replace commercial fungicides in cauliflower cropping system. In addition, the presence of both lipopeptide genes (ItuD and SrfC) and iturin A in this isolate makes him potent strain for biological control application in agriculture.
Keywords: Bacillus pumilus, Sclerotinia sclerotiorum, 16S rRNA, Disease suppression, Cauliflower production
Introduction
Sclerotinia stalk rot (SSR) caused by S. sclerotiorum (Ss) is a major disease of cauliflower (Brassica oleracea var. botrytis L.) under mid-hills conditions of Himachal Pradesh. S. sclerotiorum known as white mold is a necrotrophic pathogen having wide host range including important vegetable and oil yielding crops (Bolton et al. 2006) thus causing substantial yield losses. Cauliflower curd and stem is the primary site of infection during generative phase. Disease first appears as wet soft lesion on curd which enlarges into a watery rotten mass of tissues covered by a white silvery appearance. Affected curds causes another plant parts to wilt and later die. Cool temperature and high humidity conditions in mid-hills of Himachal Pradesh are favourable for epidemics of SSR. The standard strategies employed to overcome SSR include crop management practices such as use of clean seeds, early planting date, soil tillage, and adjustment of row width and density of plant population; however, effectiveness of these measures is very limited (Yexin et al. 2011) due to sclerotia survival in soil and high potential virulence. Over last few decades interest in biocontrol against SSR increased, because downfall of chemical pesticides controls the pathogen as well as their impact on environment (Saharan and Mehta 2008). Although several biocontrol agents have been reported to control SSR in different crops (Awais et al. 2008; Hu et al. 2013; Kamal et al. 2015), least information is available on biocontrol of SSR in cauliflower. In an earlier study, B. pumilus SQR-N43 exhibited 68% control efficacy against R. solani in cucumber (Huang et al. 2012). However, there is no report in literature which demonstrated the detrimental effects caused by S. sclerotiorum and their control by B. pumilus. Therefore, the present study was designed to evaluate the efficacy of cell suspensions, cell-free culture filtrates, and broth culture in suppressing SSR and effect of microbial control agent (bacterial isolate YSPMK11) in controlling SSR at different bloom stage of cauliflower followed by enhancing the productivity of crop under mid-hills of Himachal Pradesh.
Materials and methods
Isolation of bacterial strain
Endorhizosphere samples of cauliflower plants were collected from four different naturally growing agro climatic zones of Himachal Pradesh. Each zone comprises four samples, collectively yielding 16 samples. A total of 108 bacterial strains were isolated following modified replica plating technique. Streak plate method was employed to purify the isolated strains and thus stored in nutrient agar slants at 4 °C for further screening activities. Identification of isolates was done on the basis of morphological characteristics and as per the criteria Bergey’s Manual of Determinative Bacteriology.
Screening of bacterial isolate
All 108 bacterial isolates were screened for different multifarious plant growth promoting attributes. Phosphate solubilization potential was detected utilizing PVK broth. Solubilizing efficiency E (E = diameter of bacterial growth/diameter of clearing zone × 100) was calculated as per the method described by Bray and Kurtz (1945). Quantitative estimation of water extractable free inorganic P (Pi) was then carried out (Dubey and Maheshwari 2011). IAA, HCN (cyanogen), and siderophore production were determined as per methods described by Costacurta et al. (2006), Bakker and Schippers (1987) and Schwyn and Neilands (1987), respectively.
In vitro antagonistic activities of bacterial isolate YSPMK11 against S. sclerotiorum
Based on multifarious plant growth promoting attributes, bacterial isolate YSPMK11 (showing maximum activities) was selected to study antagonistic activities against S. sclerotiorum. To study antifungal activity, common and locally active plant disease causing fungal species S. sclerotiorum was procured from the Department of Mycology and Plant Pathology, University of Horticulture and Forestry, Nauni, Solan, and maintained on malt extract agar (MEA) at 4 °C.
A single colony of bacterial strain grown in Luria–Bertani (LB) broth with constant shaking at 150 rpm for 48 h at room temperature. Bacterial culture centrifuged at 6000 rpm for 10 min and bacterial cells resuspended in phosphate buffer (100 mM, pH 7.0). Cell concentration was adjusted spectrophotometrically to 9 × 108 CFU/ml (Fernando et al. 2007). Inhibitory effect of culture filtrate of isolate was studied using agar dilution technique (Warnock 1989).
Production of antifungal antibiotics
The culture supernatant was filter sterilized and used as crude antifungal antibiotic preparation. Yeast extract malt broth (YEM) medium was used to determine antifungal antibiotic production by antagonists.
Effect of inoculum concentration, time course, temperature, and pH on antifungal antibiotic production
Inoculum concentrations 5.0, 10.0, and 15.0% (w/v); incubation periods (0, 24, 48, 72, 96, and 120 h); temperatures (30, 35, and 40 °C); and pH in the range of 5–8 were studied consequently on antifungal antibiotic production.
Molecular characterization of bacterial isolate YSPMK11
Isolation of genomic DNA was done with CTAB method (Wahyudi et al. 2010). Amplification of 16S-rRNA gene was carried out by Polymerase Chain Reaction (PCR) using specific primer (Bf-5′-GCAAGTCGAGCGGACAGATGGGAGC-3′) and (Br-5′-AACTCTCGTGGTGTGACGGGCGGTG-3′) which yielded 1375 bp DNA fragment. PCR was carried out; all PCR products were purified using Real Genomic DNA Extraction Kit and sequenced using ABI 310 (Perkin Elmer, USA). Similarity of each 16S-rRNA sequence was aligned against GeneBank database sequences using BLASTN program. The query sequence was aligned with ClustalW program for constructing a phylogenetic tree and comparison was done with those of other phylogenetically close microbial relatives.
