Abstract
Introduction
We compared insulin antibody response (IAR) profiles in patients with type 1 diabetes (T1D) or type 2 diabetes (T2D) who received LY2963016 insulin glargine (LY IGlar) or Lantus® insulin glargine (IGlar) and evaluated the potential relationship between higher IARs and clinical and safety outcomes with a focus on patients who exhibited antibody responses in the upper quartile.
Methods
Data from ELEMENT-1 (52-week open-label in T1D) and ELEMENT-2 (24-week, double-blind study in T2D) were analyzed. Maximum postbaseline IAR levels and proportions of patients in the upper quartile of maximum antibody percent binding (UQMAPB; patients with maximum postbaseline percent binding in the highest 25% of maximum values observed) were compared for differential treatment effects on clinical efficacy outcomes and incidence of adverse events. Continuous outcomes were analyzed by analysis of covariance. Categorical data were analyzed by the Cochran–Mantel–Haenszel or Breslow–Day test.
Results
In both studies (N = 532 evaluable patients with T1D; N = 730 with T2D), no statistically significant differences between LY IGlar and IGlar were observed for maximum antibody percent binding (MAPB) levels or for proportions of patients in the respective UQMAPB. No statistically significant differential treatment effects were observed in the relationship between MAPB and clinical efficacy and safety outcomes.
Conclusions
Maximum postbaseline IAR levels and the proportion of patients with high IAR levels were similar for LY IGlar and IGlar. High antibody levels did not affect clinical outcomes. These results add further evidence supporting similar IARs of LY IGlar and IGlar.
Funding
Eli Lilly and Company and Boehringer-Ingelheim.
Keywords: Insulin antibody response, Insulin glargine, LY2963016 insulin glargine
Introduction
LY2963016 insulin glargine (LY IGlar; Eli Lilly and Company, Indianapolis, IN, USA) is the first biosimilar insulin approved in the European Union [1] and Japan in 2014 [2] and was authorized as a follow-on biologic in the USA [3]. LY2963016 insulin glargine and Lantus® insulin glargine (IGlar; recombinant DNA origin; Sanofi-Aventis, Paris, France) have identical primary amino acid sequences, the same pharmaceutical form and strength, and the pharmacokinetic/pharmacodynamic, efficacy, and safety profiles of LY IGlar are highly similar to those of IGlar [4–7].
Insulin products can elicit the formation of insulin antibodies, often without clinical consequences [8, 9]. However, rare instances exist where the presence of insulin antibodies may have clinically relevant effects on efficacy and safety [9–11]. We have previously shown that the immunogenicity profile of LY IGlar is similar to that of IGlar [7], and that both LY IGlar and IGlar have comparable insulin antibody response (IAR) profiles, with no observed effect on efficacy and safety outcomes [7]. While the median antibody levels were low (i.e., less than 5%) and similar between treatment groups, there was variation in patient-to-patient IARs [7]. Because high IARs may provide additional insight into potential immune-mediated effects on efficacy or safety, and these effects may be diluted by looking at all patients, this study focuses on patients who elicited the highest quartile of maximum IARs observed in the LY IGlar phase 3 studies to determine how LY IGlar and IGlar compared in this subgroup of patients and assess whether their IARs were related to clinical outcomes. Thus, we assessed and compared IAR profiles of LY IGlar and IGlar treatment in patients with type 1 diabetes mellitus (T1D) or type 2 diabetes mellitus (T2D), assessed the frequencies of higher IARs among patients treated with either insulin glargine product, and evaluated the potential relationship between higher IARs and clinical and safety outcomes.
Methods
Data from the ELEMENT-1 and ELEMENT-2 studies were analyzed. Both trials were registered at Clinical Trials.gov (NCT01421147 and NCT01421459). Detailed study methods and results for the primary endpoints for both studies have been previously described [5, 6]. Briefly, ELEMENT-1 was a 52-week (24-week treatment period plus 28-week extension period), open-label study of patients with T1D being treated with basal and bolus insulin [5]. ELEMENT-2 was a 24-week, double-blind study of patients with T2D who were insulin-naïve or previously treated with IGlar [6]. In both studies, patients were randomized to receive an LY IGlar or IGlar dose that was equivalent to their prestudy insulin dose except for patients with T2D who were insulin-naïve and started with 10 units of LY IGlar or IGlar. The LY IGlar or IGlar dose was titrated on the basis of daily blood glucose levels [5, 6]. The primary efficacy outcome in both studies was to demonstrate the non-inferiority (0.4% and then 0.3% margin) of LY IGlar to IGlar as measured by change in HbA1c from baseline to 24 weeks [5, 6]. Both of these studies were done in accordance with the International Conference on Harmonisation Guidelines for Good Clinical Practice and the Declaration of Helsinki [12], and written informed consent was obtained from all patients [5, 6].
