Abstract
Introduction
Using the Indian and rest of world (ROW) injection technique questionnaire (ITQ) data, we address key insulin injection complications.
Methods
In 2015 we conducted an ITQ survey throughout India involving 1011 patients. Indian values were compared with those from 41 other countries participating in the ITQ, known here as ROW.
Results
More than a quarter of Indian insulin users described lesions consistent with lipohypertrophy (LH) at their injection sites and approximately 1 in 5 were found to have LH by the examining nurse (using visual inspection and palpation). Just over half of Indian injectors report having pain on injection. Of these, 4 out of 5 report having painful injections only several times a month or year (i.e., not with every injection). Doctors and diabetes educators in India (as opposed to nurses) have a larger role in teaching patients how to inject than they do in ROW. Despite this specialized approach, a very high percentage of patients report that they have not been trained (at least cannot remember being trained) in a wide range of essential injection topics. Only about 30% of Indian injectors get their sites checked at least annually, with nearly a third only having sites checked when they specifically complained and nearly 4 out of 10 never having had their sites checked.
Conclusion
Indian HCPs can clearly do a better job covering all the vital topics essential to proper injection habits.
Keywords: Infusions, Injections, Insulin, Lipodystrophy, Lipohypertrophy, Needles, Needlestick, Subcutaneous
Introduction
In a previous paper we introduced the Indian injection technique questionnaire (ITQ) survey patient population and injecting practice [1]. In conjunction with the results, we gave evidence-based Indian best practice recommendations. Here we use the same approach for injecting complications.
Methods
Our previous paper [1] described the methods, materials, centers, and patients who participated in the study.
All procedures followed were in accordance with the ethical standards of the Indian Council of Medical Research, the responsible committee on human experimentation (institutional and national), and the Helsinki Declaration of 1964, as revised in 2013. Informed consent was obtained from all patients for being included in the study.
Results
Lipohypertrophy (LH)
Patients were asked: “Do you have any swelling or lumps under the skin at your usual injection sites that have been there for some time (weeks, months, or years)?” Table 1 gives the results for both the patients’ answers and the nurses’ examination of all patient injecting sites. Indian results are given beside rest of world (ROW). The latter constitute the values from the 41 other ITQ participating countries combined (excluding India).
Table 1.
Lipohypertrophy in India vs ROW
| Presence of lypohypertrophy | % India (N = 873) | % ROW (N = 7657) |
|---|---|---|
| As per patient report | 25.9 | 29.2 |
| As per nurse examination | 21.9 | 31.8 |
ROW (rest of world) constitutes the values from the 41 other ITQ participating countries combined (excluding India)
Nurses examined each of the patient’s injection sites both visually and by palpation and reported any LH (Table 2). Percentages for both visual and palpated LH in India were slightly lower than in ROW. When nurses found LH they were asked to measure the lesions along their longest dimension in millimeters. Table 3 shows that LH size in Indian patients was on average slightly lower in the abdomen and thigh and slightly higher in the arm. Whenever nurses found LH they asked the patient if they were still injecting into it and 18.9% of Indian patients said yes. They were then asked how often they did so (Table 4). Patients who injected into LH were also asked why they did so (Table 5). More than half of Indian patients answered ‘Don’t know’ to that question.
Table 2.
Nurse-reported lipohypertrophy in Indian and ROW patients
| Site | Exam type | % India (N = 837) | % ROW (N = 7565) |
|---|---|---|---|
| Abdomen | Visual | 6.8 | 17.3 |
| Palpation | 9.3 | 21.1 | |
| Thigh | Visual | 6.9 | 9.8 |
| Palpation | 8.7 | 11.2 | |
| Buttocks | Visual | 3.4 | 2.1 |
| Palpation | 0.0 | 2.8 | |
| Arm | Visual | 9.4 | 11.2 |
| Palpation | 10.1 | 13.4 |
Table 3.
Size of nurse-measured lipohypertrophy for Indian and ROW patients
| Size of lipohypertrophy (mm) | Mean India | Mean ROW | N India | N ROW |
|---|---|---|---|---|
| Abdominal | 39.1 | 44.7 | 62 | 1258 |
| Thigh | 40.4 | 42.0 | 27 | 460 |
| Arm | 39.0 | 35.5 | 21 | 372 |
Table 4.
Frequency of injection into lipohypertrophy in Indian and ROW patients
| Frequency | % India (N = 118) | % ROW (N = 1964) |
|---|---|---|
| Every injection | 6.8 | 16.7 |
| Frequently (daily) | 28.8 | 39.5 |
| Occasionally (weekly) | 44.1 | 30.3 |
| Seldom (monthly) | 20.3 | 13.5 |
Table 5.
Reasons patients report injecting into lipohypertrophy in Indian and ROW patients
| Reason | % India (N = 158) | % ROW (N = 2160) |
|---|---|---|
| It is convenient | 4.2 | 16.3 |
| It is less painful | 13.3 | 22.3 |
| Just a habit | 29.7 | 34.9 |
| Do not know | 52.8 | 26.5 |
The worldwide ITQ data [2, 3] showed a strong association between the presence of LH and the total daily dose (TDD) of insulin. Over 10 IU of insulin on average was consumed in the LH+ population vs LH−. In T2DM patients, this average TDD difference is 13.5 IU. In T1DM patients, the average TDD difference is 5.4 IU. The presence of LH is associated with higher HbA1c values, an average difference of 0.55. The worldwide data also showed that LH is associated with incorrect rotation of injection sites, using smaller injecting zones, more years on insulin, and reusing pen needles. The higher the number of times the needle is reused, the more frequently reported LH is.
We defined “hypoglycemia” as the occurrence of at least one symptom of low sugar (e.g., palpitations, tiredness, sweating, strong hunger, dizziness, tremor) and a confirmed blood glucose meter reading of no greater than 60 mg/dL (3.3 mM/L). We defined “frequent unexplained hypoglycemia” as hypoglycemia occurring one or more times weekly in the absence of a definable precipitating event such as a change in medication, diet, or activity. We defined “glycemic variability” as the presence of blood glucose oscillations from less than 60 mg/dL (3.3 mM/L) to more than 250 mg/dL (13.9 mM/L) at least three times a week in an unpredictable and unexplained fashion and evidence of such a pattern for at least the previous 6 months.
