Abstract
Introduction
Published evaluations of sensor glucose monitoring use in insulin treated type 2 diabetes are limited. The aim of this study was to assess the impact of flash glucose-sensing technology as a replacement for self-monitoring of blood glucose (SMBG) over a 12-month period in participants with type 2 diabetes who were on intensive insulin therapy.
Methods
An open-label, randomized, controlled study in adults with type 2 diabetes on intensive insulin therapy from 26 European diabetes centers aimed at assessing flash glucose sensing technology was conducted. Participants (N = 224) were randomized (1:2 respectively) to a control group (n = 75) that used SMBG (FreeStyle Lite™) or to an intervention group (n = 149) which used sensor glucose data (FreeStyle Libre™ Flash Glucose Monitoring System) for self-management over 6 months. All intervention group participants who completed the 6-month treatment phase continued into an additional 6-month open-access phase.
Results
A total of 139 intervention participants completed the 6-month treatment phase and continued into the open-access phase. At 12 months (end of open-access period), time in hypoglycemia [sensor glucose <3.9 mmol/L (70 mg/dL)] was reduced by 50% compared to baseline [−0.70 ± 1.85/24 h (mean ± standard deviation); p = 0.0002]. Nocturnal hypoglycemia [2300 to 0600 hours, <3.9 mmol/L (70 mg/dL)] was reduced by 52%; p = 0.0002. There was no change in time in range [sensor glucose 3.9–10.0 mmol/L (70–180 mg/dL)]. SMBG testing fell from a mean of 3.9 (median 3.9) times/day at baseline to 0.2 (0.0), with an average frequency of sensor scanning of 7.1 (5.7) times/day at 12 months, and mean sensor utilization was 83.6 ± 13.8% (median 88.3%) during the open-access phase. During this 6-month extension period no device-related serious adverse events were reported. Nine participants reported 16 instances of device-related adverse events (e.g. infection, allergy) and 28 participants (20.1%) experienced 134 occurrences of anticipated skin symptoms/sensor-insertion events expected with device use (e.g. erythema, itching and rash).
Conclusion
The use of flash glucose-sensing technology for glycemic management in individuals with type 2 diabetes treated by intensive insulin therapy over 12 months was associated with a sustained reduction in hypoglycemia and safely and effectively replaced SMBG.
Trial Registration
ClinicalTrials.gov identifier, NCT02082184.
Electronic supplementary material
The online version of this article (doi:10.1007/s13300-017-0255-6) contains supplementary material, which is available to authorized users.
Keywords: Flash sensor glucose technology, Glucose monitoring, Insulin, Type 2 diabetes
Introduction
The management of hyperglycemia remains a primary focus of diabetes management in patients with type 2 diabetes. Current management strategies balance optimization of glucose control with potential risks from the therapy, especially hypoglycemia [1]. In both type 1 and type 2 diabetes, increased hypoglycemic risk is associated with the duration of diabetes and insulin use [2, 3] and not with glycated hemoglobin (HbA1c) level [3]. Prandial insulin carries a higher risk for non-severe hypoglycemia than treatment with basal insulin alone [4], and intensive insulin treatment for the management of type 2 diabetes further increases the risk for severe hypoglycemia [5]. Therefore, enhanced detection of dysglycemia for patients with type 2 diabetes managed with multiple daily injections or continuous subcutaneous insulin infusion (CSII) is essential and can be challenging with self-monitoring of blood glucose (SMBG) as neither hypo- nor hyperglycemia are easily detected [6]. Continuous glucose monitoring (CGM) can offer enhanced assessment of glycemic issues; however, current guidance for CGM use and benefit in the patient population with type 2 diabetes excludes the use of intensive insulin therapy [7] due to the scarcity of published data in this population [8].
Our results from the randomized controlled trial “Novel Glucose-sensing Technology as a Replacement for Blood Glucose Monitoring for the Management of Insulin-treated Type 2 Diabetes (REPLACE)”, which compared the safety and efficacy of the new flash glucose-sensing technology to SMBG over a 6-month period have been published [9]. This study included an additional 6-month open-access phase for all participants in the intervention group. The aim of the open-access phase was twofold: to evaluate (1) the safety of the new device in day-to-day use over an extended time period by assessing changes in glycemic measures between baseline and 12 months and (2) device-related adverse events.