Antibody determination methods have been described in greater detail previously [5–7]. Briefly, samples used in this study for antibody determination were those collected before randomization (baseline) and at study endpoint. Insulin antibodies were quantified as percent binding using a classic radioimmunoassay format. The anti-LY IGlar antibody assay has cross-reactivity to IGlar and human insulin; hence, antibodies to LY IGlar and IGlar were measured using the same assay [5–7]. Insulin antibody testing was conducted by Millipore (St. Charles, MO, USA).
In each study, patients in the upper quartile of maximum antibody percent binding (UQMAPB) were determined as follows: among all patients with postbaseline insulin antibodies, the maximum postbaseline insulin antibody level (percent binding) per patient was identified. Patients whose maximum postbaseline percent binding belonging to the highest 25% of the maximum values observed comprised the UQMAPB. Patients in the lower 75% and patients who did not have any detectable antibodies postbaseline comprised those patients not in the UQMAPB. Postbaseline maximum antibody percent binding (MAPB) levels and the proportions of patients who exhibited IARs in the UQMAPB with either treatment were evaluated to compare the IAR profiles of LY IGlar and IGlar. Separate analyses for each study also compared LY IGlar to IGlar for clinical outcomes [changes in glycated hemoglobin (HbA1c) (%), basal insulin dose (U/kg/day), weight (kg), incidence of adverse events, and incidence of total hypoglycemia] among patients who did or did not exhibit IARs in the UQMAPB. The potential differential treatment effects of UQMAPB status on clinical and safety outcomes were also assessed by interaction tests. Hypoglycemia was defined in accordance with American Diabetes Association [13] and European Medicine Agency [14] guidelines as having a blood glucose no greater than 3.9 mmol/L (70 mg/dL) or signs or symptoms attributable to low blood glucose levels. Analyses were also performed with hypoglycemia defined as having a blood glucose of less than 3.0 mmol/L (54 mg/dL), a cutoff that had been recommended in the past [15].
Statistical Analysis
The analysis population was comprised of all randomized patients who took at least one dose of study drug (full analysis set) and also had a baseline and at least one postbaseline insulin antibody level assessment. Fisher’s exact test was used to analyze binary data.
Relationships between UQMAPB status (UQMAPB or not UQMAPB) and continuous outcomes were analyzed by an analysis of covariance model with adjustments for design factors—with baseline HbA1c serving as a covariate; and country, time of basal insulin injection (daytime vs. evening/bedtime), sulfonylurea use (ELEMENT-2 only), treatment, UQMAPB status, and treatment-by-UQMAPB interaction as fixed effects. Relationships between UQMAPB status and categorical outcomes for adverse events were analyzed. The Cochran–Mantel–Haenszel test was used for between-treatment comparisons of odds ratios within UQMAPB status subgroups. The Breslow–Day test was used to assess the homogeneity of odds ratios for the interaction between treatment and UQMAPB status. Data were analyzed using SAS version 9.1.3 (SAS Drug Development, Cary, NC, USA).
Results
IAR Profiles of LY IGlar and IGlar Treatment
Of the 535 patients with T1D (ELEMENT-1), 265 LY IGlar and 267 IGlar patients were evaluable. Of the 756 patients with T2D (ELEMENT-2), 365 LY IGlar and 365 IGlar patients were evaluable. No significant differences were observed in either study between the medians for MAPB levels for LY IGlar vs. IGlar or for the proportions of patients in the respective UQMAPB with either treatment (Table 1).
Table 1.