Nurses were asked to review the records of each subject in the ITQ and assess how many qualified as having “frequent unexplained hypoglycemia” and “glucose variability”. Approximately 1 out of 4 Indian insulin injectors had frequent unexpected hypoglycemia and more than 1 out of 3 had glucose variability (Table 6). These findings are proportionally similar in India to ROW. In general, LH is associated with higher rates of unexplained hypoglycemia and higher rates of glycemic variability as well as more frequent diabetic ketoacidosis (DKA). Nurses also assessed injection sites for lipoatrophy and redness, and Table 7 gives the results for India and ROW.
Table 6.
Frequency of unexpected hypoglycemia and glucose variability in Indian patients
| % India | % ROW | N India | N ROW | |
|---|---|---|---|---|
| Unexpected hypoglycemia | ||||
| Yes | 24.1 | 19.4 | 236 | 1580 |
| No | 75.9 | 80.6 | 745 | 6558 |
| Glucose variability | ||||
| Yes | 37.3 | 35.4 | 369 | 2872 |
| No | 62.7 | 64.6 | 621 | 5251 |
Table 7.
Nurse-reported lipoatrophy and redness in Indian and ROW patients
| Site | Finding | % India (N = 837) | % ROW (N = 7565) |
|---|---|---|---|
| Abdomen | Lipoatrophy | 0.4 | 0.6 |
| Redness | 5.7 | 3.3 | |
| Thigh | Lipoatrophy | 1.2 | 0.5 |
| Redness | 5.4 | 2.8 | |
| Buttocks | Lipoatrophy | 0.0 | 0.2 |
| Redness | 3.4 | 0.4 | |
| Arm | Lipoatrophy | 0.7 | 0.4 |
| Redness | 2.4 | 3.6 |
Rotation of Injecting Sites
Correct site rotation is defined as always injecting at least 1 cm from a previous injection. Worldwide ITQ data shows that patients who rotate correctly tend to have less hyperglycemia, less LH, less unexplained hyperglycemia, and less glucose variability. (Data not shown for the aforementioned, but all differences were significant to a p < 0.05.) HbA1c is lower in those who correctly rotate by an average of 0.53. Correct rotation is also associated with lower TDD by an average of 4.7 IU. Table 8 shows that 68.1% of Indian injectors were found to correctly rotate site, a value similar to that in ROW.
Table 8.
Lipohypertrophy and correct rotation: India vs ROW
| India (N = 873) | ROW (N = 7657) | |
|---|---|---|
| Practice correct rotation | 68.1 | 71.0 |
Bleeding, Bruising, Pain, and Leakage
Indian patients were asked if they ever observed bleeding or bruising from their injection sites and 41.4% said they did. They were then asked about the frequency and only 0.9% said it was “always”, 7.8% said “often” (several times a week), 53.6% said “sometimes” (several times a month), and 37.7% said it was “almost never” (several times a year).
We found that just over half of Indian injectors report having pain on injection. Of these, 4 out of 5 report having painful injections only several times a month or year (i.e., not with every injection). Pain seems also to be commonly associated with bleeding. Approximately 1 out of 5 Indian patients report leakage of insulin from the skin. Of these, approximately 85% say it occurs rarely (several times a month or a year).
Indian patients were asked who gave them their injection training. Table 9 makes clear that doctors and diabetes educators in India have a larger role in teaching patients how to inject than they do in ROW. Pharmacists and industry representatives are also more engaged in India than ROW. Health care in India is often delivered in very rapid physician visits and diabetes care is no exception; it is common for a physician to see from 50 to 100 outpatients in a day. Injection training is still considered the prevue of the doctor in India.
Table 9.
Professional who gave patient injection training
| Injection instructor | % India (N = 986) | % ROW (N = 9440) |
|---|---|---|
| General nurse | 13.9 | 22.9 |
| Diabetes nurse | 15.7 | 46.7 |
| Diabetes educator | 23.4 | 12.3 |
| Doctor (general practitioner) | 15.3 | 5.1 |
| Doctor (diabetes specialist) | 17.1 | 10.0 |
| Pharmacist | 7.8 | 2.0 |
| A representative of the pen or needle manufacturer | 6.7 | 1.0 |
Indian patients were asked how often their injection sites were checked by their HCP. Table 10 shows that in India, as in ROW, the goal of checking injection sites at least once a year is not being met for the majority of patients. Patients were asked when they last received instruction or advice on injections. Table 11 shows that India is actually performing slightly better than ROW in giving advice within the last year.
Table 10.
Frequency with which injection sites checked
| Frequency | % India (N = 867) | % ROW (N = 12,505) |
|---|---|---|
| Routinely every visit | 19.6 | 28.3 |
| Once a year | 11.1 | 12.6 |
| Only if I complain of a problem at a site | 31.7 | 20.2 |
| I cannot remember my sites ever being checked | 37.6 | 38.9 |
Table 11.
Last time patient given instructions or advice on injections
| Frequency | % India (N = 970) | % ROW (N = 9598) |
|---|---|---|
| Within the past 6 months | 42.9 | 37.4 |
| Within the past 6–12 months | 19.4 | 17.6 |
| Sometime in the last 1–5 years | 14.9 | 21.5 |
| Sometime in the last 5–10 years | 4.2 | 13.5 |
| Never | 18.6 | 10.0 |
Indian patients were asked to report which injection topics they could not remember ever being trained in. Table 12 shows that for all topics, a higher percentage of Indian patients could not remember being trained than in ROW, with some percentages sometimes being 2–3 times higher in India.
Table 12.
Topics patients cannot remember ever being trained in
| Topic | % India (N = 988) |
% Row (N = 8790) |
|---|---|---|
| Injection sites (e.g., thigh, arm, buttock, abdomen) | 37.7 | 11.6 |
| Skin thickness and appropriate depth of injection | 57.2 | 27.2 |
| Length of needle | 57.2 | 25.6 |
| How to do a skinlift or “pinch up” the skin | 43.6 | 18.2 |
| How long to hold a skinlift or “pinch up” | 49.7 | 25.7 |
| Angle of needle entry | 37.9 | 16.1 |
| How long to keep the needle in the skin after injection | 43.5 | 16.4 |
| Rotating within an injection site | 48.7 | 18.4 |
| Prevention of air bubbles (syringe) or proper priming of pen needle | 52.0 | 19.7 |
| Mixing insulin in a syringe (for syringe users) | 53.2 | 30.3 |
| Re-suspension of cloudy insulin | 55.1 | 25.0 |
| Single use of pen needle/syringe | 61.0 | 19.0 |
| Safe disposal of sharps (pen needles, syringes) | 65.3 | 28.2 |
The Indian HCPs filling out the ITQ were asked to identify themselves. Table 13 shows that a much higher percentage of diabetes educators and specialist doctors filled out the ITQ forms in India than in ROW. Indian HCPs were then asked if they were aware that there were new injection recommendations that had been published and 94.1% (64 of 68) said yes. Of these 92.3% (60 of 65) said they had changed their practice as a consequence of these recommendations.