The flash glucose-sensing technology used was FreeStyle Libre™, a sensor-based flash glucose monitoring system (Abbott Diabetes Care, Witney, UK). This small, single-use, factory-calibrated, on-body sensor utilizes wired enzyme technology (osmium mediator and glucose oxidase enzyme co-immobilized on electrochemical sensor) to continuously monitor interstitial glucose levels. The sensor is worn on the back of the arm for up to 14 days and automatically stores glucose data every 15 min. A real-time glucose level may be obtained as often as each minute by scanning the sensor with the reader. A glucose trend arrow (indicating rate and direction of change in glucose levels) and a graphical trace of glucose values for the previous 8-h period is also displayed on the screen. Data are transferred wirelessly by radio frequency identification from the sensor to the reader memory which stores historical sensor data for 90 days. Data can be uploaded using the device software to generate summary glucose reports (including an ambulatory glucose profile) for review by the patient at home or in the clinic with their healthcare professional [10].
Methods
Details on the rationale, methodology and results of the treatment phase (6 months) of the REPLACE study have been described previously [9]. Briefly, this was a 6-month, prospective, open-label, non-masked, two-arm, randomized controlled study that was conducted at 26 European diabetes centers (8 in France, 10 in Germany, 8 in the UK). The treatment phase of the study was designed to compare the use of novel flash glucose sensing technology with SMBG in participants with type 2 diabetes treated with multi-dose insulin therapy. Following completion of the 6-month treatment phase, intervention group participants continued using flash sensing technology for a further 6 months during the open-access period.
At each study center, any potentially eligible patient from the general diabetes population was invited to participate in the study if they were ≥18 years of age with type 2 diabetes treated with insulin for at least 6 months and on their current regimen (prandial only or prandial and basal multi-dose-insulin therapy or CSII therapy) for ≥3 months; had an HbA1c level of 58–108 mmol/mol (7.5–12.0%); had self-reported regular blood glucose testing data (more than 10/week for at least 2 months prior to study entry); were considered by the investigator to be technically capable of using the flash sensor-based glucose monitoring system.
Participants were not included for the following reasons: if they had any other insulin regimen to that described above; had a total daily dose of insulin ≥1.75 U/kg on study entry; had severe hypoglycemia (requiring third-party assistance) [8], diabetic ketoacidosis or hyperosmolar–hyperglycemic state in the preceding 6 months; had a known allergy to medical-grade adhesives; used continuous glucose monitoring within the previous 4 months; were pregnant or planning pregnancy; were receiving steroid therapy for any condition; were considered by the investigator to be unsuitable to participate.
Approval was given by the appropriate competent authorities in each country. All procedures were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. Informed consent was obtained from all patients for participation in the study.
Following 2 weeks of blinded sensor wear, the subjects were randomized (centrally, using biased-coin minimization dependent on study center and insulin administration) to the control group (SMBG) or to the intervention group (glucose-sensing technology). For the 6-month treatment phase (post-randomization), the sensor-based glucose monitoring system was un-blinded for the participants in the intervention group so that they could continuously use sensor glucose data for self-management, including insulin dose decisions, in accordance with the product labeling. No training was provided to these participants for interpretation of glucose sensor data. Their historical data was uploaded at subsequent study visits, and glucose reports [including ambulatory profile reports (AGP)] were generated for review by the healthcare professional with the participant, using the device software [10].
At 6 months (day 208), all control participants concluded their involvement in the study while intervention participants entered an open-label, open-access study phase for a further 6 months. For the open-access phase participants continued to use the sensor-based glucose monitoring system for their day-to-day glucose management and also to record any events in their event diary. These participants had a review of their glucose reports with the clinician at 3-month intervals; at the beginning of the open-access phase and after a further 3 months (day 284). Similar to the visits during the intervention phase, at these visits the effect of life-style/diet on glucose levels and insulin doses were discussed and any management changes agreed upon. In order to continue to reflect “real world” conditions there was no pre-set algorithm for insulin adjustments mandated by the protocol. However, common principles continued to be applied, including avoidance of hypoglycemia, optimization of fasting glucose and reduction of postprandial glucose excursions.