Maximum antibody percent binding (MAPB) characteristics of evaluable patients
| Outcome | ELEMENT-1 (T1D) | ELEMENT-2 (T2D) | ||
|---|---|---|---|---|
| LY IGlar | IGlar | LY IGlar | IGlar | |
| Evaluable patients, N | 265 | 267 | 365 | 365 |
| MAPB levels, % | (n = 107) | (n = 105) | (n = 56) | (n = 40) |
| Minimum | 0.26 | 0.27 | 0.27 | 0.26 |
| 25th percentile | 0.46 | 0.50 | 0.39 | 0.44 |
| Mean (SD) | 2.11 (3.71) | 1.83 (3.15) | 3.94 (7.75) | 3.59 (5.78) |
| Median | 0.84a | 0.90a | 0.56b | 0.78b |
| 75th percentile | 2.13 | 1.66 | 3.29 | 3.90 |
| Maximum | 30.41 | 20.20 | 37.70 | 24.11 |
| Patients in the upper quartile of MAPB, n (%) | 29 (10.9)c | 24 (9.0)c | 14 (3.8)d | 10 (2.7)d |
| Patients in the lower 3 quartiles of MAPB, n (%) | 236 (89.1) | 243 (91.0) | 351 (96.2) | 355 (97.3) |
IGlar insulin glargine, LY IGlar LY2963016 insulin glargine, MAPB maximum antibody percent binding, SD standard deviation, T1D type 1 diabetes, T2D type 2 diabetes
aWilcoxon rank sum, p = 0.959
bWilcoxon rank sum, p = 0.489
cTreatment comparison for the proportion of patients in the UQMAPB, p = 0.472
dTreatment comparison for the proportion of patients in the UQMAPB, p = 0.534
Relationship Between IAR and Clinical and Safety Outcomes
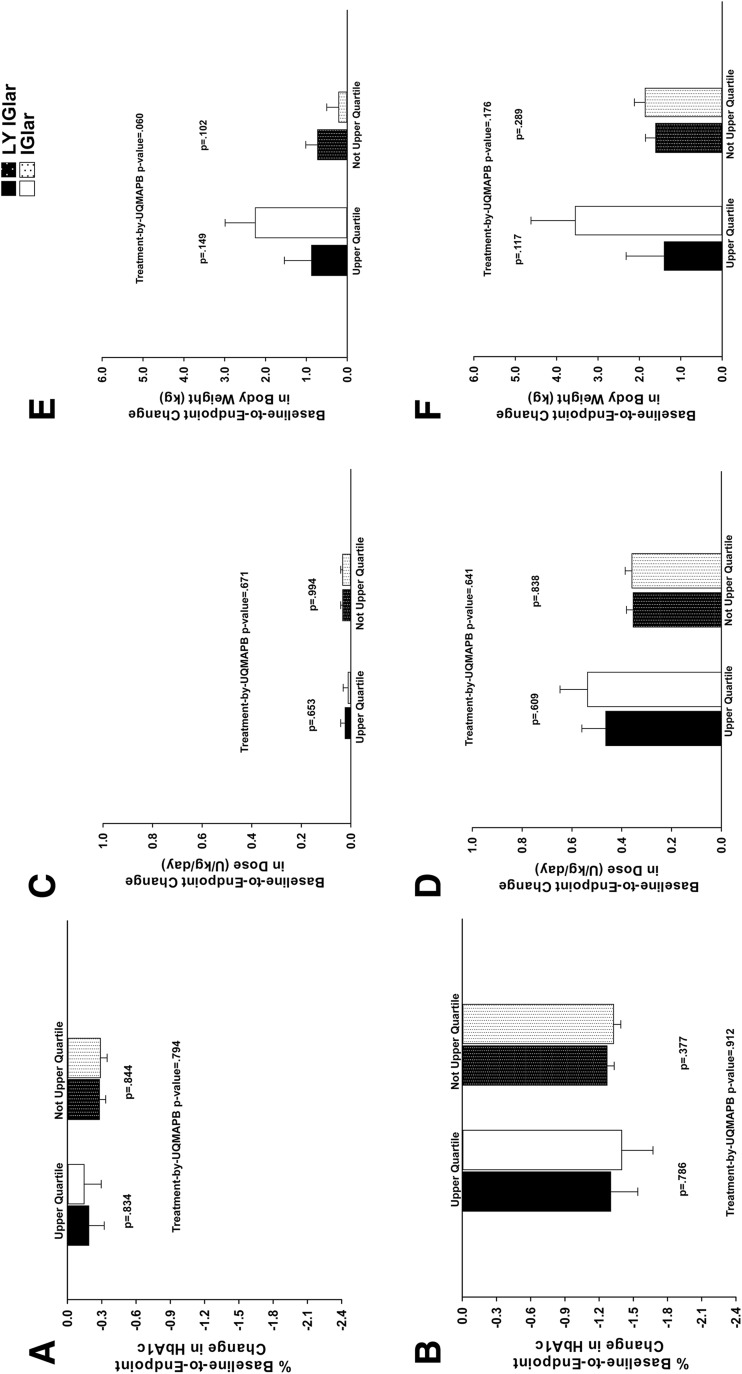
There were no significant between-treatment differences in baseline and endpoint values for HbA1c, basal insulin dose, and body weight among patients in UQMAPB or not in UQMAPB (Table 2). Baseline-to-endpoint changes in HbA1c and basal insulin dose were similar between LY IGlar and IGlar irrespective of UQMAPB status (Fig. 1a–d). While significant treatment-by-UQMAPB interactions were noted for the change from baseline to endpoint in total (basal + prandial) insulin dose (p = 0.008) and in prandial insulin dose (p = 0.027) in ELEMENT-1, there were no significant treatment-by-UQMAPB interactions for endpoint values of total insulin dose (p = 0.105) or prandial insulin dose (p = 0.143) in ELEMENT-1. More importantly there were no between-treatment