Table 13.
Professional who filled out the ITQ
| Professional | % India (N = 72) | % ROW (N = 1263) |
|---|---|---|
| General nurse | 9.7 | 17.1 |
| Diabetes nurse | 11.1 | 56.1 |
| Diabetes educator | 63.9 | 22.8 |
| Doctor (general practitioner) | 2.8 | 1.0 |
| Doctor (specialist) | 12.5 | 3.0 |
Discussion
Lipohypertrophy (LH)
LH is a thickened, “rubbery” lesion that appears in the subcutaneous (SC) tissue of injecting sites in up to half of patients who inject insulin. In some patients the lesions can be hard or scar-like [4, 5]. Detection of LH requires both visualization and palpation of injecting sites, as some lesions can be more easily felt than seen [6]. Making two ink marks at opposite edges of the LH (at the junctions between normal and “rubbery” tissue) will allow the lesion to be measured, recorded, and followed long-term.
More than a quarter of our Indian patients described lesions consistent with LH at their injection sites (Table 1) and approximately 1 in 5 were found to have LH by the examining nurse (using visual inspection and palpation) at the time of the ITQ (Tables 1, 2). Figure 1 illustrates visible LH in a woman who had injected in the same two locations below the umbilicus for 12 years. Figure 2 illustrates the detection of palpable LH by comparing a fold of normal skin (arrow tips close together) with lipohypertrophic tissue (arrow tips spread apart). Normal skin can be pinched tightly together, while lipohypertrophic lesions cannot [7].
Fig. 1.
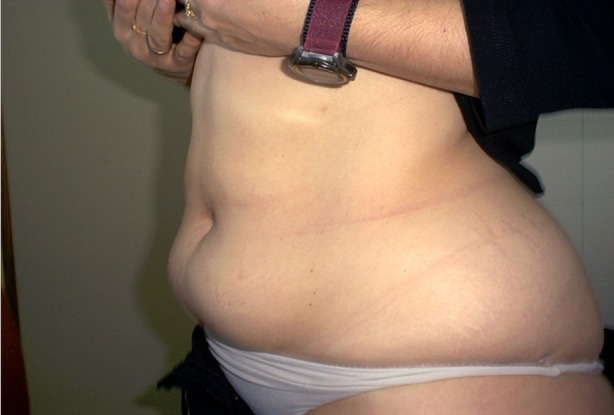
Two visible lipohypertrophic lesions below the umbilicus
Fig. 2.
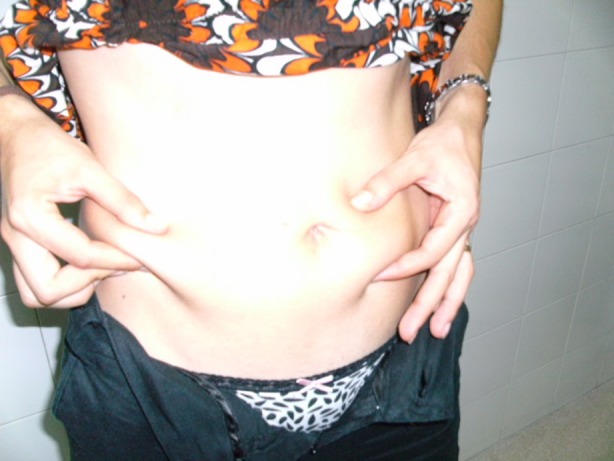
The different “pinch” characteristics of normal (left) vs lipohypertrophic (right) tissue
LH in India is slightly less frequent than in ROW (Table 1). This may reflect the fact that Indian injectors have been using insulin only 5.5 years on average, while patients in ROW have been using it on average 9.0 years (Table 3 of our first ITQ paper [1]); or it may mean that intensive therapy with multiple daily injections (MDI) is still not common in India. (Both time on insulin and number of injections/day are known to be risk factors for LH.)
As in ROW, Indian HCPs who examined injection sites in our study found more LH lesions by palpation than visually (Table 2), emphasizing the importance of carefully examining sites with the hands. HCPs should lubricate their hands before the exam with gel (ultrasound gel or equivalent) and use a circular motion, similar to that employed when examining the breast. LH lesions in India average about 40 mm, a size which is usually easy to detect, if one looks for it (Table 3).
Of some consolation is that Indian patients with LH do not inject into these lesions as frequently as in ROW (Table 4). When asked why they continued to do so, convenience and pain were less frequently cited by Indian patients than those in ROW (Table 5). Most patients simply did not know why they did so, suggesting that habit or mindlessness is at play.
Upwards of a quarter of Indian patients have LH. This should probably be considered an underestimate, since HCPs participating in the ITQ did not get any special training in LH detection. The prevalence rates of LH amongst insulin-injecting patients in other countries varies significantly. In five recent studies it ranged from lowest to highest: 14.5% (Hajheydari et al. [8]); 27.1% (Raile et al. [9]); 34.5% (Partanen and Rissanen [10]); 48.0% (Kordonouri et al. [11]); 64% (Blanco et al. [12]). Blanco et al. studied 430 patients from 19 Spanish centers and found that LH was more common in T1DM (72.3%) than in T2DM (53.4%). Grassi et al. [13] studied 388 patients from 18 Italian centers and found a prevalence of 48.7%. A Chinese study [14] of 401 patients in four centers found an overall prevalence of 53.1% (95% CI 48.2, 58.0%). By body site, LH was found in 52.4% of abdomens examined, 15.5% of thighs, and 9.4% of arms.
Lipohypertrophy has also been reported to be frequent in continuous subcutaneous insulin infusion (CSII) patients. A cross-sectional study [15] of 50 consecutive patients with T1DM using CSII for more than 6 months (26 female; age, 13.3 ± 3.5 years; CSII duration, 2.8 ± 1.7 years; HbA1c, 7.7% ± 1.1%) examined the skin for complications associated with therapy and 42% of these patients had LH. A similar survey [16] of 91 adult CSII patients revealed that the commonest infusion site problem was lipohypertrophy (26.1%), which occurred more often in those with long duration of CSII (4.8 [2.38–9.45] vs 3.0 [1.50–4.25] years; p = 0.01).