Outcomes
The primary outcomes were changes in sensor-derived glycemic measures between baseline and 12 months post-baseline. The sensor-derived glycemic measures were number and duration of hypoglycemic events [glucose <3.9 mmol/L (70 mg/dL)] and number and duration of hyperglycemic events [glucose >13.3 mmol/L (240 mg/dL)].
Pre-specified secondary endpoints included sensor-derived glycemic measures between baseline and 12 months post-baseline; frequency of glucose finger-sticks and sensor scans per day during the study period; and total daily dose of insulin. Sensor-derived glycemic measures included number and duration of hypoglycemic events [glucose <3.1 mmol/L (55 mg/dL)]; time in glucose range 3.9–10.0 mmol/L (70–180 mg/dL), number and duration of hyperglycemic events [glucose >10.0 mmol/L (180 mg/dL)]; mean and standard deviation (SD) glucose. An event was defined as at least two consecutive readings, at 15-min intervals, outside the predefined glucose range (the end of an episode was 1 reading at or inside the predefined range).
Safety endpoints incorporated all adverse events, including severe hypoglycemia (requiring third-party assistance [2]), hypoglycemic events and sensor insertion or sensor wear-related symptoms, diabetic ketoacidosis or hyperosmolar hyperglycemic state episodes and cardiac events.
Statistical Analysis
Differences between post-baseline and baseline measurements were evaluated using a paired t test. Sensor-derived glycemic endpoint values were excluded from the analysis if <72 h of sensor results were available from the final 14-day sensor wear (days 374–388). Confidence intervals were calculated for the mean difference from baseline.
The results presented here are for the full analysis set. Data analysis was performed by a contract research organization (ICON PLC; Dublin, Ireland, managed by Abbott Diabetes Care) and by Abbott Diabetes Care. We used SAS version 9.2 or higher for all analyses (SAS Institute, Cary, NC).
The trial is registered with ClinicalTrials.gov, number NCT02082184.
Results
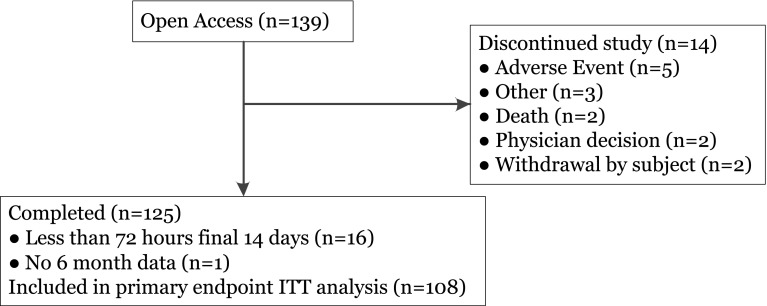
All 139 (100%) intervention participants completing the treatment phase continued into the open-access phase, of whom 125 completed the open-access phase (Fig. 1). The primary reason for discontinuation was skin reaction at the sensor site. Participants’ baseline characteristics are summarized in Table 1. Sensor-derived glycemic endpoint values were included for 108 participants who had ≥72 h of sensor results from the three (baseline and 6 and 12 months post-baseline) 14-day sensor wear periods.
Fig. 1.
Trial profile. ITT Intention to treat
Table 1.
