Abstract
Introduction
Sodium glucose co-transporter 2 (SGLT2) inhibitors increase urinary glucose excretion (UGE) by reducing the renal threshold for glucose excretion, which results in decreased serum glucose concentrations in patients with type 2 diabetes mellitus (T2D). However, no study to date has determined whether larger increases in UGE after SGLT2 inhibitor treatment correspond to larger reductions in glycated hemoglobin (HbA1C).
Methods
We enrolled participants who were newly prescribed an SGLT2 inhibitor (dapagliflozin 10 mg or ipragliflozin 50 mg, once daily) as an add-on therapy. Patients were tested for HbA1C and first morning spot urinary-creatinine and -glucose concentrations immediately prior to administration of the SGLT2 inhibitor and at a 12-week follow-up appointment. We investigated the relationship between increases in morning spot UGE and decreases in HbA1C.
Results
A total of 101 participants with T2D were enrolled. The median age and diabetes duration were 61.0 and 12.8 years, respectively, and the median HbA1C was 8.10%. SGLT2 inhibitors significantly lowered the HbA1C level, with a median change from baseline to week 12 of −0.60% (p < 0.001). Robust increases from baseline were seen for the morning spot urinary glucose-to-creatinine ratio (UGCR), with a median change at week 12 of 47.3 mg/mg. In the correlation analysis, the ∆HbA1C level showed a significant positive correlation with ∆morning spot UGCR (r = 0.395, p < 0.001). In other words, a greater reduction in HbA1C was correlated with a smaller increase in UGE. After adjusting for confounding variables, ∆HbA1C was significantly associated with ∆morning spot UGCR.
Conclusions
Although SGLT2 inhibitor treatment leads to a reduced HbA1C level by augmenting UGE, larger increases in UGE do not correlate to larger reductions in HbA1C. This suggests that the increase in UGE might not be an indicator of the degree of reductions in blood glucose.
Electronic supplementary material
The online version of this article (doi:10.1007/s13300-017-0248-5) contains supplementary material, which is available to authorized users.
Keywords: SGLT2 inhibitors, Sodium glucose co-transporter 2 inhibitors, Type 2 diabetes mellitus, Urinary glucose excretion, Urinary glucose-to-creatinine ratio
Introduction
The renal threshold for glucose excretion (RTG) is the serum glucose concentration at which tubular reabsorption of glucose begins to saturate and glucose first appears in the urine [1]. The cutoff values of RTG are dependent on sodium glucose co-transporter 2 (SGLT2), which is the protein responsible for the majority of filtered glucose reabsorption from the proximal renal tubules. Patients with type 2 diabetes mellitus (T2D) have increased expression of SGLT2 and elevated renal glucose absorption, which may contribute to persistent hyperglycemia [2]. The advent of SGLT2 inhibitors, which prevent glucose reabsorption from the proximal renal tubules, thereby promoting urinary glucose excretion (UGE) and decreasing the serum glucose level, adds to the wide range of pharmacotherapeutic options for treating patients with diabetes [3].
In previous studies [4, 5], it was shown that an SGLT2 inhibitor reduces RTG, increases UGE, and decreases the serum glucose concentration in patients with T2D. Increases in UGE, along with improvements in blood glucose indices, have been deemed pharmacological effects of SGLT2 inhibitors. Furthermore, in multiple-dose clinical studies of both healthy subjects and patients with diabetes, SGLT2 inhibitors dose-dependently decreased RTG, leading to a dose-dependent increase in UGE [4–9]. However, there are no studies to date as to whether larger increases in UGE after SGLT2 inhibitor treatment translate to larger reductions in glycated hemoglobin (HbA1c).
In this study, we aimed to investigate the relationship between increases in morning spot UGE, which showed good agreement with overnight 8-h UGE in a previous study [10], and decreases in HbA1C in patients with T2D after SGLT2 inhibitor treatment.