Vardar and Kizilci [17] identified, by logistic regression analysis, three independent risk factors for LH: duration of insulin use, with longer use associated with more LH (p = 0.001); site rotation, with a failure to rotate associated with higher LH risk (p = 0.004); changing needles, with needle reuse also associated with LH (p = 0.004). Two other studies [12, 18] have identified similar factors. Immunologic factors in LH are poorly understood, although antibodies seem to have a role in pediatric and adolescent patients with T1DM [9]. Needle length has not been shown to be a risk factor. It is also not known what the impact of different needle lengths is on insulin absorption from injections into LH.
Histopathologically, LH lesions are shown to be entirely formed of adipocytes. These cells are often hypertrophied to two or three times the size of normal adipocytes. They can be seen invading the adjacent reticular dermis, engulfing lipid droplets, proliferating or manifesting other signs of metabolic activation [19]. This anabolic activity is presumably initiated by trauma from repeat injections in the same place and time coupled with the growth-promoting properties of insulin. There may be genetic factors but these have not yet been elucidated.
Almost all studies of patients injecting into LH [20–23] show insulin absorption to be delayed or erratic, potentially worsening diabetes management.
Franzen and Ludvigsson [24] evaluated children with diabetes who were injecting into clinically detectable LH. The children received simple but direct instructions: rotate injection sites; and do not reuse your needles. In 3 months 90% of LH lesions in these children had resolved and were undetectable. HbA1c was improved significantly and insulin requirements had decreased.
Blanco et al. [12] showed that unexplained hypoglycemia and glycemic variability were also greatly increased (6- to 7-fold) in those with versus those without LH. In our study we found that unexpected hypoglycemia affected more than a quarter of Indian injecting patients, while glucose variability was present in over a third. In both cases, these adverse outcomes were more common than in ROW (Table 6). Both have many causes, and injecting into LH is one of them.
A critical finding of a Spanish study is the correlation of total daily dose (TDD) of insulin to the presence of LH and its derived cost to the health care system. Subjects with LH had significantly higher TDD, overall and in both T1DM and T2DM groups. T2DM patients had the highest TDD differences (approximately 20 additional units daily). Such patients tend to have increased weight and insulin resistance compared to T1DM patients, and these factors probably contributed to their greater TDD; however, the T2DM patients with LH had similar weight and BMI as those with T2DM but without LH. Another major contributor is the practice of injecting into LH where the absorption properties of insulin are distorted. The cost of the additional insulin consumed by injecting into LH was calculated (based on prevalence of LH, number of insulin-injecting patients in Spain, differences in TDD, and the cost in Euros per unit of insulin) to be over 122 million euros in Spain. This is an obvious opportunity for savings to both patients and health care payers. The one weakness of the Spanish study is that HbA1c levels were unfortunately not collected from the subjects examined. A Chinese study similar to the Spanish one showed remarkably similar results,1 with the addition that patients with LH had significantly higher HbA1c values (8.2 ± 1.8) than those without LH (7.7 ± 1.5) (p < 0.003) [14, 25]
The impact of LH on insulin PK-PD is rather poorly documented in the literature. While there are case reports indicating reductions in insulin consumption with improvements in HbA1c when patients with LH were taught to inject into normal areas, and a small number of studies that evaluated insulin PK-PD when patients injected into areas of LH vs normal areas, the overall quality of such studies is poor and/or they were substantially underpowered. It is assumed that LH reduces and/or slows insulin uptake, and perhaps increases PK-PD variation, but this has not been proven rigorously.
Two closely related studies have addressed these questions, using state-of-the-art methodologies. A glucose clamp study [26] in patients with LH has shown that both insulin absorption and action are substantially blunted and considerably more variable when insulin is injected into areas with LH.2 A separate, mixed meal study in the same subjects confirmed the slower absorption and decreased action of insulin when injected into LH compared to normal adipose tissue, with much greater post-meal glycemic excursions shown [27].
Site Rotation
Several studies have demonstrated that the best way to safeguard normal tissue is to properly and consistently rotate injecting sites [28–30]. Injection can be rotated from one body region to another (abdomen to thigh, to buttock, to arm) but it must be remembered that absorption characteristics change depending on the type of insulin given. Analogues can be given at any injection site with similar uptake and action (PK-PD), but human insulins (regular, NPH) vary substantially—absorption being fastest from the abdomen and slowest from the buttock. However, correct rotation involves spacing injections a least 1 cm. apart even within an injection zone. Table 8 shows that 68.1% of Indian injectors were found to correctly rotate site, a value similar to ROW. This may be why the LH rate in India is lower than in ROW (Table 1).
Some clinicians are offering the single-use skin safe marker pen to patients to keep and use to make a dot on the skin where they inject and use this as a reference point for the next injection. This seems to work very well for some patients and the marker from the pen washes off and fades to nothing in about 5 days.
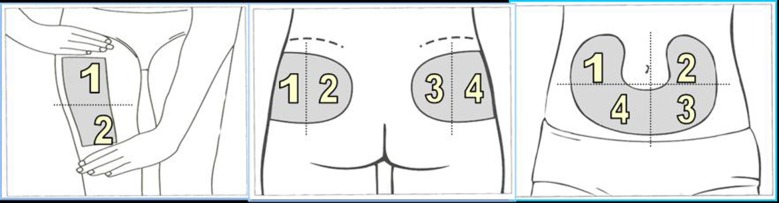
One scheme with proven effectiveness involves dividing the injection site into quadrants (or halves when using the thighs or buttocks), using one quadrant per week and moving always clockwise, as shown by Figs. 3 and 4 [7]. Injections within any quadrant or half should be spaced at least 1 cm from each other in order to avoid repeat tissue trauma. Pump cannulae should be placed at least 3 cm away from previous sites. HCPs should verify that the rotation scheme is being followed at each visit and give help and advice where needed.
Fig. 3.
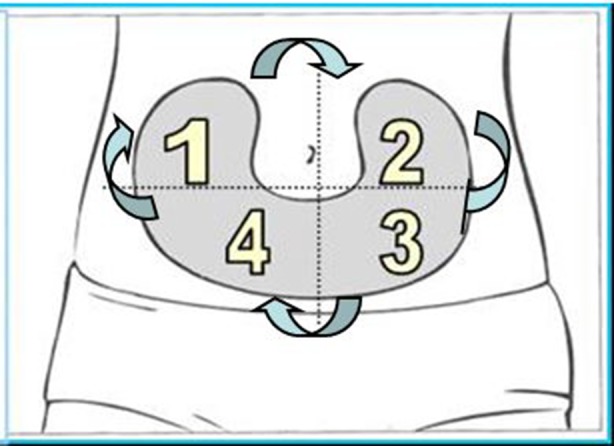
Abdominal rotation pattern by quadrants
Fig. 4.