Amplification of lipopeptide genes
The genomic DNA of isolate YSPMK11 was subjected for the identification of two lipopeptide genes, i.e., surfactin (srfC) and iturin A (ituD), as described earlier by Gond et al. (2015). The primer sequences used were: ItuD1F (GATGCGATCTCCTTGGATGT), ItuD1R (ATCGTCATGTGCTGCTTGAG), Sur3F (ACAGTATGGAGGCATGGTC), and Sur3R (TTCCGCCACTTTTTCAGTTT). PCR amplification of ituD gene yielded 647 bp fragment, whereas PCR amplification of srfC gene yielded 441 bp DNA fragment. The PCR condition used for the amplification of the genes includes the initial denaturation at 95 °C for 5 min followed by 30 cycles, which consisted of denaturation at 95 °C for 1 min, annealing at 54.5 °C for 1 min, and extension at 72 °C for 1 min, and final extension was carried out at 72 °C for 10 min. The PCR product was resolved in 2% agarose gel for 2 h using 100 bp ladder.
Identification of antifungal compounds
HPLC (VARIAN Prostar 218, USA) equipped with a Pursuit Rs-5u-C18 column (150 × 21.2 mm2) was used to separate cell-free culture filtrates. Prior to HPLC injection, extracts in methanol were filtered through a 0.2 µm PTFE Acrodisc CR syringe filter (Pall Corp., East Hills, NY). Gradient conditions were used with methanol/0.05% trifluoroacetic acid-solvent A (Sigma-Aldrich) and water/0.05% trifluoroacetic acid-solvent B at 1 ml/min and A/B ratios of 10:90, 100:0, and 100:0, with run time of 0, 30, and 33 min, respectively (Pryor et al. 2006; Li et al. 2012). Samples were eluted at a flow rate of 1 ml/min, and the elution profile was monitored with UV detector at 210 nm. Fractions obtained from semi-preparative HPLC were evaporated with an N2 stream and dissolved in CH3OH for gas chromatography/mass spectrometry (GC/MS) analysis.
Bioassay of lipopeptide
Bioassay was performed using well diffusion method in which methanol extract of 100 μl lipopeptides was tested against S. sclerotiorum (Meena et al. 2014). Malt extract agar (MEA) plates were incubated at 30 °C in inverted position, and after 3 days, growth inhibition (%) was calculated by the following formula:
where DC, diameter of control; and DT, diameter of fungal colony with treatment.
Biocontrol efficacies of bacterial isolate YSPMK11 under field conditions
Based upon results obtained from in vitro, biocontrol efficacy of bacterial isolate YSPMK11 against S. sclerotiorum, it was evaluated under field conditions to replace heavy chemicals/fungicides load on soil without deteriorating crop productivity. Cauliflower seeds were seeded in pots containing sterilized soil with methyl bromide for nursery preparation before transplantation to main field. Field trials were quadruplicated (two at Regional Horticultural Research Station, Kullu, and two at Dr. Y. S. Parmar University of Horticulture and Forestry, Nauni, Solan) during 2010–2011. The experiment was conducted in field situated at 32°N latitude and 77°E longitude with following treatments, T1 (uninoculated control), T2 (YSPMK11-50% bloom), T3 (YSPMK11-30% and 50% bloom), T4 (YSPMK11-30% bloom), T5 (carboxamide), T6 (benomyl), T7 (vinclozolin), T8 (tebuconazole), T9 (thiophanate methyl), and T10 (iprodione) arranged in randomized complete block design (RCBD) replicated thrice with plot size of 5 × 5 m in every trial. Fungicide treatments (seed treatment and soil drench at 1.25 g/L) were done at 30 and 50% bloom stage (singly in T2 and T4; twice in T3 plot). 72 h old bacterial culture was grown in 10% nutrient broth for the preparation of bacterial inoculum and a final concentration of 9 × 108 CFU/ml was made. Before nursery sowing seed treatment were given at this concentration for 30 min at 1 ml/seed. Furthermore, 72 h old bacterial cultures were grown in nutrient broth at 30 °C in an orbital shaker (150 rev/min) for 24 h. Cultures were then centrifuged at 6000 rpm for 20 min in 50 ml sterile plastic tubes. Pellets were resuspended in nutrient broth again to obtain a final concentration of 9 × 108 CFU/ml. The liquid cultures of isolate were then used for bacterial inoculations as soil drench at 30 and 50% bloom stage (singly in T2 and T4; twice in T3 plot) at 10 ml/plant. The incidence of SSR was determined based on the percentage of stems with disease symptoms among 20 randomly selected cauliflower plants from each treatment plots. Disease severity and control efficacy were calculated as follows (Anonymous 2000):
Results
Bacterial isolation
Presumptive identification of all 108 rhizobacterial isolates were done on the basis of Bergey’s Manual of Determinative Bacteriology which presented strain as circular colonies having entire margin, Gram-positive, short rods, non-motile with cream pigment which matched to genera Bacillus.
Screening of bacterial isolate
When subjected to screening activities, it was found that among all the 108, only the rhizobacterial isolate YSPMK11 (Bacillus spp.) showed halo zone around its colony on Pikovskaya agar plates indicating the P solubilization. Maximum solubilization efficiency (183.60%) showed on 4th day of incubation which started to decrease from 5th day onwards. Furthermore, this strain was tested for quantitative estimation of phosphate solubilization and showed maximum solubilized P (156.3 ppm) after 24 h of incubation.