Methods
Study Participants
This was a retrospective, longitudinal study of the patients with T2D who presented to Severance Hospital Diabetes Center between January 2015 and October 2016 and who were newly prescribed an SGLT2 inhibitor (dapagliflozin 10 mg or ipragliflozin 50 mg, once daily) as an add-on therapy to lower inadequately controlled serum glucose. We enrolled participants who had been tested for blood glucose parameters, including serum fasting glucose and HbA1C, and first morning spot urinary creatinine and glucose concentrations immediately prior to the administration of the SGLT2 inhibitor and at a 12-week follow-up visit. We used the following inclusion criteria: (1) patients who regularly visit the outpatient clinic for blood glucose control and (2) patients who were newly prescribed an SGLT2 inhibitor (dapagliflozin 10 mg or ipragliflozin 50 mg, once daily) as an add-on therapy. Participants who met the following criteria were excluded: (1) <20 years of age; (2) type 1 diabetes; (3) pregnant women; (4) active infectious disease including urinary tract infection; (5) estimated glomerular filtration rate (eGFR) <60 ml/min/1.73 m2.
Age, sex, weight, height, waist circumference, blood pressure, duration of diabetes, and pre-existing use of oral hypoglycemic agents or insulin were recorded. Body mass index (BMI) was calculated as weight divided by height squared (kg/m2).
Compliance with Ethics Guidelines
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The Institutional Review Board at Severance Hospital approved this study protocol (4-2016-0998). Written informed consent for this study was not required by the Institutional Review Board because the database was only accessed for analysis purposes and personal information was not used.
Measurements of Blood and Urinary Gluco-Metabolic Parameters
To exclude external factors such as food intake on glucose homeostasis, participants were advised to keep fasting after dinner, but could drink water. Following an overnight fast, blood tests for glucose, HbA1C, lipid profiles, and creatinine were performed, and a routine fasting morning urine sample was collected, both immediately prior to the administration of the SGLT2 inhibitor and at a 12-week follow-up visit. The morning urine and blood were sampled on the same day. In our previous study [10], we determined that the overnight 8-h UGE, overnight 8-h urinary glucose-to-creatinine ratio (UGCR), and morning spot UGCR showed good agreement with each other, suggesting that morning spot UGCR can be used as a simple index for estimating overnight amounts of UGE in patients with T2D. HbA1C was measured by an immunoassay using an Integra 800 CTS (Roche, Hercules, CA, USA). Serum glucose and creatinine were measured using the Hitachi 7600 analyzer (Hitachi Ltd., Tokyo, Japan). For serum creatinine, a compensated kinetic Jaffé method (Clinimate CRE, Sekisui Medical Co., Ltd., Tokyo, Japan) was used in which the creatinine concentration was standardized to isotope dilution-mass spectrometry. The eGFR was derived using the guidelines from the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) [11]. The urinary glucose levels were measured using the hexokinase method with an AU680 chemistry analyzer (Beckman Coulter, Brea, CA, USA). The urinary creatinine levels were measured using the AU680 analyzer (Beckman Coulter, Inc.) with the kinetic Jaffé method. Urinary glucose levels were expressed as UGCR to minimize the influence of variations of kidney function using the following formula: morning spot UGCR (mg/mg) = [first-voided morning spot urine glucose (mg/dl)/first-voided morning spot urine creatinine (mg/dl)].
Statistical Analysis
All statistical analyses were performed using SPSS version 23.0 for Windows (IBM Corp., Armonk, NY, USA). A normality test was performed for all continuous variables. The data are presented as mean ± standard deviation (SD) for normally distributed continuous variables and as median (interquartile range) for non-normally distributed continuous variables. Categorical data are expressed as numbers and percentages. The characteristics of the study participants were analyzed according to the change in HbA1C after 12 weeks of SGLT2 inhibitor treatment using the two-sample Student’s t test or the Mann-Whitney U test for continuous variables and Pearson’s χ 2 test for categorical variables. The laboratory parameters at baseline and at week 12 of SGLT2 inhibitor treatment were compared using a paired t test or the Wilcoxon signed-rank test. The changes of the major parameters from baseline to 12 weeks were defined as follows: ∆HbA1C (%) = [HbA1C at week 12 (%)—HbA1C at baseline (%)]; ∆morning spot UGCR (mg/mg) = [morning spot UGCR at week 12 (mg/mg)—morning spot UGCR at baseline (mg/mg)]. Correlations between ∆morning spot UGCR and either baseline HbA1C or ∆HbA1C were analyzed using Spearman’s correlation coefficient. Multiple linear regression analyses were performed on logarithm-transformed values of ∆morning spot UGCR to model the relationship between the change in urinary glycemic indices and metabolic parameters including ∆HbA1C. All P values <0.05 were considered statistically significant.