Thigh and buttocks rotational pattern by halves
Lipoatrophy
Lipoatrophy (LA) has been reported in all injecting sites [31–36]. It is now considered a relatively unusual condition, prompting case reporting. Risk factors are not understood. Some authors have suggested that young women with other autoimmune disorders may be at higher risk. LA is felt to be a local immune reaction against fat cells provoked by insulin crystals. Consequently LA is rarer today than it was when less pure insulins were given. But LA is still observed even with short- and long-acting analogues. LA causes significant variability in insulin absorption when injections are given into it.
Treatment for LH is not evidence-based because of the lack of trials. Several approaches, however, have been recommended: changing the insulin formulation (e.g., aspart to lispro, or lispro to glulisine, etc.), changing injection sites, or shifting to CSII and possibly cortisone injected into the LA. LA may or may not resolve with time, but this depends on the individual patient. LA has been seen with the short-acting analogues, lispro and aspart, as well as the long-acting ones, glargine and detemir. It may also be associated with non-rotation of injection sites and needle reuse. LA is both a cosmetic problem (disfiguring) and a clinical one (erratic and abnormal insulin absorption).
Fortunately lipoatrophy is now much less common than it used to be in the days of animal insulins. In our study it affected only about 2% of Indian injectors, a figure compatible with ROW (Table 7).
Injection Pain
We found that just over half of Indian injectors report having pain on injection. Of these, 4 out of 5 report having painful injections only several times a month or year (i.e., not with every injection).
As the above data suggests, most insulin injections are not painful, except in the infrequent event that the needle comes into direct contact with a nerve ending. Some patients, however, are exceptionally sensitive to sensations they describe as painful. Patient awareness of injection discomfort has been studied extensively and is related to three key factors: needle length (and tissue level penetrated); needle diameter; and injection context. Injection context is defined by environment (including noise and the presence of other people), view of the needle, and the apprehension of HCPs, both professional and family. The more apprehension the latter display, the greater the pain and anxiety felt by the patient [37, 38]. This reverse transference places a large responsibility on carers to assess their own attitudes towards injection pain. Some patients complain of discomfort when injecting insulins which have a low pH. This seems, anecdotally, to be reported more commonly in children. Glargine is an example of an acidic insulin.
Heise et al. [39] showed that injection speed (150, 300, and 450 μl/s; equivalent to 15–45 IU/s of U100 insulin) makes no difference in pain. But injection volume does, with higher volumes (≥1200 μl or 120 IU of U100 insulin) causing more pain. His group also found that injections in the thigh appear to hurt more than those in the abdomen in adults. Anderson et al. [40] and Jorgensen et al. [41] also found that higher injected volumes cause more pain. Hofman et al. [42] as well showed in both children and adults that thigh injections are more painful than abdominal ones. Nevertheless, Heise et al. [39] found that most patients say the pain is acceptable regardless of volume or injection site. So we can reasonably conclude that injection pain, though felt under certain circumstances by certain patients, is mild enough to be acceptable to most of them, particularly with today’s very thin, short needles.
This survey shows that at present injection training in India is performed mainly by diabetes educators and physicians (Table 9). Despite this specialized approach, a very high percentage of patients report that they have not been trained (at least cannot remember being trained) in a wide range of essential injection topics (Table 12).
The latest version of the Indian insulin injection recommendations (from 2015 [43]) has specific guidelines for each one of these topics, and many others.
We know that injecting training works, even when delivered only one time. In a recent Italian study [13], 346 patients with diabetes from 18 ambulatory centers throughout northern Italy who had been injecting insulin for at least 4 years received a thorough evaluation of their injection technique (IT). Their doctors and nurses then examined all injection sites for the presence of LH, followed by an individualized training session in which suboptimal IT practices were addressed. All patients were taught to rotate sites correctly in order to avoid LH and were begun on 4-mm pen needles to increase potential injection sites, while avoiding intramuscular (IM) injections. Patients were also instructed not to reuse needles. Results showed that 49% of patients had LH at study entry. After 3 months, patients had mean reductions in HbA1c of 0.58%, in fasting blood glucose of 14 mg/dL, and in total daily insulin dose of 2.0 IU (from baseline 50.5 IU), all statistically significant at p < 0.05. Follow-up questionnaires showed that significant numbers of patients recognized the importance of IT and were performing their injections more optimally. The majority found the 4-mm pen needle convenient and comfortable.
The relationship between diabetes education and glucose control is far from simple. A recent study suggested that education alone (including empowerment) may not be sufficient to ensure behavioral change and improved glycemic control, at least in T2DM patients managed in primary care [44].
Therapeutic Education
It is clear that “education”, defined as the simple imparting of information, is not enough. In diabetes we must employ “therapeutic education” [45]. Therapeutic education is above all patient-centered, focusing on individual needs, resources, and values. It tailors individual strategies to the patient, and the individual patient helps shape these strategies. Patients participate actively in their treatment, eventually virtually taking it over. Therapeutic education takes into account a whole array of psychological, social, and biologic factors. It tackles the hardest things human beings must do—change behavior. It uses the approaches and brings to bear all power of cognitive behavioral therapy. It focuses on motivation, step-by-step change, and compassion with oneself in the face of failure. Injection training must be rooted in therapeutic education to be effective.
Studies have shown that not all patients receive therapeutic education about injections and for those who do, not all critical topics are covered [46–48]. This was very clear from our study (Table 12). One principle of therapeutic education is that decisions should be made in a discussion context in which the patient is a partner and the HCP offers experience and advice [49, 50]. Therapeutic education can also occur in a group setting, and there is evidence that lower subsequent HbA1c values and better adherence are achieved if the HCP has formal training as an educator [6].
Educational guidelines for injectors recommend checking injection sites at least annually and more frequently when the risk for LH or other complication is high. Our study showed that only about 30% of Indian injectors get their sites checked this frequently (Table 10), with nearly a third only having sites checked when they specifically complained and nearly 4 out of 10 never having had their sites checked. This is clearly an area in which therapeutic education in India can be improved.
Conversely, India is doing a somewhat better job than ROW in giving advice about injections. Nearly two-thirds of injecting patients had been given instruction in the last year (Table 11). But Indian HCPs can clearly do a better job covering all the vital topics essential to proper injection habits (Table 12).