The rhizobacterial isolate YSPMK11 (Bacillus spp.) also displayed higher IAA, HCN, and siderophore production. Development of pink colour with and without addition of tryptophan in culture broth was observed in the cell-free supernatant. Higher concentration of IAA (30.67 µg/ml) was produced in the presence of 3 mg/ml tryptophan. In the presence of glycine and FeCl3, the deep brown colour of filter paper was observed, giving a clear indication of HCN production by isolate YSPMK11. A bright zone (17.56 mm) of yellowish colour around the bacterial colony on Chrome-azurol-S (CAS) medium was produced by isolate YSPMK11 (Bacillus spp.) which indicate the production of siderophore. Quantitative analysis revealed that isolate YSPMK11 (Bacillus spp.) produced 57.63% siderophore unit as well as higher (59.38 × 105 cfu/ml) viable count after 72 h of incubation.
In vitro antagonistic activities of bacterial isolate YSPMK11 (Bacillus spp.) against S. sclerotiorum
Inhibition zone ranged from 20.2 to 22.4 mm for S. sclerotiorum. Antifungal antibiotic activity of isolate YSPMK11 (Bacillus spp.) tested against S. sclerotiorum by culture filtrate (5–15%) method. The results showed that culture filtrate exhibited inhibition zone against S. sclerotiorum which ranged from 20.2 to 22.4 mm.
Effect of inoculum concentration
Increase in concentration of culture filtrate from 5 to 15% increased percent growth inhibition with maximum of 81.50% (Table 1). The interaction studies revealed 86.71% growth inhibition (15% CF) after 72 h incubation. Thus, 15% inoculum was used for further experimentation.
Table 1.
Effect of inoculum concentration of isolate YSPMK11 on antifungal antibiotic activity against S. sclerotiorum
| Sample | Sample details | Antifungal antibiotic activity (% growth inhibition) | Mean | ||
|---|---|---|---|---|---|
| 5% | 10% | 15% | |||
| Control | MEA | 0.00 (1.00) | 0.00 (1.00) | 0.00 (1.00) | 0.00 (1.00) |
| Test | MEA + CF (5%) | 65.08 (8.12) | 75.94 (8.77) | 78.76 (8.93) | 73.26 (8.60) |
| MEA + CF (10%) | 73.90 (8.65) | 77.53 (8.86) | 82.93 (9.16) | 78.12 (8.89) | |
| MEA + CF (15%) | 77.34 (8.85) | 80.43 (9.02) | 86.71 (9.36) | 81.50 (9.07) | |
| Mean | 54.08 (6.65) | 58.48 (6.91) | 62.10 (7.11) | ||
Figures in parentheses are square root transformed values
Least square difference (LSD) (T) = 1.23 LSD (I) = 1.06 LSD (T × I) = 2.12
Values are the mean of three replications
MEA malt extract agar
Duration of incubation period
Antifungal antibiotic production was monitored for 120 h in nutrient broth (Table 2). The antifungal antibiotic activity increased gradually up to 72 h with maximum (60.20%) growth inhibition of S. sclerotiorum later followed a decreasing trend. Results showed that 15% culture filtrate (CF) showed more mean percent growth inhibition (62.74%) as compared to 5% culture filtrate (48.44%). The interaction studies revealed maximum (84.72%) growth inhibition at 72 h incubation with 15% CF. Thus, 72 h incubation was selected as the best time course for antifungal antibiotic production.
Table 2.
Effect of incubation period on antifungal antibiotic activity of isolate YSPMK11 against S. sclerotiorum in nutrient broth
| Sample | Sample details | Antifungal antibiotic activity (% growth inhibition) | Mean | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 h | 24 h | 48 h | 72 h | 96 h | 120 h | ||||
| Control | MEA | 0.00 (1.00) | 0.00 (1.00) | 0.00 (1.00) | 0.00 (1.00) | 0.00 (1.00) | 0.00 (1.00) | 0.00 (1.00) | |
| Test | MEA + CF (5%) | 0.00 (1.00) | 14.47 (3.92) | 57.86 (7.67) | 73.26 (8.61) | 72.81 (8.59) | 72.21 (8.55) | 48.44 (6.39) | |
| MEA + CF (10%) | 0.00 (1.00) | 34.65 (5.96) | 68.78 (8.35) | 82.85 (9.15) | 82.33 (9.12) | 81.92 (9.10) | 58.42 (7.11) | ||
| MEA + CF (15%) | 0.00 (1.00) | 47.25 (6.94) | 75.82 (8.76) | 84.72 (9.24) | 84.55 (9.25) | 84.09 (9.22) | 62.74 (7.40) | ||
| Mean | 0.00 (1.00) | 24.09 (4.45) | 50.62 (6.44) | 60.20 (7.00) | 59.92 (6.99) | 59.56 (6.96) | |||
Figures in parentheses are square root transformed values
LSD (T) = 0.96 LSD (I) = 1.18 LSD (T × I) = 2.37
Values are the mean of three replications
MEA malt extract agar
Variation of temperature and pH
In the present study, it was observed that percent growth inhibition increased with increase in temperature from 30 to 35 °C; however, further increase in incubation temperature (40 °C) adversely affected antibiotic production and growth inhibition of tested fungus (Table 3). Increase in pH from acidic towards neutral increased percent growth inhibition from 44.67 to 57.48% (Table 4). The maximum percent growth inhibition displayed at temperature of 35 °C at pH 7 with 15% CF.
Table 3.