Results
Characteristics of the Study Participants
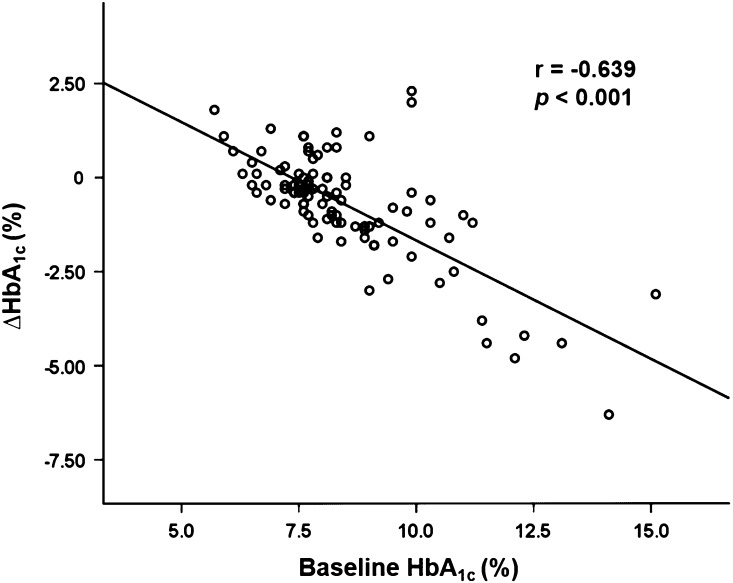
A total of 101 participants were recruited in this study. The demographics and laboratory characteristics of the participants are shown in Table 1. The median age and duration of diabetes among the study participants were 61.0 and 12.8 years, respectively. The participants were divided into two groups according to whether HbA1C decreased (N = 77) or increased (N = 24) after 12 weeks of treatment with SGLT2 inhibitors. The median value of ∆HbA1C [HbA1C at week 12 (%)—HbA1C at baseline (%)] in the two groups was −0.90% and 0.75%, respectively. There were no significant differences in age, sex, BMI, waist circumference, blood pressures, or duration of diabetes between the two groups. The proportions of participants taking oral hypoglycemic agents (except thiazolidinediones) or insulin were similar between the two groups. With respect to laboratory indices, baseline HbA1C and serum fasting glucose were significantly higher in the group with decreased HbA1C than in the group with increased HbA1C. In addition, the baseline HbA1C level showed a significant negative correlation with ∆HbA1C level (Fig. 1), which suggests that patients with higher baseline HbA1C have greater reductions of HbA1C after taking an SGLT2 inhibitor. The eGFR values calculated using the CKD-EPI equation did not significantly differ between the two groups. Regarding urinary glycemic indices, neither the morning spot urine glucose concentration nor morning spot UGCR differed significantly between the two groups. In the group with increased HbA1C, only one participant showed a slightly lower morning spot UGCR value after SGLT2 inhibitor treatment compared to baseline (∆morning spot UGCR = −0.70 mg/mg).
Table 1.