Baseline characteristics
| Baseline characteristics | Open-access phase intervention participants (N = 139) |
|---|---|
| Age (years) | 59.3 ± 9.6 [33, 77] |
| Weight (kg) | 97 ± 20 [51, 170] |
| Body mass index (kg/m2) | 33.1 ± 6.0 [18.8, 54.1] |
| Duration of diabetes (years) | 17 ± 8 [2, 43] |
| Duration of insulin use (years) | 9 ± 6 [0, 40] |
| Screening HbA1c | |
| mmol/mol | 71.8 ± 10.5 [59, 103] |
| % | 8.72 ± 0.96 [7.5, 11.6] |
| Self-reported blood glucose frequency per day | 3.6 ± 1.29 [1, 10] |
| Insulin (total daily dose) | |
| Basal units (n = 124) | 38.0 ± 21.0 |
| Bolus units (n = 115) | 51.7 ± 30.4 |
| CSII units (n = 5) | 56.5 ± 39.5 |
| Open-access phase intervention participants N (%) |
|
|---|---|
| Gender (male) | 88 (63%) |
| Ethnicity | |
| White | 134 (96%) |
| Black | 1 (1%) |
| Asian/Pacific Islander | 1 (1%) |
| Other | 3 (2%) |
| Diabetes management | |
| Insulin pen device | 130 (94%) |
| CSII | 8 (6%) |
| Insulin syringe | 1 (1%) |
| Previous CGM use | 10 (7%) |
| Employment status | |
| Employed (n = 136) | 56 (41%) |
| Not employed/retired/other (n = 136) | 80 (59%) |
| Insulin management training | |
| <1 year ago | 41 (29%) |
| >1 year ago | 93 (67%) |
| Carbohydrate counting training | |
| <1 year ago | 40 (29%) |
| >1 year ago | 49 (35%) |
| Bolus dose titration | |
| Based on meal content (n = 138) | 89 (64%) |
| Based on current glucose level (n = 138) | 108 (78%) |
| Using sliding scale (n = 138) | 53 (38%) |
Values in table are presented as the mean ± standard deviation (SD) with the range in square brackets or as the number with the percentage in parenthesis, as appropriate
CGM Continuous glucose monitoring, CSII continuous subcutaneous insulin infusion, HbA1c glycated hemoglobin
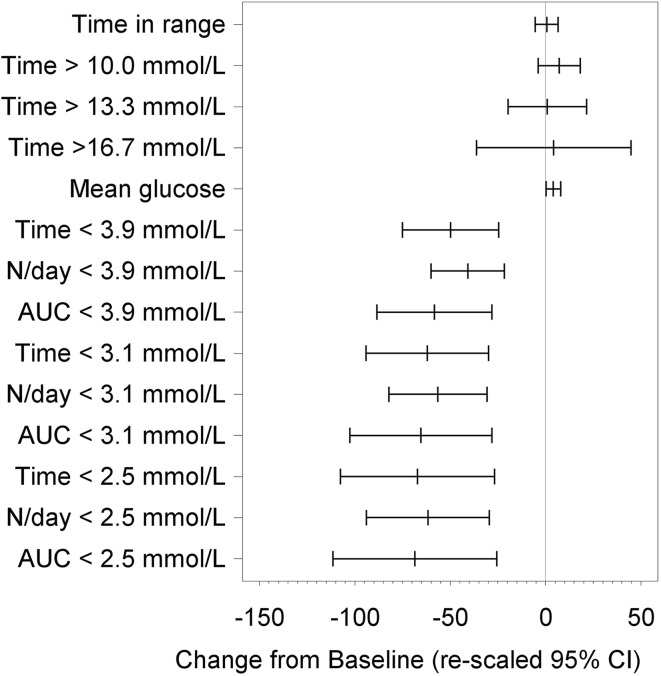