differences in total insulin or in prandial insulin dose among patients in UQMAPB or not in UQMAPB (Table 2) which is consistent with the absence of between-treatment differences in basal insulin dose among patients by UQMAPB subgroups (Fig. 1c, d). Although IGlar-treated patients who demonstrated UQMAPB showed a greater weight gain than LY IGlar-treated patients or IGlar-treated patients who exhibited lower MAPB, this trend was not found to be statistically significant in either study (Fig. 1e, f).
Table 2.
Baseline and endpoint values for HbA1c, insulin dose, and body weight by treatment-by-upper quartile of the maximum antibody percent binding
| UQMAPB–yes | UQMAPB–no | |||||
|---|---|---|---|---|---|---|
| LY IGlar | IGlar | p value | LY IGlar | IGlar | p value | |
| ELEMENT-1 (T1D) | ||||||
| Evaluable patientsa | N = 265 | N = 267 | N = 265 | N = 267 | ||
| Patients in UQMAPB | (Yes) n = 29 | (Yes) n = 24 | (No) n = 236 | (No) n = 243 | ||
| Baseline HbA1c (%) | 7.71 (0.20) | 7.84 (0.22) | 0.663 | 7.75 (0.07) | 7.78 (0.07) | 0.722 |
| Endpoint HbA1c (%) | 7.58 (0.14) | 7.62 (0.15) | 0.834 | 7.49 (0.06) | 7.48 (0.06) | 0.844 |
| Baseline basal insulin dose (U/kg/day) | 0.36 (0.03) | 0.31 (0.03) | 0.277 | 0.32 (0.01) | 0.31 (0.01) | 0.305 |
| Endpoint basal insulin dose (U/kg/day) | 0.39 (0.03) | 0.34 (0.03) | 0.158 | 0.38 (0.01) | 0.36 (0.01) | 0.321 |
| Baseline basal insulin lispro dose (U/kg/day) | 0.37 (0.04) | 0.38 (0.04) | 0.833 | 0.40 (0.01) | 0.40 (0.02) | 0.790 |
| Endpoint basal insulin lispro dose (U/kg/day) | 0.45 (0.04) | 0.38 (0.04) | 0.181 | 0.36 (0.02) | 0.37 (0.02) | 0.530 |
| Baseline total insulin dose (U/kg/day) | 0.69 (0.05) | 0.69 (0.05) | 0.956 | 0.72 (0.02) | 0.71 (0.02) | 0.664 |
| Endpoint total insulin dose (U/kg/day) | 0.84 (0.05) | 0.71 (0.06) | 0.081 | 0.74 (0.02) | 0.74 (0.02) | 0.908 |
| Baseline body weight (kg) | 77.5 (3.0) | 74.1 (3.3) | 0.453 | 75.7 (1.05) | 74.8 (1.0) | 0.547 |
| Endpoint body weight (kg) | 77.6 (2.8) | 72.2 (3.1) | 0.179 | 74.2 (1.2) | 73.3 (1.2) | 0.506 |