Baseline characteristics of the study participants according to change in HbA1C after 12 weeks of treatment with SGLT2 inhibitors (N = 101)
| Baseline characteristics | Total (N = 101) |
Decreased HbA1c (N = 77) |
Increased HbA1c (N = 24) |
P
value |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) | 61.0 (51.0–68.0) | 58.0 (50.5–67.5) | 64.0 (51.3–73.0) | 0.133 |
| Male Sex [n (%)] | 50 (49.5) | 37 (48.1) | 13 (54.2) | 0.601 |
| BMI (kg/m2) | 26.4 ± 3.66 | 26.6 ± 3.76 | 25.9 ± 3.31 | 0.433 |
| Waist circumference (cm) | 92.0 ± 9.47 | 92.2 ± 9.41 | 91.5 ± 9.89 | 0.789 |
| Systolic blood pressure (mmHg) | 125.5 (116.0–133.0) | 126.0 (115.3–132.8) | 124.5 (116.0–135.8) | 0.910 |
| Diastolic blood pressure (mmHg) | 74.9 ± 9.75 | 74.9 ± 9.73 | 74.8 ± 9.99 | 0.992 |
| Duration of diabetes (years) | 12.8 (8.88–17.8) | 11.8 (8.88-17.4) | 16.8 (9.06–18.5) | 0.322 |
| Laboratory indices | ||||
| HbA1C (%) | 8.10 (7.55–9.05) | 8.30 (7.60–9.45) | 7.60 (6.63–8.05) | <0.001 |
| Fasting glucose (mg/dl) | 150.0 (126.5–180.0) | 155.0 (130.5–191.0) | 136.5 (111.0–155.8) | 0.013 |
| Total cholesterol (mg/dl) | 150.0 (126.0–175.0) | 152.0 (132.0–179.0) | 131.0 (113.5–156.8) | 0.035 |
| Triglyceride (mg/dl) | 123.0 (83.3–167.0) | 127.0 (89.0–174.0) | 90.0 (61.0–157.0) | 0.043 |
| HDL cholesterol (mg/dl) | 43.0 (37.0–52.0) | 43.0 (37.0–52.0) | 44.5 (36.5–54.8) | 0.415 |
| LDL cholesterol (mg/dl) | 79.4 ± 38.6 | 81.3 ± 41.0 | 73.0 ± 28.9 | 0.373 |
| BUN (mg/dl) | 15.3 ± 3.95 | 15.2 ± 3.96 | 15.6 ± 3.98 | 0.635 |
| Creatinine (mg/dl) | 0.73 (0.59–0.87) | 0.74 (0.59–0.88) | 0.67 (0.60–0.85) | 0.641 |
| eGFR CKD-EPI (ml/min/1.73 m2) | 97.1 ± 18.3 | 96.8 ± 17.6 | 98.1 ± 20.8 | 0.766 |
| Urinary glycemic indices | ||||
| Morning spot urine glucose (mg/dl) | 22.0 (8.50–149.0) | 24.0 (8.50–165.0) | 16.0 (9.00–123.3) | 0.655 |
| Morning spot UGCR (mg/mg) | 0.19 (0.08–1.36) | 0.19 (0.08–1.51) | 0.20 (0.08–1.37) | 0.955 |
| Antidiabetic drugs | ||||
| Metformin [n (%)] | 86 (85.1) | 65 (84.4) | 21 (87.5) | >0.999 |
| Sulfonylurea [n (%)] | 52 (51.5) | 38 (49.4) | 14 (58.3) | 0.490 |
| DPP-IV inhibitor [n (%)] | 48 (47.5) | 36 (46.8) | 12 (50.0) | 0.818 |
| Thiazolidinediones [n (%)] | 9 (8.90) | 4 (5.20) | 5 (20.8) | 0.033 |
| α-Glucosidase inhibitors [n (%)] | 11 (10.9) | 7 (9.10) | 4 (16.7) | 0.286 |
| Insulin [n (%)] | 37 (36.6) | 30 (39.0) | 7 (29.2) | 0.471 |
Continuous variables are described as mean ± SD for parametric variables and median (interquartile range) for nonparametric variables
Bold denotes statistical significance at P < 0.05
HbA 1C glycated hemoglobin, SGLT2 sodium-glucose co-transporter 2, BMI body mass index, HDL high-density lipoprotein, LDL low-density lipoprotein, BUN blood urea nitrogen, eGFR estimated glomerular filtration rate, CKD-EPI Chronic Kidney Disease Epidemiology Collaboration, UGCR urinary glucose-to-creatinine ratio, DPP-IV dipeptidyl peptidase-IV, SD standard deviation
Fig. 1.