Conclusions
The first Indian insulin injection technique recommendations were published in Indian Journal of Endocrinology & Metabolism, November 2012 issue. Addenda were published in the November 2013 and November 2014 issues of that journal. The latest version of these recommendations were published in 2015 [43]. That version provides the following guidelines for preventing LH:
Regular inspection and palpation of insulin sites
Do not reuse needles
Follow correct site rotation policy
Use larger injection surface areas
Do not inject into LH sites
Reduce dose of insulin in habitual LH site injections when shifting to normal SC tissue
Rule out LH as a cause of poor glycemic control, hypoglycemia, and high glycemic variability
Before starting the injection therapy, the healthcare provider should ensure that patients understand each of these essential topics:
The injection regimen
The choice and management of the devices used
The choice, care, and self-examination of the devices used
Proper injection techniques (including site rotation, injection angle, and possible use of skinfolds)
Injection complications and how to avoid them
Optimal needle lengths
Healthcare professionals should spend ample time exploring patient anxieties and other concerns about the injecting process and insulin itself.
A quality management process should be put in place and make sure that the correct injection technique has been practiced regularly by patients and is also documented in the record.
All results from the ITQ survey data are available in an interactive form on Tableau Public Adam Young’s Profile website [56]. We believe the latest ITQ data support and reinforce the above recommendations. Every diabetes center in India should be familiar with the ITQ results and should be scrupulous in following the official Indian insulin injection recommendations.
Acknowledgements
No funding or sponsorship was received for this study or publication of this article. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published. Our sincerest thanks go to the 20 Indian centers with their 72 health care professionals and 1011 patients for performing the ITQ study. They have given an invaluable gift to the world of Indian diabetes.
Disclosures
Kenneth W. Strauss is an employee of BD, a manufacturer of injecting devices.
Laurence J. Hirsch is an employee of BD, a manufacturer of injecting devices.
Vandita Gupta is an employee of BD, a manufacturer of injecting devices.
Sanjay Kalra, Ambrish Mithal, Rakesh Sahay, Mathew John, AG Unnikrishnan, Banshi Saboo, Sujoy Ghosh and Debmalya Sanyal have nothing to disclose.
Compliance with Ethics Guidelines
All procedures followed were in accordance with the ethical standards of the Indian Council of Medical Research, the responsible committee on human experimentation (institutional and national), and the Helsinki Declaration of 1964, as revised in 2013. Informed consent was obtained from all patients for being included in the study.
Data Availability
All results from the ITQ survey data are available in an interactive form on Tableau Public Adam Young’s Profile website [56].
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Specifically, LH was present in 52.9% of participants. Patients were an average of 59.6 (SD = 11.5) years old and took insulin for 5.6 (SD = 4.6) years, averaging 33.0 (SD = 18.4) U/day. HbA1c was 8.2% (1.8) and 7.7% (1.5), respectively, in those with and without LH (p = 0.003). LH was associated with higher daily insulin dose (38.1 vs 27.1 U, p < 0.001) and cost [the Chinese currency, the Renminbi (RMB 8.2 vs 5.8, p < 0.001)]. Those with LH averaged 2.3 (2.2) nodes, had higher frequency of pen needle (PN) reuse (median 13.0 vs 7.5, p = 0.003), and greater total 6-month direct costs (RMB 5506.9 vs 5258.0, p = 0.037). With 8.4 million insulin injectors in China, the estimated excess annual direct cost of LH is RMB 2.2 billion ($360 million). Average pain scores (0–10) were higher if LH was present (2.7 vs 2.0, p = 0.021), if ≥3 nodes were present (3.8 vs 2.3, p < 0.001), and if PNs were not reimbursed (2.8 vs 1.7, p < 0.001). Patient satisfaction decreased as presence, number, and size of LH nodes increased (all p < 0.05).
Specifically the study investigated insulin exposure and pharmacodynamics of insulin lispro injected into abdominal areas with LH or normal adipose tissue. Thirteen T1DM with LH (confirmed by palpation and ultrasound) received single doses of 0.15 U/kg LIS approximately every 6 h, twice into a region with LHT and twice into normal tissue. Comparing LHT with NAT injection, LS-mean INS concentrations were comparable during the first 30 min (AUCINS0–0.5 h 8.8 vs 9.4 h mU/L), but significantly lower thereafter (AUCINS0–1 h 29.3 vs 41.5 h mU/L, AUCINS.0–4 h 97 vs 154 h mU/L, all p < 0.02). Maximum INS exposure was reduced by 34% (CINSmax 49.7 vs 75.4 mU/L, p < 0.002). The PD effect in the first 4 h was 27% lower with LHT injection (AUCGIR0–4 h: 529 vs 720 mg/kg, p < 0.05), whereas maximum GIR was comparable (GIRmax 5.5 vs 6.0 mg/kg/min, p = 0.378). Intrasubject variability was substantially higher after dosing into LHT (coefficients of variation 52% vs 11% [AUCINS.0–4 h] and 57% vs 23%, [AUCGIR0–4 h], all p < 0.002).
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/A2F7F0605653FD50.
References
- 1.Kalra S, Mithal A, Sahay R, et al. Indian Injection technique study: population characteristics and injection practices. Diabetes Ther. 2017. doi:10.1007/s13300-017-0243-x. [DOI] [PMC free article] [PubMed]
- 2.Frid AH, et al. Worldwide injection technique questionnaire study: population parameters and injection practices. Mayo Clinic Proc. 91(9):1212–1223. http://www.mayoclinicproceedings.org/article/S0025-6196(16)30322-6/fulltext. [DOI] [PubMed]
- 3.Frid AH, et al. Worldwide injection technique questionnaire study: injecting complications and role of the professional. Mayo Clinic Proc. 91(9):1224–1230. http://www.mayoclinicproceedings.org/article/S0025-6196(16)30326-3/fulltext. [DOI] [PubMed]
- 4.Thow JC, Johnson AB, Marsden S, Taylor R, Home PH. Morphology of palpably abnormal injection sites and effects on absorption of isophane (NPH) insulin. Diabet Med. 1990;7:795–799. doi: 10.1111/j.1464-5491.1990.tb01494.x. [DOI] [PubMed] [Google Scholar]
- 5.Richardson T, Kerr D. Skin-related complications of insulin therapy: epidemiology and emerging management strategies. Am J Clin Dermatol. 2003;4:661–667. doi: 10.2165/00128071-200304100-00001. [DOI] [PubMed] [Google Scholar]
- 6.Seyoum B, Abdulkadir J. Systematic inspection of insulin injection sites for local complications related to incorrect injection technique. Trop Doct. 1996;26:159–161. doi: 10.1177/004947559602600406. [DOI] [PubMed] [Google Scholar]
- 7.Photographs courtesy of Lourdes Saez-de Ibarra and Ruth Gaspar, Diabetes Nurses and Specialist Educators from La Paz Hospital, Madrid, Spain.