| ELEMENT-2 (T2D) | ||||||
| Evaluable patientsa | N = 365 | N = 365 | N = 365 | N = 365 | ||
| Patients in UQMAPB | (Yes) n = 14 | (Yes) n = 10 | (No) n = 351 | (No) n = 355 | ||
| Baseline HbA1c (%) | 8.86 (0.29) | 8.38 (0.34) | 0.285 | 8.33 (0.06) | 8.33 (0.06) | 0.969 |
| Endpoint HbA1c (%) | 7.04 (0.24) | 6.94 (0.28) | 0.786 | 7.07 (0.07) | 7.02 (0.07) | 0.377 |
| Baseline basal insulin dose (U/kg/day) | 0.12 (0.06) | 0.02 (0.08) | 0.298 | 0.16 (0.01) | 0.14 (0.01) | 0.184 |
| Endpoint basal insulin dose (U/kg/day) | 0.52 (0.10) | 0.51 (0.11) | 0.921 | 0.50 (0.03) | 0.48 (0.03) | 0.437 |
| Baseline body weight (kg) | 79.9 (5.2) | 84.9 (6.2) | 0.540 | 90.8 (1.1) | 90.3 (1.1) | 0.744 |
| Endpoint body weight (kg) | 80.2 (5.1) | 87.6 (5.9) | 0.324 | 85.7 (1.4) | 85.0 (1.4) | 0.611 |
Data are least squares mean ± standard error
HbA1c glycated hemoglobin, IGlar insulin glargine, LY IGlar LY2963016 insulin glargine, UQMAPB upper quartile of maximum antibody percent binding, T1D type 1 diabetes, T2D type 2 diabetes
aDefined as patients having “detected” or “nondetected” insulin antibody levels at baseline and postbaseline visit (patients at risk of treatment-emergent antibody response)
Fig. 1.
Baseline-to-endpoint changes in glycated hemoglobin (HbA1c) in patients with a type 1 diabetes (T1D) or b type 2 diabetes (T2D), basal insulin dose in patients with c T1D or d T2D, weight in patients with e T1D or f T2D. Data are least squares mean ± standard error. HbA1c glycated hemoglobin, IGlar insulin glargine, LY IGlar LY2963016 insulin glargine, UQMAPB upper quartile of maximum antibody percent binding
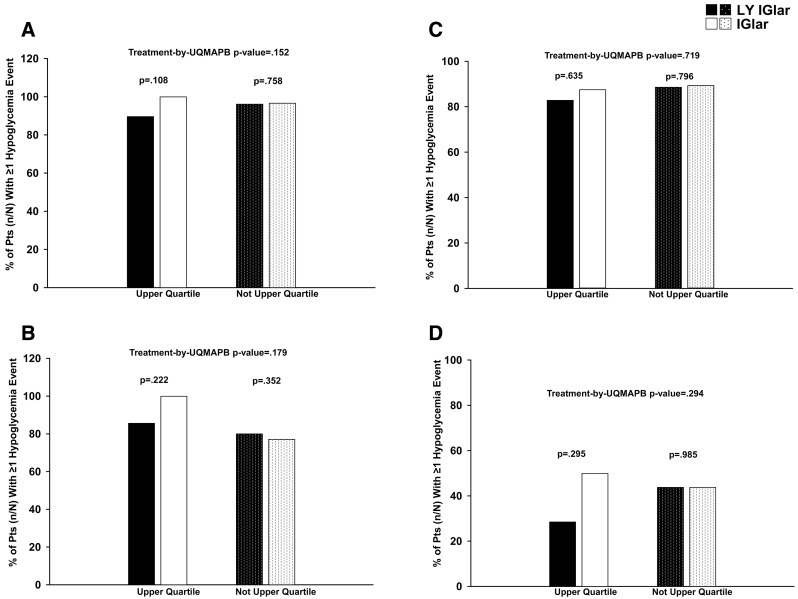
In both studies, both treatment groups exhibited similar frequencies of allergic reactions, injection site reactions, and serious adverse events irrespective of UQMAPB status (Table 3). Likewise, the overall incidence of hypoglycemia events was similar between both treatment groups irrespective of patient UQMAPB status (Fig. 2).
Table 3.