Correlation between baseline HbA1C and ∆HbA1C (N = 101). ∆HbA1C (%) = [HbA1C at week 12 (%)—HbA1C at baseline (%)]. HbA 1C glycated hemoglobin
Changes in Laboratory Parameters from Baseline to Week 12
Changes in laboratory parameters from baseline to week 12 of SGLT2 inhibitor treatment are shown in Table 2. SGLT2 inhibitors (i.e., dapagliflozin or ipragliflozin) used as an add-on therapy significantly lowered the HbA1C level, with a median change from baseline to week 12 of −0.60% (p < 0.001). Similar to HbA1C, serum fasting glucose was also significantly decreased at week 12 compared to the baseline values, with a median change of −25.0 mg/dl (p < 0.001). There was no significant change in the eGFR at week 12 compared to baseline. Robust increases from baseline were seen for both the morning spot urine glucose concentration and morning spot UGCR, with median changes at week 12 of 3837 mg/dl and 47.3 mg/mg, respectively.
Table 2.
Changes in laboratory parameters from baseline to week 12 (N = 101)
| Baseline | Week 12 | P | |
|---|---|---|---|
| HbA1C (%) | 8.10 (7.55–9.05) | 7.50 (7.05–8.25) | <0.001 |
| Fasting glucose (mg/dl) | 150.0 (126.5–180.0) | 125.0 (108.5–148.0) | <0.001 |
| eGFR CKD-EPI (ml/min/1.73 m2) | 96.4 ± 17.3 | 95.9 ± 18.3 | 0.415 |
| Morning spot urine glucose (mg/dl) | 22.0 (8.50–149.0) | 3859.0 (2581.0–5158.5) | <0.001 |
| Morning spot UGCR (mg/mg) | 0.19 (0.08–1.36) | 47.5 (30.6–66.3) | <0.001 |
Continuous variables are described as mean ± SD for parametric variables and median (interquartile range) for nonparametric variables
Bold denotes statistical significance at P < 0.05
HbA 1C glycated hemoglobin, eGFR estimated glomerular filtration rate, CKD-EPI Chronic Kidney Disease Epidemiology Collaboration, UGCR urinary glucose-to-creatinine ratio
Correlations between Change in Urinary Glycemic Indices and Change in HbA1C Level
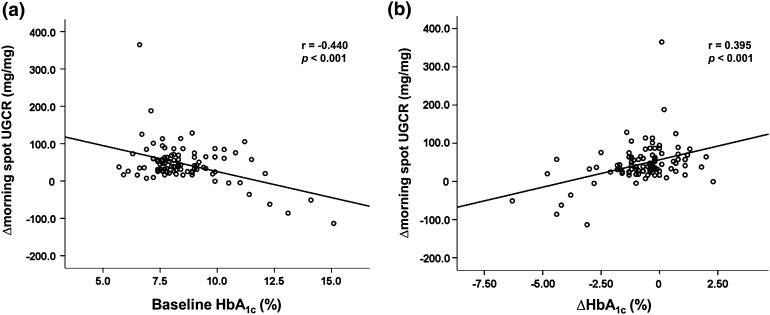
In the correlation analyses, the baseline HbA1C level (Fig. 2a) showed a significant negative correlation with ∆morning spot UGCR. Regarding the ∆HbA1C level (Fig. 2b), it showed a significant positive correlation with ∆morning spot UGCR. In other words, larger reductions in HbA1C were correlated with smaller increases in UGE.
Fig. 2.
Correlations between ∆morning spot UGCR and baseline HbA1C (a) or ∆HbA1C (b) (N = 101). ∆morning spot UGCR (mg/mg) = [morning spot UGCR at week 12 (mg/mg)—morning spot UGCR at baseline (mg/mg)]; ∆HbA1C (%) = [HbA1C at week 12 (%)—HbA1C at baseline (%)]. UGCR urinary glucose-to-creatinine ratio, HbA 1C glycated hemoglobin
Multiple Linear Regression Analysis for Factors Affecting Changes in Urinary Glycemic Indices
To determine which factors predicted changes in urinary glycemic indices after SGLT2 inhibitor treatment, we performed multiple linear regression analyses (Table 3) using log-transformed ∆morning spot UGCR as the dependent variable. In the univariate model, age and eGFR were significantly associated with ∆morning spot UGCR. After adjusting for age, sex, eGFR, baseline morning spot UGCR, and ∆HbA1C, both eGFR and ∆HbA1C were significantly associated with ∆morning spot UGCR.