Effect of temperature on antifungal antibiotic activity of isolate YSPMK11 against S. sclerotiorum
| Sample | Sample details | Antifungal antibiotic activity (% growth inhibition) | Mean | ||
|---|---|---|---|---|---|
| 30 °C | 35 °C | 40 °C | |||
| Control | MEA | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) |
| Test | MEA + CF (5%) | 39.03 (38.62) | 82.50 (65.43) | 36.29 (37.02) | 52.61 (47.03) |
| MEA + CF (10%) | 40.93 (39.75) | 89.79 (71.38) | 42.97 (40.93) | 57.90 (50.69) | |
| MEA + CF (15%) | 46.39 (42.93) | 91.47 (73.03) | 51.45 (45.83) | 63.10 (53.93) | |
| Mean | 31.59 (30.33) | 65.94 (52.46) | 32.68 (30.95) | ||
Figures in parentheses are arc sine transformed values
LSD (T) = 3.55 LSD (I) = 3.07 LSD (T × I) = 6.13
Values are the mean of three replications
MEA malt extract agar
Table 4.
Effect of pH on antifungal antibiotic activity of isolate YSPMK11 against S. sclerotiorum
| Sample | Sample details | Antifungal antibiotic activity (% growth inhibition) | Mean | |||
|---|---|---|---|---|---|---|
| pH 6 | pH 7 | pH 8 | pH 9 | |||
| Control | MEA | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) |
| Test | MEA + CF (5%) | 51.67 (45.96) | 71.60 (57.80) | 37.02 (37.43) | 16.91 (23.88) | 44.30 (41.27) |
| MEA + CF (10%) | 59.76 (50.63) | 75.08 (60.07) | 44.85 (42.04) | 32.64 (34.84) | 53.08 (46.90) | |
| MEA + CF (15%) | 67.26 (55.10) | 83.26 (65.86) | 56.97 (49.01) | 38.08 (38.10) | 61.39 (52.02) | |
| Mean | 44.67 (37.92) | 57.48 (45.93) | 34.71 (32.12) | 21.91 (24.21) | ||
Figures in parentheses are arc sine transformed values
LSD (T) = 2.38 LSD (I) = 2.38 LSD (T × I) = 4.75
Values are the mean of three replications
MEA malt extract agar
Molecular characterization of isolate YSPMK11
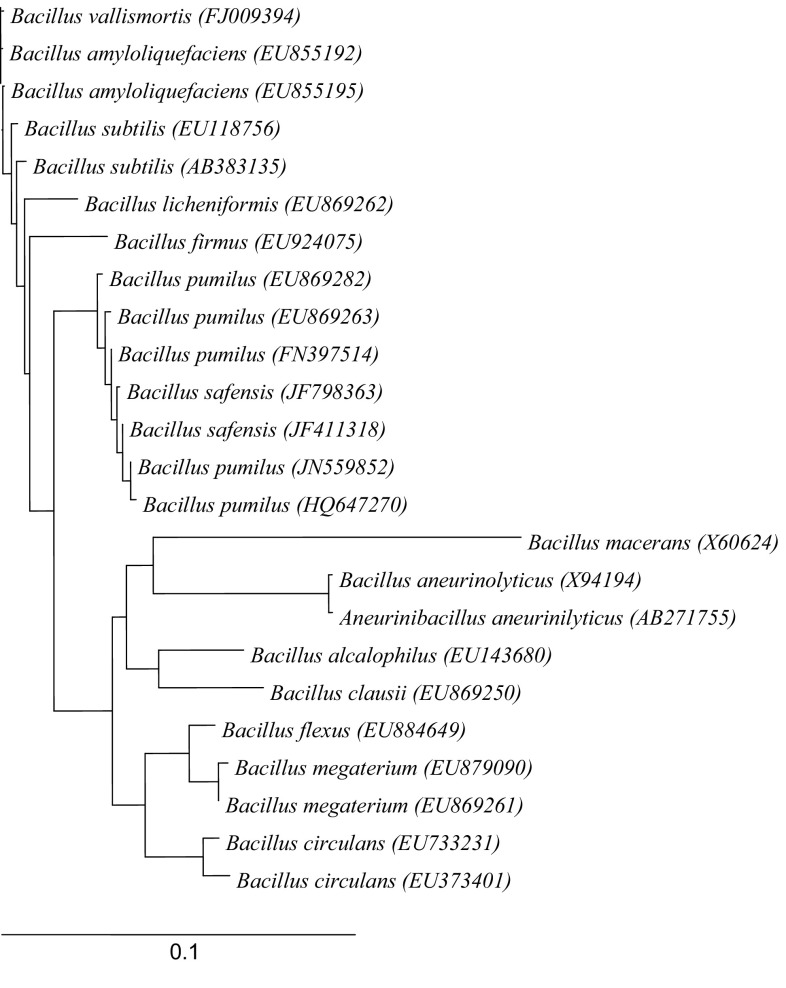
Designed primers were used successfully for amplification of 16S rDNA from bacterial isolate YSPMK11 which yielded amplicon of expected size, i.e., ~1375 bp. PCR product was eluted from gel and sequenced using PCR primers used for amplification. Blasting of 16S rRNA sequence of Bacillus isolate YSPMK11 showed 99% homology with B. pumilus. Dendogram based on phylogenetic analysis presented in Fig. 1 showed that Bacillus isolate YSPMK11 is clustered with B. pumilus. The sequence of respective isolate was submitted to the National Center for Biotechnology Information (NCBI) under accession No. JN559852.
Fig. 1.