Treatment reactions and serious adverse events
| UQMAPB–yes | UQMAPB–no | INT p valued | |||||
|---|---|---|---|---|---|---|---|
| LY IGlar | IGlar | p valueb | LY IGlar | IGlar | p valuec | ||
| ELEMENT-1 (T1D) | |||||||
| Evaluable patientsa | N = 265 | N = 267 | N = 265 | N = 267 | |||
| Patients in UQMAPB | (Yes) n = 29 | (Yes) n = 24 | (No) n = 236 | (No) n = 243 | |||
| Allergic reactions, n (%) | 2 (6.9) | 1 (4.2) | 0.672 | 18 (7.6) | 10 (4.1) | 0.102 | 0.927 |
| Injection site reactions, n (%) | 0 (0.0) | 0 (0.0) | NA | 7 (3.0) | 3 (1.2) | 0.186 | NA |
| Serious adverse events n (%) | 3 (10.3) | 1 (4.2) | 0.401 | 17 (7.2) | 23 (9.5) | 0.372 | 0.282 |
| ELEMENT-2 (T2D) | |||||||
| Evaluable patientsa | N = 365 | N = 365 | N = 365 | N = 365 | |||
| Patients in UQMAPB | (Yes) n = 14 | (Yes) n = 10 | (No) n = 351 | (No) n = 355 | |||
| Allergic reactions, n (%) | 1 (7.1) | 1 (10.0) | 0.807 | 19 (5.4) | 25 (7.0) | 0.371 | 0.954 |
| Injection site reactions, n (%) | 2 (14.3) | 1 (10.0) | 0.759 | 11 (3.1) | 10 (2.8) | 0.804 | 0.830 |
| Serious adverse events n (%) | 0 (0.0) | 0 (0.0) | 14 (4.0) | 17 (4.8) | 0.604 | NA | |
p values are not shown if the total number of patients with events for the combined subgroups and treatments is <10. If the total number of events within a subgroup is 0, the interaction test is not performed. Patients may be counted in more than one category of reaction or event
IGlar insulin glargine, INT interaction, LY IGlar LY2963016 insulin glargine, NA not assessed, T1D type 1 diabetes, T2D type 2 diabetes, UQMAPB upper quartile of maximum antibody percent binding
aDefined as patients having “detected” or “nondetected” insulin antibody levels at baseline and postbaseline visit (patients at risk of treatment-emergent antibody response)
b p values are from the Mantel–Haenszel test for comparison of LY IGlar (yes) vs. IGlar (yes)
c p values are from the Mantel–Haenszel test for comparison of LY IGlar (no) vs. IGlar (no)
dInteraction p value assessed using the Breslow–Day test
Fig. 2.
Overall incidence of hypoglycemia (blood glucose no greater than 3.9 mmol/L) in patients with a type 1 diabetes (T1D) or b type 2 diabetes (T2D), hypoglycemia (blood glucose less than 3.0 mmol/L) in patients with c T1D or d T2D. IGlar insulin glargine, LY IGlar LY2963016 insulin glargine, Pts patients, UQMAPB upper quartile of maximum antibody percent binding
Discussion
This study shows that patients with T1D or T2D who received LY IGlar or IGlar exhibited similar levels and frequencies of IARs. The number of patients exhibiting IARs in the UQMAPB was similar for LY IGlar and IGlar. Median antibody levels of those patients in the UQMAPB were comparable between both treatment groups and low (i.e., less than 5%). No significant differential treatment effects were observed between LY IGlar and IGlar for clinical efficacy or safety outcomes among patients who did or did not exhibit UQMAPBs for IARs. Clinical efficacy and safety outcomes were similar among patients treated with LY IGlar or IGlar regardless of IAR levels.
LY IGlar has an identical primary amino acid sequence, similar pharmacokinetic/pharmacodynamic, efficacy, and safety profiles as IGlar [4–6] and is considered a biosimilar insulin in certain jurisdictions. In previous studies, IGlar has been shown to exhibit a low immunogenic potential compared to neutral protamine Hagedorn (NPH) insulin. In a 28-week study that compared IGlar to NPH insulin, 1–2% of patients with T1D in either group exhibited clinically relevant [at least 20 U, % bound/total (B/T)] antibodies [16]. Similarly, IGlar was significantly less immunogenic than NPH insulin in a 52-week study of patients with T2D [17].
Our finding of no relationship between immune response and efficacy or safety with LY IGlar or IGlar is also consistent with previous studies of IGlar. Although some of these studies comparing IGlar and NPH insulin did not specifically analyze any relationship between anti-IGlar antibodies and clinical outcomes, IGlar exhibited similar or greater glycemic control than NPH [15–17]. In addition, treatment-related adverse events were comparable between IGlar and NPH insulin [17, 18]. A recent retrospective analysis of seven published registration studies that evaluated the immunogenicity of IGlar and NPH in relation to clinical outcomes found that the proportion of patients with clinically relevant changes in anti-IGlar antibody levels (at least 20 U, %B/T) was low. In addition, antibody levels were not associated with any clinically relevant effects on HbA1c, insulin dose, and hypersensitivity [19].