Table 3.
Multiple linear regression models for change in morning spot UGCR (N = 101)
| Variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| STD β | P | STD β | P | |
| ∆HbA1C (%) | 0.177 | 0.098 | 0.269 | 0.007 |
| Age (years) | −0.308 | 0.003 | 0.000 | 0.999 |
| Male sex | −0.069 | 0.518 | −0.045 | 0.633 |
| eGFR CKD-EPI (ml/min/1.73 m2) | 0.464 | <0.001 | 0.501 | 0.001 |
| Baseline morning spot UGCR (mg/mg) | 0.024 | 0.820 | −0.030 | 0.747 |
Logarithm-transformed values of ∆morning spot UGCR were used as the dependent variable
∆HbA1C (%) = [HbA1C at week 12 (%)—HbA1C at baseline (%)]; ∆morning spot UGCR (mg/mg) = [morning spot UGCR at week 12 (mg/mg)—morning spot UGCR at baseline (mg/mg)]
Bold denotes statistical significance at P < 0.05
UGCR urinary glucose-to-creatinine ratio, HbA 1c glycated hemoglobin, eGFR estimated glomerular filtration rate, CKD-EPI Chronic Kidney Disease Epidemiology Collaboration, STD standardized
Discussion
Despite numerous reports on the clinical efficacy and safety of SGLT2 inhibitors, a debate remains regarding the direct causality between the pharmacologic efficacy of SGLT2 inhibitors in UGE and their clinical effectiveness in lowering the glucose level as assessed by several glycemic indices since few observational studies based on routine care have been performed. We hypothesized that the increased glucose excretion into the urine might decrease glycemic parameters, such as HbA1C or glycated albumin, in the blood. Based on these hypotheses, this study aimed to investigate the relationship between increases in morning spot urine glucose parameters and decreases in HbA1C in patients with T2D after taking SGLT2 inhibitors.
In this retrospective, observational study of SGLT2 inhibitors in Korean patients with poorly controlled T2D, we demonstrated three main findings. First, based on the increased urinary glucose levels after starting SGLT2 inhibitor therapy, the pharmacological mode of action of SGLT2 inhibitors on renal tubules is intact and active. Second, based on the HbA1C results, we determined that, despite the pharmacological activity of SGLT2 inhibitors, there are responders and non-responders among the subjects with T2D. Third, based on the increased morning spot urine glucose parameters and decreased HbA1C, the relation between morning spot urine glucose parameters and HbA1C is not as simple as hypothesized.
In previous studies [6, 8], SGLT2 inhibitors induced sustained glycosuria and improvements in blood glucose. In a study of the addition of dapagliflozin treatment for patients with T2D that was inadequately controlled on pioglitazone [8], the beneficial effects of dapagliflozin on hyperglycemia were maintained for almost 1 year and were consistent with a sustained pharmacodynamic activity toward glycosuria. Consistent with these previous reports, our study participants also showed a significant increase in morning spot UGCR, indicating that controlled glycosuria was induced after 12 weeks of SGLT2 inhibitor treatment. However, of the 101 participants, 8 had negative ∆morning spot UGCR values after SGLT2 inhibitor treatment. This suggests that the glycosuria can be divided into two categories. The first category is natural-overflow glycosuria, where UGE is caused by elevated blood glucose and glomerular-filtered glucose levels. The other category is chemically induced glycosuria for managing hyperglycemia using an SGLT2 inhibitor. In this study, those eight patients who had negative ∆morning spot UGCR values had very high baseline HbA1C levels of more than 9.90% with mean 12.2% (Table S1 for details). Therefore, we postulate that, in patients with very high HbA1C levels, the initial total glycosuria (natural overflow plus chemically induced glycosuria) might be more augmented by SGLT2 inhibitor treatment, resulting in decreased serum glucose levels. After the stable state of pharmacologic efficacy of SGLT2 inhibitor, the decreased levels of serum glucose in turn decreased the urinary glucose excretion. The net degree of chemically induced glycosuria caused by the SGLT2 inhibitors might be lower than the initial natural-overflow glycosuria.