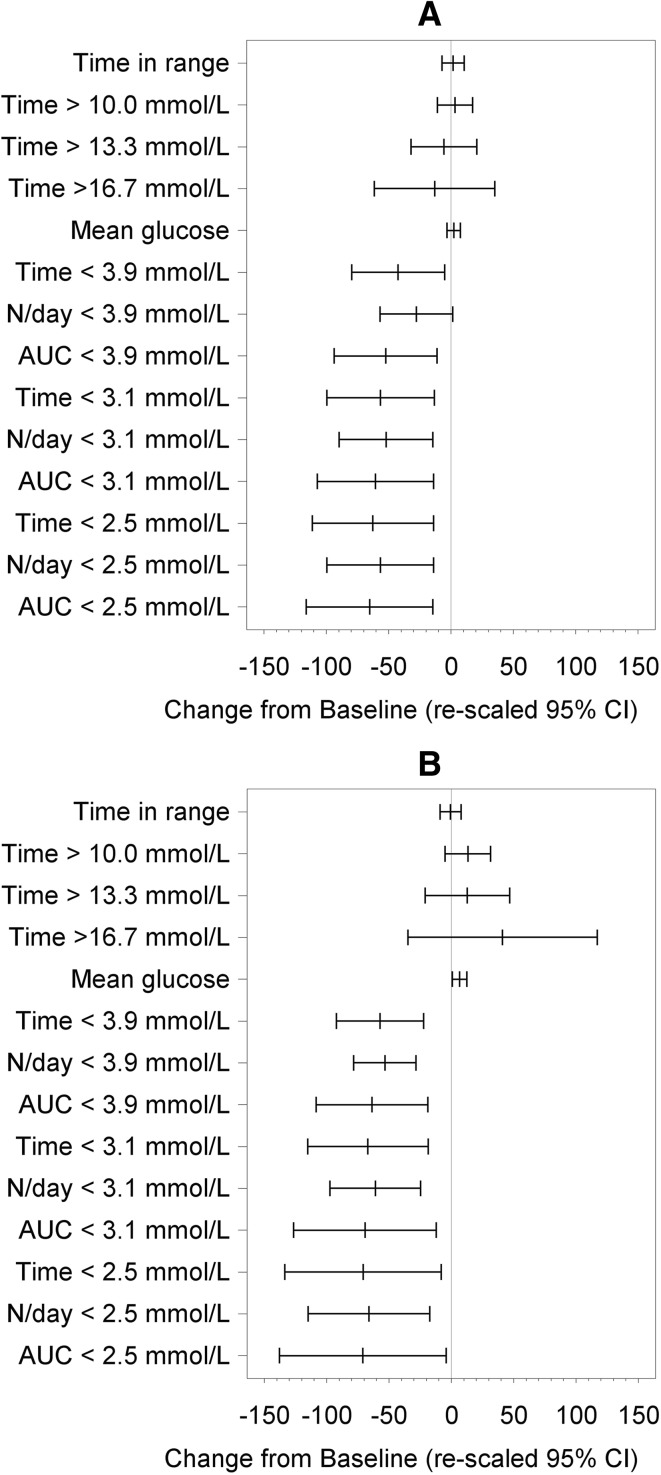
Significant reductions in all sensor measures of time spent in hypoglycemia [glucose <3.9 mmol/L (70 mg/dL), <3.1 mmol/L (55 mg/dL) and <2.5 mmol/L (45 mg/dL)], number of events and area under the curve were observed for participants at the end of the open-access phase (12 months) compared to the baseline phase (Table 2; Figs. 2, 3).
Table 2.
Glycemic measures
| Measure | Participants (N = 139) | |||||
|---|---|---|---|---|---|---|
| Baseline(Days 1–15) (n = 108)Mean (SD) | 6-months(Days 194–208) (n = 108)Mean (SD) | Open Access(Days 374–388) (n = 108) Mean (SD) | Open Access % Change from Baseline | Open Access Change fromBaseline Mean (SD) | Open Access Change from Baseline p-value | |
| Time with glucose 3.9–10.0 mmol/L (70–180 mg/dL) (h) | 14.0 (4.4) | 13.6 (4.5) | 14.1 (4.0) | 0.6 | 0.1 (4.4) | 0.8519 |
| Mean Glucose (mg/dL) | 163.3 (32.7) | 175.7 (31.6) | 169.9 (27.5) | 4.0 | 6.6 (33.1) | 0.0409 |
| Mean Glucose (mmol/L) | 9.1 (1.8) | 9.8 (1.8) | 9.4 (1.5) | 4.0 | 0.4 (1.8) | 0.0409 |
| SD Glucose (mg/dL) | 55.4 (13.0) | 54.7 (13.7) | 53.7 (14.3) | −3.1 | −1.7 (11.7) | 0.1324 |
| SD Glucose (mmol/L) | 3.1 (0.7) | 3.0 (0.8) | 3.0 (0.8) | −3.1 | −0.1 (0.7) | 0.1324 |
| Glucose <3.9 mmol/L (70 mg/dL) within 24 h | ||||||
| Events | 0.67 (0.66) | 0.33 (0.36) | 0.40 (0.44) | −40.8 | −0.27 (0.67) | <0.0001 |
| Time (h) | 1.40 (1.91) | 0.47 (0.57) | 0.70 (0.94) | −49.9 | −0.70 (1.85) | 0.0002 |
| AUC (h x mg/dL) | 21.84 (37.45) | 5.21 (7.28) | 9.11 (14.70) | −58.3 | −12.73 (34.53) | 0.0002 |
| Glucose <3.9 mmol/L (70 mg/dL) at night (23.00–06.00) within 7 h | ||||||
| Events | 0.26 (0.29) | 0.13 (0.16) | 0.16 (0.22) | −37.8 | −0.10 (0.33) | 0.0021 |
| Time (h) | 0.60 (0.90) | 0.20 (0.29) | 0.28 (0.42) | −52.3 | −0.31 (0.84) | 0.0002 |