One study limitation is the sample size; because the registration studies were originally designed to demonstrate noninferiority in HbA1c change [5, 6], some may argue that the sample is relatively small to detect differences in rare events, such as immune reactions. Notably, the parent studies included 535 patients with T1D and 756 patients with T2D, and the findings in this study are consistent with what was shown in analyses including the total study populations [7]. Furthermore, no relationship between UQMAPB status and clinical outcomes was observed for patients with the highest IARs. A second limitation is the study length which limits our findings to anti-insulin antibody formation for up to 24 or 52 weeks. However, it should be noted that a previous 52-week study has shown that the level of antibodies against insulin glargine plateaus after approximately 20 weeks of treatment [17], a time frame covered by both studies in this analysis.
Conclusions
Patients treated with LY IGlar and IGlar had similar maximum antibody responses, with similar proportions of patients having high IARs. High antibody levels were not associated with effects on clinical outcomes. These findings reduce concerns of immunogenicity responses that may affect efficacy and safety with insulin glargine treatment and provide additional support for the similarity between LY IGlar and IGlar.
Acknowledgements
The study was funded by Eli Lilly and Company (Indianapolis, IN, USA) and Boehringer-Ingelheim (Ridgefield, CT, USA). Eli Lilly and Company (Indianapolis, IN, USA) and Boehringer-Ingelheim (Ridgefield, CT, USA) also funded the article processing charges.
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
The authors acknowledge the investigators and patients who participated in the ELEMENT-1 and ELEMENT-2 studies. The authors also acknowledge Eileen Girten, MS, and Michelle Carey, PhD, both of inVentiv Health Clinical, for assistance with the preparation of this manuscript. Support for this assistance was funded by Eli Lilly and Company (Indianapolis, IN, USA) and Boehringer-Ingelheim (Ridgefield, CT, USA). This work was previously published as an abstract presented at the American Diabetes Association’s 75th Scientific Sessions, Boston, Massachusetts, June 2015 (1029-P).
Disclosures
Liza L. Ilag is an employee of and holds stock in Eli Lilly and Company. Robyn K. Pollom is an employee of and holds stock in Eli Lilly and Company. Timothy M. Costigan is an employee of and holds stock in Eli Lilly and Company. Curtis L. Chang is an employee of and holds stock in Eli Lilly and Company.Robert J. Konrad is an employee of and holds stock in Eli Lilly and Company. Mark A. Deeg is an employee of and holds stock in Eli Lilly and Company. Melvin J. Prince is a retiree of Eli Lilly and Company.
Compliance with Ethics Guidelines
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. Informed consent was obtained from all patients for being included in the study.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced Content
To view enhanced content for this article go to http://www.medengine.com/Redeem/79F7F0601290EA61.
References
- 1.Eli Lilly and Company. European Commission grants Lilly and Boehringer Ingelheim’s insulin glargine product marketing authorisation in Europe (news release). Indianapolis, IN: Eli Lilly and Company, Boehringer Ingelheim; September 10, 2014. http://lilly.mediaroom.com/index.php?s=9042&item=137348. Accessed 18 May 2015.
- 2.Pharmaceuticals and Medical Devices Agency. New drugs approved in FY 2014. http://www.pmda.go.jp/files/000206818.pdf. Accessed 10 Dec 2015.
- 3.US Food and Drug Administration. FDA approves Basaglar, the first “follow-on” insulin glargine product to treat diabetes (news release). December 16, 2015. http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm477734.htm. Accessed 02 Nov 2016.