Phylogenetic tree constructed by neighbor-joining method derived from analysis of the 16S rRNA gene sequences of native isolate YSPMK11 and related sequences
Detection of lipopeptide genes
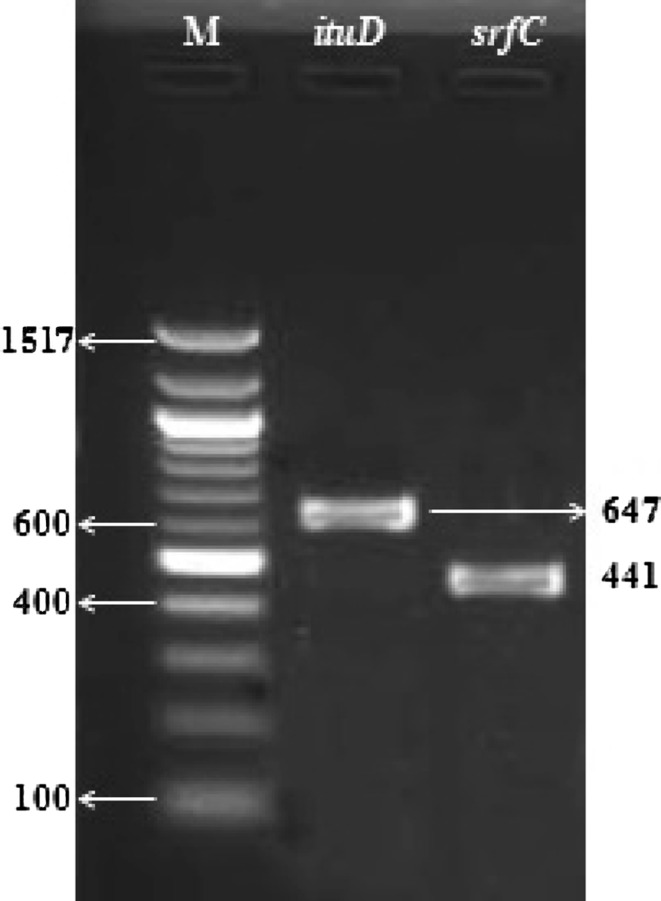
The isolate YSPMK11 was further subjected to detect the presence of lipopeptide genes, i.e., ItuD and SrfC at molecular level. Results of PCR amplification showed the presence of 647 and 441 bp DNA fragments which confirmed the presence of ItuD and SrfC genes, respectively, in this isolate (Fig. 2).
Fig. 2.
PCR amplification of lipopeptide genes shows the presence of ituD and srfC gene in native isolate YSPMK11 (M 100 bp molecular weight marker)
Identification of antifungal compounds
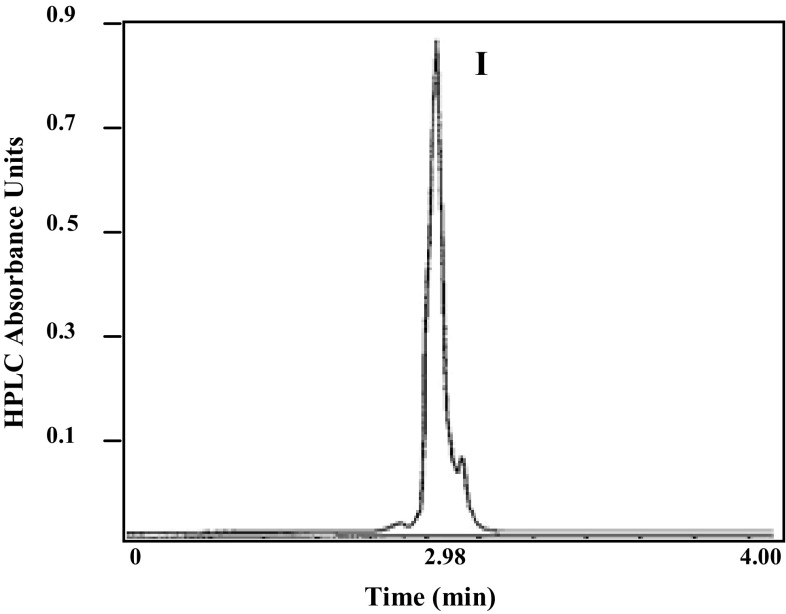
The culture filtrate fraction was analyzed by GC–MS and there was only one potential antifungal compounds detected.
The retention time of this metabolite was 2.98 min and designated as I (Fig. 3). The potential antifungal substance was identified as iturin A as major components of fraction.
Fig. 3.
GC spectrum of identified compound from B. pumilus extract
Bioassay of lipopeptide (iturin A)
Methanol extract (100 μl) was bioassayed on MEA plates which displayed 82.6% mycelial growth inhibition of S. sclerotiorum (Fig. 4).
Fig. 4.