- 8.Hajheydari Z, Kashi Z, Akha O, Akbarzadeh S. Frequency of lipodystrophy induced by recombinant human insulin. Eur Rev Med Pharmacol Sci. 2011;15(10):1196–1201. [PubMed] [Google Scholar]
- 9.Raile K, Noelle V, Landgraf R, Schwarz HP. Insulin antibodies are associated with lipoatrophy but also with lipohypertrophy in children and adolescents with type 1 diabetes. Exp Clin Endocrinol Diabetes. 2001;109(8):393–396. doi: 10.1055/s-2001-18991. [DOI] [PubMed] [Google Scholar]
- 10.Partanen TM, Rissanen A. Insulin injection practices. Pract Diabetes Int. 2000;17:252–254. doi: 10.1002/pdi.91. [DOI] [Google Scholar]
- 11.Kordonouri O, Lauterborn R, Deiss D. Lipohypertrophy in young patients with type 1 diabetes. Diabetes Care. 2002;25(3):634. doi: 10.2337/diacare.25.3.634. [DOI] [PubMed] [Google Scholar]
- 12.Blanco M, Hernández MT, Strauss KW, Amaya M. Prevalence and risk factors of lipohypertrophy in insulin-injecting patients with diabetes. Diabetes Metab. 2013;39(5):445–453. doi: 10.1016/j.diabet.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 13.Grassi G, Scuntero P, Trepiccioni R, et al. Optimizing insulin injection technique and its effect on blood glucose control. J Clin Transl Endocrinol. 2014;1:145–150 [DOI] [PMC free article] [PubMed]
- 14.Ji L, Li Q, Wei G. Lipohypertrophy—prevalence, risk factors and clinical characteristics of insulin-requiring patients in China. Abstract, EASD Vienna 2014, Tracking Number: A-14-747.
- 15.Conwell LS, Pope E, Artiles AM, Mohanta A, Daneman A, Daneman D. Dermatological complications of continuous subcutaneous insulin infusion in children and adolescents. J Pediatr. 2008;152:622–628. doi: 10.1016/j.jpeds.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 16.Pickup J, Yemane N, Brackenridge A, Pender S. Nonmetabolic complications of continuous subcutaneous insulin infusion: a patient survey. Diabetes Technol Ther. 2014;16:145–149. doi: 10.1089/dia.2013.0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vardar B, Kizilci S. Incidence of lipohypertrophy in diabetic patients and a study of influencing factors. Diabetes Res Clin Pract. 2007;77:231–236. doi: 10.1016/j.diabres.2006.12.023. [DOI] [PubMed] [Google Scholar]
- 18.Saez-de Ibarra L, Gallego F. Factors related to lipohypertrophy in insulin-treated diabetic patients: role of educational intervention. Pract Diab Int. 1998;15:9–11. doi: 10.1002/pdi.1960150108. [DOI] [Google Scholar]
- 19.Fujikura J, Fujimoto M, Yasue S, et al. Insulin-induced lipohypertrophy: report of a case with histopathology. Endocr J. 2005;52(5):623–628. doi: 10.1507/endocrj.52.623. [DOI] [PubMed] [Google Scholar]
- 20.Young RJ, Hannan WJ, Frier BM, Steel JM, Duncan LJ. Diabetic lipohypertrophy delays insulin absorption. Diabetes Care. 1984;7:479–480. doi: 10.2337/diacare.7.5.479. [DOI] [PubMed] [Google Scholar]
- 21.Chowdhury TA, Escudier V. Poor glycaemic control caused by insulin induced lipohypertrophy. Brit Med J. 2003;327:383–384. doi: 10.1136/bmj.327.7411.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johansson UB. Impaired absorption of insulin aspart from lipohypertrophic injection sites. Diabetes Care. 2005;28:2025–2027. doi: 10.2337/diacare.28.8.2025. [DOI] [PubMed] [Google Scholar]
- 23.Frid A, Linden B. Computed tomography of injection sites in patients with diabetes mellitus. Injection and absorption of insulin, Thesis. Stockholm; 1992.
- 24.Franzen I, Ludvigsson J. Specific instructions gave reduction of lipomas and improved metabolic control in diabetic children. Diabetologia. 1997;40(Suppl 1):A615. [Google Scholar]
- 25.Li Q, Ji L, Sun Z, et al. Lipohypertrophy (LH) prevalence varies widely between chinese cities—need for consistent LH diagnostic methods. Abstract submitted to 2015 ADA (2015).
- 26.Famulla S, Hövelmann U, Fischer A, et al. Lipohypertrophy (LHT) leads to blunted, more variable insulin absorption and action in patients with type 1 diabetes (T1DM). Abstract, 2015 ADA in Boston, USA (2015).
- 27.Hovelmann U, Famulla S, Hermanski L, et al. Insulin injection into regions with lipohypertrophy (LHT) worsens postprandial (PP) blood glucose (BG) versus injections into normal adipose tissue (NAT). Abstract, 2015 ADA in Boston, USA (2015).