In multiple-dose clinical studies of SGLT2 inhibitors in both healthy subjects and patients with diabetes, SGLT2 inhibitors provided dose-dependent reductions in RTG and increases in UGE [4–9]. A study comparing 50-, 100-, and 300-mg doses of canagliflozin showed that the mean 24-h RTG values decreased in a dose-dependent manner in T2D patients [9]. In healthy euglycemic subjects, canagliflozin dose-dependently decreased mean 24-h RTG and increased mean 24-h UGE [7]. However, the association between the dose of SGLT2 inhibitor and the degree of improvement in blood glucose is not consistent in previous studies. In a study of five dapagliflozin doses in T2D patients [6], changes in fasting serum glucose were dose-related; however, there was little evidence of a dose response for either postprandial glucose or HbA1C level. In a study of canagliflozin as an add-on to metformin treatment [5], reductions in HbA1C and fasting serum glucose showed dose-dependent patterns. However, no study to date has determined whether larger increases in UGE after SGLT2 inhibitor treatment correlate with larger reductions in HbA1C. We hypothesized that the increased UGE might cause decreased glycemic parameters, such as HbA1C or glycated albumin, in the blood. In the correlation and regression analyses, unlike our hypothesis, the ∆HbA1C level and ∆morning spot UGCR showed significant positive correlations. In other words, a larger reduction in HbA1C was correlated with a smaller increase in UGE. We postulate that the pharmacological effects of SGLT2 inhibitors result in increases in chemically induced UGE and improvements in blood glucose, and these improved blood glucose levels trigger less chemically induced UGE and markedly decrease natural-overflow glycosuria.
Our study has some potential limitations. Because the cutoff point for maximum glucose reabsorption in the renal tubules (maximal reabsorptive capacity, TmG) is not abrupt [12], assessing RTG was impossible in this study design. Therefore, we could not demonstrate a clear relationship between the plasma glucose concentration and urinary glucose amount. Also, we did not collect information about the time, type, or last meal consumed by the participants, which might have an influence on UGCR and glucagon levels. When serum glucose levels are decreasing during fasting state, glucagon is secreted from pancreatic α-cells and signals the liver or kidney to increase glucose output through gluconeogenesis [13]. Furthermore, in subjects with diabetes, it was reported that SGLT2 inhibitor increased endogenous glucose and raised levels of the glucagon hormone, which prompts the body to produce glucose [14]. The participants in our study were newly prescribed an SGLT2 inhibitor as an add-on therapy, and preexisting oral hypoglycemic agents or insulin was maintained throughout study period in each participant. Therefore, improvement in blood glucose was thought to be mainly caused by SGLT2 inhibitor treatment. However, further studies are needed to be investigated on the potential underlying pathophysiology on urinary glucose homeostasis and pharmacologic influences of SGLT2 inhibitors including glucagon levels and food effects on UGCR in accurate research settings for better management in T2D.
Conclusion
Although SGLT2 inhibitor treatment leads to reduced HbA1C levels by augmenting UGE (i.e., medication efficacy), larger increases in UGE after SGLT2 inhibitor treatment do not correlate to larger reductions in HbA1C (i.e., effectiveness). This suggests that the relation between morning spot urine glucose parameters and HbA1C is not as simple as hypothesized and increases in UGE might not be an indicator of the degree of reductions in blood glucose.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Acknowledgements
No funding or sponsorship was received for this study or publication of this article. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published.
Disclosures
So Ra Kim, Yong-ho Lee, Eun Seok Kang, Bong-Soo Cha, and Byung-Wan Lee have nothing to disclose.
Compliance with Ethics Guidelines
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The Institutional Review Board at Severance Hospital approved this study protocol (4-2016-0998). Written informed consent for this study was not required by the Institutional Review Board because the database was only accessed for analysis purposes and personal information was not used.
Data Availability
All data generated or analyzed during this study are included in this published article.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced Content
To view enhanced content for this article go to http://www.medengine.com/Redeem/A6F7F0607498CA43.
Contributor Information
Yong-ho Lee, Email: yholee@yuhs.ac.
Byung-Wan Lee, Email: bwanlee@yuhs.ac.