Antifungal activity—dual culture test (by extracted lipopeptides). a Sclerotinia sclerotiorum positive control with methanol. b Sclerotinia sclerotiorum growth inhibition by extracted lipopeptides (iturin A)
Field evaluation of B. pumilus strain YSPMK11 as biocontrol agent
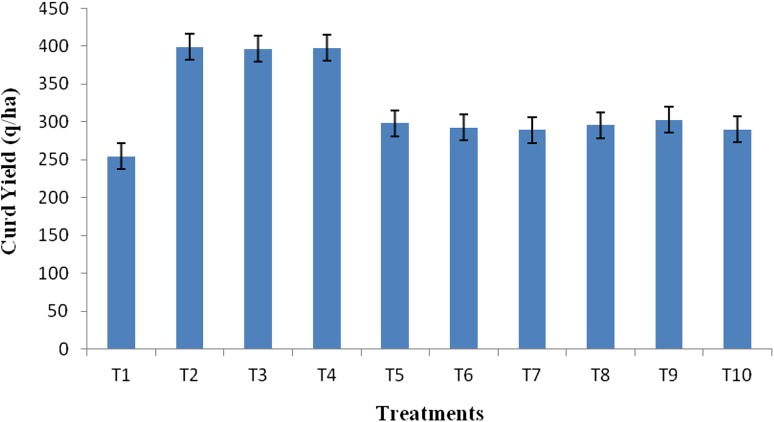
SSR incidence and severity greatly reduced with use of microbial control agent (B. pumilus strain YSPMK11) as compared to control and regularly employed fungicide. Results showed that before 7 days of harvesting the crop, incidence and severity of SSR in uninoculated control plots were 6.03 and 9.23%, respectively, while those having fungicide treatment ranged from 2.46 to 2.96% (incidence) and 1.26–1.48% (severity). However, plots received bioinoculation of B. pumilus strain YSPMK11 had incidence and severity of SSR of 1.96 and 0.77% (50% bloom), 1.83 and 0.73% (30 and 50% bloom), and 2.00 and 0.89% (30% bloom), respectively. Bioinoculated plots also observed reduced incidence and severity by 92.60–93.70%, respectively, at 30 and 50% bloom stages suggesting that B. pumilus strain YSPMK11 exhibited better disease control efficacy over the commercially available fungicides (86.03–89.46%) (Table 5). Thus, bioinoculation of B. pumilus strain YSPMK11 at 30 and 50% bloom stages exhibited maximum (93.70%) control efficacy. Curd yield was increased by all three applied treatments (having bioinoculation of B. pumilus strain YSPMK11) over uninoculated controls with highest (398.8 q/ha) recorded of T2 which was significantly at par with T3 (396.4 q/ha) and T4 (397.4 q/ha) (Fig. 5). However, cauliflower yield increased by 36% with B. pumilus strain YSPMK11 (T2–T4) compared to uninoculated control (T1). When compared to other fungicide treatments, yield was 24–27% greater with the use of B. pumilus strain YSPMK11.
Table 5.
Control of Sclerotinia stalk rot ± standard deviation by rhizobacterial strain YSPMK11 of Bacillus
| Treatments | Disease incidence (%) | Disease severity (%) | Control efficacy (%) |
|---|---|---|---|
| T1 | 6.03 ± 0.32 | 9.23 ± 0.20 | 29.83 ± 1.63 |
| T2 | 1.96 ± 0.12 | 0.77 ± 0.01 | 93.13 ± 0.41 |
| T3 | 1.83 ± 0.31 | 0.73 ± 0.009 | 93.70 ± 1.09 |
| T4 | 2.00 ± 0.30 | 0.89 ± 0.03 | 92.60 ± 1.25 |
| T5 | 2.86 ± 0.17 | 1.26 ± 0.31 | 89.46 ± 0.42 |
| T6 | 2.93 ± 0.37 | 1.27 ± 0.28 | 86.86 ± 3.20 |
| T7 | 2.96 ± 0.21 | 1.47 ± 0.37 | 86.03 ± 2.51 |
| T8 | 2.46 ± 0.23 | 1.41 ± 0.36 | 89.03 ± 3.48 |
| T9 | 2.46 ± 0.31 | 1.32 ± 0.24 | 88.76 ± 2.46 |
| T10 | 2.70 ± 0.32 | 1.48 ± 0.27 | 87.16 ± 2.77 |
| CD | 0.806 | 0.608 | 6.582 |
Values are the mean of quadruplicate trials conducted at two places
Fig. 5.
Effect of bioinoculation of native isolate YSPMK11 and fungicides on curd yield of cauliflower
Discussion
In the present study, strain YSPMK11 was isolated from cauliflower endorhizosphere and evaluated its potential against S. sclerotiorum in vitro and in vivo. Solubilization of insoluble TCP by the strain YSPMK11 was associated with a decline in pH value. In our study, strain YSPMK11 was found as the best solubilizer of inorganic phosphates due to the production of organic acid, which is considered to be the chief mechanism of P solubilization (Sanjotha et al. 2011). Higher concentration of IAA was produced in the presence of 3 mg/ml tryptophan which is directly correlated with growth promotion of plants. HCN and siderophore production by isolate YSPMK11 proves its plant growth promoting properties and antagonistic nature against S. sclerotiorum. When subjected to test antagonism against S. sclerotiorum, zone formation was observed by isolate YSPMK11 which may be due to secretion of antifungal substance that strongly inhibited radial growth of phytopathogenic fungi on agar. Isolate YSPMK11 also exhibited excellent control efficacy because of their ability to form endospores and the broad-spectrum activity of their antimicrobial compounds. The maximum antifungal metabolite produced by isolate YSPMK11 in culture filtrate was observed after 72 h of incubation at 35 °C and at pH 7 in nutrient broth (Tables 1, 2, 3, 4). The physical factors that have been reported to affect antimicrobial metabolite production are temperature (Shanahan et al. 1992) and pH (Ownley et al. 1992). Isolate YSPMK11 was further subjected to molecular analysis. 16S rRNA sequence was highly homologous to B. pumilus. The PCR analysis further confirms the presence of ItuD and SrfC lipopeptide genes in isolate YSPMK11 which were responsible for the antifungal activities. Iturin A has reported to have strong haemolytic activity which kills wide range of fungal phytopathogens, whereas surfactin help in swarming, cell spreading, and biofilm production which favours the bacteria to colonize the root cells of plants (Moran et al. 2009; Yuan et al. 2011). In this study, we also found the potential antifungal compounds produced by B. pumilus. It suggested that a greater proportion of the fraction containing iturin A was responsible for the antifungal activities which result into inhibit the growth of S. sclerotiorum.