| AUC (h x mg/dL) | 10.29 (19.42) | 2.40 (4.34) | 4.07 (6.89) | −60.5 | −6.22 (17.68) | 0.0004 |
| Glucose <3.1 mmol/L (55 mg/dL) within 24 h | ||||||
| Events | 0.36 (0.53) | 0.11 (0.17) | 0.16 (0.27) | −56.5 | −0.20 (0.49) | <0.0001 |
| Time (h) | 0.65 (1.20) | 0.13 (0.22) | 0.25 (0.45) | −62.0 | −0.40 (1.09) | 0.0002 |
| AUC (h x mg/dL) | 6.54 (14.00) | 0.95 (1.81) | 2.26 (4.67) | −65.4 | −4.28 (12.76) | 0.0007 |
| Glucose <3.1 mmol/L (55 mg/dL) at night (23.00–06.00) within 7 h | ||||||
| Events | 0.16 (0.23) | 0.05 (0.09) | 0.07 (0.12) | −55.5 | −0.09 (0.21) | <0.0001 |
| Time (h) | 0.30 (0.63) | 0.06 (0.14) | 0.12 (0.22) | −62.1 | −0.19 (0.57) | 0.0008 |
| AUC (h x mg/dL) | 3.30 (7.99) | 0.48 (1.26) | 1.10 (2.40) | −66.8 | −2.21 (7.28) | 0.0021 |
| Glucose <2.5 mmol/L (45 mg/dL) within 24 h | ||||||
| Events | 0.21 (0.39) | 0.04 (0.07) | 0.08 (0.17) | −61.7 | −0.13 (0.35) | 0.0002 |
| Time (h) | 0.34 (0.79) | 0.04 (0.08) | 0.11 (0.25) | −67.2 | −0.23 (0.73) | 0.0013 |
| AUC (h x mg/dL) | 1.63 (3.96) | 0.16 (0.37) | 0.51 (1.22) | −68.5 | −1.12 (3.67) | 0.0021 |
| Glucose <2.5 mmol/L (45 mg/dL) at night (23.00–06.00) within 7 h | ||||||
| Events | 0.09 (0.18) | 0.02 (0.07) | 0.04 (0.08) | −56.0 | −0.05 (0.15) | 0.0008 |
| Time (h) | 0.17 (0.46) | 0.02 (0.07) | 0.05 (0.14) | −68.8 | −0.12 (0.42) | 0.0032 |
| AUC (h x mg/dL) | 0.88 (2.45) | 0.09 (0.31) | 0.25 (0.69) | −71.5 | −0.63 (2.26) | 0.0045 |
| Time in hyperglycemia | ||||||
| Time >10.0 mmo/L (180 mg/dL) within 24 h | 8.58 (5.84) | 9.98 (4.68) | 9.20 (4.29) | 7.2 | 0.62 (4.94) | 0.1981 |
| Time >13.3 mmo/L (240 mg/dL) within 24 h | 2.94 (3.04) | 3.54 (3.48) | 2.96 (2.81) | 0.9 | 0.03 (3.17) | 0.9533 |
| Time >16.7 mmo/L (300 mg/dL) within 24 h | 0.72 (1.45) | 1.01 (1.93) | 0.75 (1.36) | 4.3 | 0.03 (1.53) | 0.8349 |
Fig. 2.