- 28.Ahern J, Mazur ML. Site rotation. Diabetes Forecast. 2001;54:66–68. [Google Scholar]
- 29.Bantle JP, Weber MS, Rao SM, Chattopadhyay MK, Robertson RP. Rotation of the anatomic regions used for insulin injections day-to-day variability of plasma glucose in type 1 diabetic subjects. JAMA. 1990;263:1802–1806. doi: 10.1001/jama.1990.03440130090031. [DOI] [PubMed] [Google Scholar]
- 30.Davis ED, Chesnaky P. Site rotation…taking insulin. Diabetes Forecast. 1992;45:54–56. [Google Scholar]
- 31.Del Olmo MI, Campos V, Abellán P, Merino-Torres JF, Piñón F. A case of lipoatrophy with insulin detemir. Diabetes Res Clin Pract. 2008;80(1):e20–e21. doi: 10.1016/j.diabres.2007.12.026. [DOI] [PubMed] [Google Scholar]
- 32.Arranz A, Andia V, López-Guzmán A. A case of lipoatrophy with lispro insulin without insulin pump therapy. Diabetes Care. 2004;27(2):625–626. doi: 10.2337/diacare.27.2.625. [DOI] [PubMed] [Google Scholar]
- 33.Breznik V, Kokol R, Luzar B, Miljković J. Insulin-induced localized lipoatrophy. Acta Dermatovenerol Alp Pannonica Adriat. 2013;22(4):83–85. [PubMed] [Google Scholar]
- 34.Swelheim HT, Westerlaken C, van Pinxteren-Nagler E, Bocca G. Lipoatrophy in a girl with type 1 diabetes: beneficial effects of treatment with a glucocorticoid added to an insulin analog. Diabetes Care. 2012;35(3):e22. doi: 10.2337/dc11-2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Babiker A, Datta V. Lipoatrophy with insulin analogues in type I diabetes. Arch Dis Child. 2011;96(1):101–102. doi: 10.1136/adc.2010.183731. [DOI] [PubMed] [Google Scholar]
- 36.Holstein A, Stege H, Kovacs P. Lipoatrophy associated with the use of insulin analogues: a new case associated with the use of insulin glargine and review of the literature. Expert Opin Drug Saf. 2010;9(2):225–231. doi: 10.1517/14740330903496402. [DOI] [PubMed] [Google Scholar]
- 37.Brady KA, Avner JR, Khine H. Perception and attitude of providers towards pain and anxiety associated with pediatric vaccine injection. Clin Pediatr. 2011;50:140–143. doi: 10.1177/0009922810384721. [DOI] [PubMed] [Google Scholar]
- 38.Diamond S, Matok I. Pharmacists’ anticipated pain compared to experienced pain associated with insulin pen injection and fingertip. Can J Diabetes. 2011;35:282–286. doi: 10.1016/S1499-2671(11)53012-6. [DOI] [Google Scholar]
- 39.Heise T, Nosek L, Dellweg S, et al. Impact of injection speed and volume on perceived pain during subcutaneous injections into the abdomen and thigh: a single-centre, randomized controlled trial. Diabetes Obes Metab. 2014;16(10):971–976. doi: 10.1111/dom.12304. [DOI] [PubMed] [Google Scholar]
- 40.Anderson G, Meyer D, Herrman CE, et al. Tolerability and safety of novel half milliliter formulation of glatiramer acetate for subcutaneous injection: an open-label, multicenter, randomized comparative study. J Neurol. 2010;257:1917–1923. doi: 10.1007/s00415-010-5779-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jorgensen JT, Romsing J, Rasmussen M, Moller-Sonnergaard J, Vang L, Musaeus L. Pain assessment of subcutaneous injections. Ann Pharmacother. 1996;30:729–732. doi: 10.1177/106002809603000703. [DOI] [PubMed] [Google Scholar]
- 42.Hofman PL, Derraik JG, Pinto TE, et al. Defining the ideal injection techniques when using 5-mm needles in children and adults. Diab Care. 2010;33(9):1940–1944. doi: 10.2337/dc10-0871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tandon N, Kalra S, Balhara YS, et al. Forum for injection technique (FIT), India: the Indian recommendations 2.0, for best practice in insulin injection technique, 2015. Indian J Endocr Metab. 2015;19(3):317–331. doi: 10.4103/2230-8210.152762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mujika-Zabaleta A, et al. Relationship between diabetes knowledge, glycaemic control and quality of life: pilot study. Diabetes Prim Care. 2010;12:376–381. [Google Scholar]
- 45.Golay A, Lagger G, Chambouleyron M, Carrard I, Lasserre-Moutet A. Therapeutic education of diabetic patients. Diabetes Metab Res Rev. 2008;24(3):192–196. doi: 10.1002/dmrr.798. [DOI] [PubMed] [Google Scholar]
- 46.Strauss K, De Gols H, Hannet I, Partanen TM, Frid A. A pan-European epidemiologic study of insulin injection technique in patients with diabetes. Pract Diab Int. 2002;19:71–76. doi: 10.1002/pdi.314. [DOI] [Google Scholar]
- 47.Martinez L, Consoli SM, Monnier L, et al. Studying the Hurdles of Insulin Prescription (SHIP): development, scoring and initial validation of a new self-administered questionnaire. Health Qual Life Out. 2007;5:53. doi: 10.1186/1477-7525-5-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cefalu WT, Mathieu C, Davidson J. Patients’ perceptions of subcutaneous insulin in the OPTIMIZE study: a multicenter follow-up study. Diab Tech Ther. 2008;10:25–38. doi: 10.1089/dia.2008.0249. [DOI] [PubMed] [Google Scholar]
- 49.DiMatteo RM, DiNicola DD, editors. Achieving patient compliance. The psychology of medical practitioner’s role. Oxford: Pergamon; 1982. pp. 233–256. [Google Scholar]
- 50.Joy SV. Clinical pearls and strategies to optimize patient outcomes. Diabetes Educator. 2008;34:54S–59S. doi: 10.1177/0145721708319233. [DOI] [PubMed] [Google Scholar]
- 51.Davidson M. No need for the needle (at first) Diabetes Care. 2008;31:2070–2071. doi: 10.2337/dc08-1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reach G. Patient non-adherence and healthcare-provider inertia are clinical myopia. Diab Metab. 2008;34:382–385. doi: 10.1016/j.diabet.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 53.Genev NM, Flack JR, Hoskins PL, et al. Diabetes education: whose priorities are met? Diab Med. 1992;9:475–479. doi: 10.1111/j.1464-5491.1992.tb01820.x. [DOI] [PubMed] [Google Scholar]
- 54.Klonoff DC. The pen is mightier than the needle (and syringe) Diab Tech Ther. 2001;3:631–633. doi: 10.1089/15209150152811261. [DOI] [PubMed] [Google Scholar]
- 55.Heinemann L, Hompesch M, Kapitza C, Harvey NG, Ginsberg BH, Pettis RJ. Intra-dermal insulin lispro application with a new microneedle delivery system led to a substantially more rapid insulin absorption than subcutaneous injection. Diabetologia. 2006;49:755. doi: 10.1007/s00125-005-0125-z. [DOI] [PubMed] [Google Scholar]
- 56.ITQ survey data. Tableau Public Adam Young’s Profile website. http://tabsoft.co/23V6ofi. Accessed 8 June 2016.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All results from the ITQ survey data are available in an interactive form on Tableau Public Adam Young’s Profile website [56].