References
- 1.Aires I, Fila M, Polidori D, Santos AR, Costa AB, Calado J. Determination of the renal threshold for glucose excretion in familial renal glucosuria. Nephron. 2015;129:300–304. doi: 10.1159/000381677. [DOI] [PubMed] [Google Scholar]
- 2.Bakris GL, Fonseca VA, Sharma K, Wright EM. Renal sodium-glucose transport: role in diabetes mellitus and potential clinical implications. Kidney Int. 2009;75:1272–1277. doi: 10.1038/ki.2009.87. [DOI] [PubMed] [Google Scholar]
- 3.Jung CH, Jang JE, Park JY. A novel therapeutic agent for type 2 diabetes mellitus: SGLT2 inhibitor. Diabetes Metab J. 2014;38:261–273. doi: 10.4093/dmj.2014.38.4.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Devineni D, Morrow L, Hompesch M, et al. Canagliflozin improves glycaemic control over 28 days in subjects with type 2 diabetes not optimally controlled on insulin. Diabetes Obes Metab. 2012;14:539–545. doi: 10.1111/j.1463-1326.2012.01558.x. [DOI] [PubMed] [Google Scholar]
- 5.Rosenstock J, Aggarwal N, Polidori D, et al. Dose-ranging effects of canagliflozin, a sodium-glucose cotransporter 2 inhibitor, as add-on to metformin in subjects with type 2 diabetes. Diabetes Care. 2012;35:1232–1238. doi: 10.2337/dc11-1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.List JF, Woo V, Morales E, Tang W, Fiedorek FT. Sodium-glucose cotransport inhibition with dapagliflozin in type 2 diabetes. Diabetes Care. 2009;32:650–657. doi: 10.2337/dc08-1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sha S, Devineni D, Ghosh A, et al. Canagliflozin, a novel inhibitor of sodium glucose co-transporter 2, dose dependently reduces calculated renal threshold for glucose excretion and increases urinary glucose excretion in healthy subjects. Diabetes Obes Metab. 2011;13:669–672. doi: 10.1111/j.1463-1326.2011.01406.x. [DOI] [PubMed] [Google Scholar]
- 8.Rosenstock J, Vico M, Wei L, Salsali A, List JF. Effects of dapagliflozin, an SGLT2 inhibitor, on HbA(1c), body weight, and hypoglycemia risk in patients with type 2 diabetes inadequately controlled on pioglitazone monotherapy. Diabetes Care. 2012;35:1473–1478. doi: 10.2337/dc11-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Devineni D, Curtin CR, Polidori D, et al. Pharmacokinetics and pharmacodynamics of canagliflozin, a sodium glucose co-transporter 2 inhibitor, in subjects with type 2 diabetes mellitus. J Clin Pharmacol. 2013;53:601–610. doi: 10.1002/jcph.88. [DOI] [PubMed] [Google Scholar]
- 10.Kim SR, Lee YH, Lee SG, et al. Morning spot urine glucose-to-creatinine ratios predict overnight urinary glucose excretion in patients with type 2 diabetes. Ann Lab Med. 2017;37:9–17. doi: 10.3343/alm.2017.37.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee EY, Lee YM, Choi KH, Lee HC, Lee BW, Kim BS. Comparison of two creatinine-based equations for predicting decline in renal function in type 2 diabetic patients with nephropathy in a korean population. Int J Endocrinol. 2013;2013:848963. doi: 10.1155/2013/848963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Polidori D, Sha S, Ghosh A, Plum-Morschel L, Heise T, Rothenberg P. Validation of a novel method for determining the renal threshold for glucose excretion in untreated and canagliflozin-treated subjects with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2013;98:E867–E871. doi: 10.1210/jc.2012-4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferrannini E, DeFronzo RA. Impact of glucose-lowering drugs on cardiovascular disease in type 2 diabetes. Eur Heart J. 2015;36(34):2288–2296. doi: 10.1093/eurheartj/ehv239. [DOI] [PubMed] [Google Scholar]
- 14.Cefalu WT. Paradoxical insights into whole body metabolic adaptations following SGLT2 inhibition. J Clin Investig. 2014;124(2):485–487. doi: 10.1172/JCI74297. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article.