- 4.Linnebjerg H, Lam EC, Seger ME, et al. Comparison of the pharmacokinetics and pharmacodynamics of LY2963016 insulin glargine and EU- and US-approved versions of Lantus insulin glargine in healthy subjects: three randomized euglycemic clamp studies. Diabetes Care. 2015;38:2226–2233. doi: 10.2337/dc14-2623. [DOI] [PubMed] [Google Scholar]
- 5.Blevins TC, Dahl D, Rosenstock J, et al. Efficacy and safety of LY2963016 insulin glargine compared with insulin glargine (Lantus®) in patients with type 1 diabetes in a randomized controlled trial: the ELEMENT 1 study. Diabetes Obes Metab. 2015;17:726–733. doi: 10.1111/dom.12496. [DOI] [PubMed] [Google Scholar]
- 6.Rosenstock J, Hollander P, Bhargava A, et al. Similar efficacy and safety of LY2963016 insulin glargine and insulin glargine (Lantus®) in patients with type 2 diabetes who were insulin-naïve or previously treated with insulin glargine: a randomized, double-blind controlled trial (the ELEMENT 2 study) Diabetes Obes Metab. 2015;17:734–741. doi: 10.1111/dom.12482. [DOI] [PubMed] [Google Scholar]
- 7.Ilag LL, Deeg MA, Costigan T, et al. Evaluation of immunogenicity of LY2963016 insulin glargine compared with Lantus® insulin glargine in patients with type 1 or type 2 diabetes mellitus. Diabetes Obes Metab. 2016;18:159–168. doi: 10.1111/dom.12584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dolinar RO, Edelman S, Heinemann L, et al. Impact of biosimilar insulins on clinical practice: meeting report. J Diabetes Sci Technol. 2014;8:179–185. doi: 10.1177/1932296813518267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Polimeni G, Trifirò G, Ingrasciotta Y, Caputi AP. The advent of biosimilars for the treatment of diabetes: current status and future directions. Acta Diabetol. 2015;52:423–431. doi: 10.1007/s00592-015-0771-7. [DOI] [PubMed] [Google Scholar]
- 10.Owens DR, Landgraf W, Schmidt A, Bretzel RG, Kuhlmann MK. The emergence of biosimilar insulin preparations—a cause for concern? Diabetes Technol Ther. 2012;14:989–996. doi: 10.1089/dia.2012.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shankar G, Arkin S, Cocea L, et al. Assessment and reporting of clinical immunogenicity of therapeutic proteins and peptides–harmonized terminology and tactical recommendations. AAPS J. 2014;16:658–673. doi: 10.1208/s12248-014-9599-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Medical Association Declaration of Helsinki Recommendations guiding physicians in biomedical research involving human subjects. JAMA. 1997;277:925–926. doi: 10.1001/jama.1997.03540350075038. [DOI] [PubMed] [Google Scholar]
- 13.American Diabetes Association Workgroup on Hypoglycemia Defining and reporting hypoglycemia in diabetes: a report from the American Diabetes Association Workgroup on Hypoglycemia. Diabetes Care. 2005;28:1245–1249. doi: 10.2337/diacare.28.5.1245. [DOI] [PubMed] [Google Scholar]
- 14.European Medicines Agency. Committee for Medicinal Products for Human Use (CHMP). 2012. Guideline on the clinical investigation of medicinal products in the treatment or prevention of diabetes mellitus. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2012/06/WC500129256.pdf. Accessed 09 March 2016.
- 15.European Agency for the Evaluation of Medicinal Products. Committee for Proprietary Medicinal Products (CPMP). 2002. Note for guidance on clinical investigation of medicinal products in the treatment of diabetes mellitus. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003262.pdf. Accessed 08 March 2016.
- 16.Ratner RE, Hirsch IB, Neifing JL, Garg SK, Mecca TE, Wilson CA. Less hypoglycemia with insulin glargine in intensive insulin therapy for type 1 diabetes. US Study Group of Insulin Glargine in Type 1 Diabetes. Diabetes Care. 2000;23:639–643. doi: 10.2337/diacare.23.5.639. [DOI] [PubMed] [Google Scholar]
- 17.Yki-Järvinen H, Dressler A, Ziemen M, HOE 901/300s Study Group Less nocturnal hypoglycaemia and better post-dinner glucose control with bedtime insulin glargine compared with bedtime NPH insulin during insulin combination therapy in type 2 diabetes. HOE 901/3002 Study Group. Diabetes Care. 2000;23:1130–1136. doi: 10.2337/diacare.23.8.1130. [DOI] [PubMed] [Google Scholar]
- 18.Zinman B, Philis-Tsimikas A, Cariou B, et al. Insulin degludec versus insulin glargine in insulin-naive patients with type 2 diabetes: a 1-year, randomized, treat-to-target trial (BEGIN Once Long) Diabetes Care. 2012;35:2464–2471. doi: 10.2337/dc12-1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vlajnic A, Traylor L, Frimpter J, Khatami H, Schellekens H. The immunogenicity of originator insulin glargine: incidence and absence of clinical sequelae. Diabetologia. 2014;57(Suppl1):S397. [Google Scholar]