In the present study, versatile test isolate B. pumilus strain YSPMK11 showed noticeable attributes under in vitro conditions; therefore, this isolate was examined as a biocontrol agent in cauliflower against SSR caused by S. sclerotiorum under field conditions. Application of B. pumilus strain YSPMK11 was highly effective over the 2 year experimentation. Twice application of B. pumilus strain YSPMK11 (9 × 108 CFU/ml) at 30 and 50% bloom stage controlled disease incidence (93.70%) of SSR in cauliflower, higher than that of commercially available and regularly practiced fungicides in farmers field at various agroclimatic locations of Himachal Pradesh. In another study, disease severity was greatly reduced by B. subtilis strain SB24 when applied the cell suspension, broth culture, or cell-free filtrate solution 24 h before inoculation with S. Sclerotiorum displayed significant effect (Zhang and Xue 2010). Liu et al. (2010) demonstrated better control of S. sclerotiorum by cell-free filtrate of endophytic bacteria Yc8 application on the rapeseed leaves 24 h before fungal inoculation. Under field conditions, Bacillus isolate has a potential to be used as biobacteriocide in tomato plants (Santiago et al. 2015) and for the control of red rot in sugarcane (Hassan et al. 2015). However, the presence of biologically active metabolite produced by the antagonist, which diffused in the agar media, can be indicated by the zone of inhibition seen in the dual culture plates. Although our in vitro data indicate that isolate YSPMK11 inhibits S. sclerotiorum by means of antibiosis (Kumar and Kaushik 2013). Increase in curd yield by 36% over control may be attributed to the ability of bacteria to make P assimilable, and simultaneously produce plant growth promoting substances. This suggests that increasing crop production in sustainable manner by bioinoculation with the addition of small amount of insoluble inorganic P can be used as a feasible alternative of chemical fertilizers/fungicides.
It is thus concluded that application of B. pumilus strain YSPMK11 showed the better yield of cauliflower plants through reducing the percent disease incidence of S. sclerotiorum in infested plants. The presence of surfactin and iturin antifungal compound in this isolate makes it potent strain for biological control application in agriculture. This is the first study which showed that B. pumilus strain YSPMK11 has tremendous potential to be used as effective biocontrol agent against SSR in cauliflower and, therefore, deserved to be further developed.
Acknowledgements
Authors thanks to ICAR, New Delhi for financial support provided to carry out this study.
Compliance with ethical standards
Conflict of interest
Authors declare no conflict of interest.
References
- Anonymous (2000) Pesticide—guidelines for the field efficacy trials (I). National Standards of China, Standards Press of China
- Awais M, Pervez A, Qayyum S, Saleem M. Effects of glucose, incubation period and pH on the production of peptide antibiotics by Bacillus pumilus. Afr J Microbiol Res. 2008;2:114–119. [Google Scholar]
- Bakker AW, Schippers B. Microbial cyanide production in the rhizosphere to potato yield reduction and Pseudomonas spp. mediated plant growth stimulation. Soil Biol Biochem. 1987;19:451–457. doi: 10.1016/0038-0717(87)90037-X. [DOI] [Google Scholar]
- Bolton MD, Thomma BPHJ, Nelson BD. Sclerotinia sclerotiorum (Lib.) de Bary, biology and molecular traits of a cosmopolitan pathogen. Mol Plant Pathol. 2006;7:1–16. doi: 10.1111/j.1364-3703.2005.00316.x. [DOI] [PubMed] [Google Scholar]
- Bray RH, Kurtz LT. Determination of total organic and available forms of phosphorus in soils. Soil Sci. 1945;59:39–45. doi: 10.1097/00010694-194501000-00006. [DOI] [Google Scholar]
- Costacurta A, Mazzafera P, Rosato YB. Indole-3-acetic acid biosynthesis by Xanthomonas axonopodis pv. citri is increased in the presence of plant leaf extracts. FEMS Microbiol Lett. 2006;159:215–220. doi: 10.1111/j.1574-6968.1998.tb12863.x. [DOI] [Google Scholar]
- Dubey RC, Maheshwari DK. Practical microbiology. New Delhi: S. Chand and Company; 2011. [Google Scholar]
- FernandoA WGD, Nakkeeran S, Zhang Y, Savchuk S. Biological control of Sclerotinia sclerotiorum (Lib.) de Bary by Pseudomonas and Bacillus species on canola petals. Crop Prot. 2007;26:100–107. doi: 10.1016/j.cropro.2006.04.007. [DOI] [Google Scholar]
- Gond SK, Bergen MS, Torres MS, White JF., Jr Endophytic Bacillus spp. produce antifungal lipopeptides and induce host defence gene expression in maize. Microbiol Res. 2015;172:79–87. doi: 10.1016/j.micres.2014.11.004. [DOI] [PubMed] [Google Scholar]
- Hassan MN, Namood-e-Sahar, Shah S, Zia-Ul-Husnain S, Afghan S, Hafeez FY (2015) Suppression of red rot disease by Bacillus sp. based biopesticide formulated in non-sterilized sugarcane filter cake. Biocontrol. doi:10.1007/s10526-015-9673-4
- Hu X, Roberts DP, Xie L, Maul JE, Yu C, Li Y, Zhang S, Liao X. Bacillus megaterium A6 suppresses Sclerotinia sclerotiorum on oilseed rape in the field and promotes oilseed rape growth. Crop Prot. 2013;52:151–158. doi: 10.1016/j.cropro.2013.05.018. [DOI] [Google Scholar]
- Huang X, Zhang N, Yong X, Yang X, Shen Q. Biocontrol of Rhizoctonia solani damping-off disease in cucumber with Bacillus pumilus SQR-N43. Microbiol Res. 2012;167:135–143. doi: 10.1016/j.micres.2011.06.002. [DOI] [PubMed] [Google Scholar]
- Kamal MM, Lindbeck KD, Savocchia S, Ash GJ. Biological control of sclerotinia stem rot of canola using antagonistic bacteria. Plant Pathol. 2015;64:1375–1384. doi: 10.1111/ppa.12369. [DOI] [Google Scholar]
- Kumar S, Kaushik N. Endophytic fungi isolated from oil-seed crop Jatropha curcas produces oil and exhibit antifungal activity. PLoS One. 2013;8:e56202. doi: 10.1371/journal.pone.0056202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Liu L, Zhang S, Cui W, Lv J. Identification of antifungal compounds produced by Lactobacillus casei AST18. Curr Microbiol. 2012;65:156–161. doi: 10.1007/s00284-012-0135-2. [DOI] [PubMed] [Google Scholar]
- Liu B, Huang LL, Buchenauer H, Kang ZS. Isolation and partial characterization of an antifungal protein from the endophytic Bacillus subtilis strain EDR4. Pest Biochem Physiol. 2010;98:305–311. doi: 10.1016/j.pestbp.2010.07.001. [DOI] [Google Scholar]
- Meena KR, Saha D, Kumar R. Isolation and partial characterization of iturin like lipopeptides (a bio-control agent) from a Bacillus subtilis strain. Int J Curr Microbiol App Sci. 2014;3:121–126. [Google Scholar]
- Moran S, Rai DK, Clark BR, Murphy CD. Precursor-directed biosynthesis of fluorinated iturin A in Bacillus spp. Org Biomol Chem. 2009;7(4):644–646. doi: 10.1039/b816345f. [DOI] [PubMed] [Google Scholar]
- Ownley BH, Weller DM, Thomashow LS. Influence of in situ and in vitro pH on suppression of Gaeumannomyces graminis var. tritica by Pseudomonas fluorescens 2–79. Phytopathology. 1992;82:178–184. doi: 10.1094/Phyto-82-178. [DOI] [Google Scholar]
- Pryor SW, Gibson DM, Krasnoff SB, Walker LP. Identification of antifungal compounds in a biological control product using a microplate inhibition bioassay. Am Soc Agric Biol Eng. 2006;49:1643–1649. [Google Scholar]
- Saharan GS, Mehta N. Sclerotinia diseases of crop plants, biology, ecology and disease management. The Netherlands: Springer; 2008. [Google Scholar]
- Sanjotha P, Mahantesh P, Patil CS. Isolation and screening of efficiency of phosphate solubilizing microbes. Int J Microbiol Res. 2011;3:56–58. doi: 10.9735/0975-5276.3.1.56-58. [DOI] [Google Scholar]
- Santiago TR, Grabowski C, Rossato M, Romeiro RS, Mizubuti ESG. Biological control of eucalyptus bacterial wilt with rhizobacteria. Biol Control. 2015;80:14–22. doi: 10.1016/j.biocontrol.2014.09.007. [DOI] [Google Scholar]
- Schwyn B, Neilands JB. Universal chemical assay for the detection and determination of siderophore. Anal Biochem. 1987;160:47–56. doi: 10.1016/0003-2697(87)90612-9. [DOI] [PubMed] [Google Scholar]
- Shanahan P, O’Sullivan DJ, Simpson P, Glennon JD, O’Gara F. Isolation of 2, 4-diacetylphloroglucinol from a fluorescent Pseudomonad and investigation of physiological parameters influencing its production. Appl Environ Microbiol. 1992;58:353–358. doi: 10.1128/aem.58.1.353-358.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahyudi AT, Prasojo BJ, Mubarik NR. Diversity of antifungal compounds-producing Bacillus spp. isolated from rhizosphere of soybean plant based on ARDRA and 16S rRNA. Hayati J Biosci. 2010;17:145–150. doi: 10.4308/hjb.17.3.145. [DOI] [Google Scholar]
- Warnock DW. Methods with antifungal drugs. In: Evans EGV, Richardson MD, editors. Medical mycology—a practical approach. Oxford: IEL Press; 1989. [Google Scholar]
- Yexin Z, Huqiang Q, Fengjie N, Lili H, Xiaoning G, Zhensheng K, Qingmei H. Investigation of Sclerotinia stem rot in Shaanxi Province. Plant Prot. 2011;2:116–119. [Google Scholar]
- Yuan J, Raza W, Huang Q, Shen Q. Quantification of the antifungal lipopeptide iturin A by high performance liquid chromatography coupled with aqueous two phase extraction. J Chromatogr B Anal Technol Biomed Life Sci. 2011;879:2746–2750. doi: 10.1016/j.jchromb.2011.07.041. [DOI] [PubMed] [Google Scholar]
- Zhang JX, Xue AG. Biocontrol of sclerotinia stem rot (Sclerotinia sclerotiorum) of soybean using novel Bacillus subtilis strain SB24 under control conditions. Plant Pathol. 2010;59:382–391. doi: 10.1111/j.1365-3059.2009.02227.x. [DOI] [Google Scholar]