Difference from baseline for time in range and hypoglycemia measures at 12 months. Rescaled confidence intervals are confidence intervals for the difference from baseline expressed as a percentage of the baseline mean
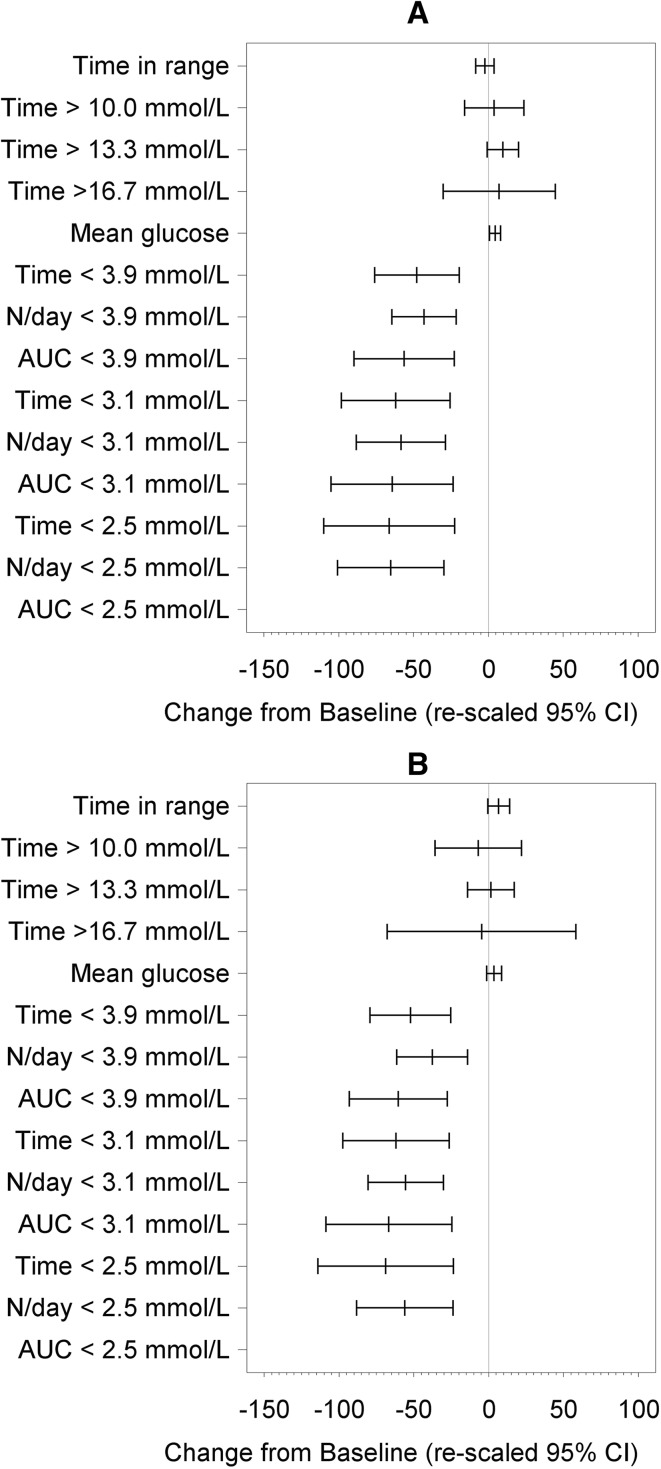
Fig. 3.
Summaries of all glycemic measures during the day (0600–2300) (a) and during the night (2300–0600) (b). Difference from baseline for glycemic measures at 12 months post-baseline. Rescaled confidence intervals are confidence intervals for the difference from baseline expressed as a percentage of the baseline mean
Time in hypoglycemia [glucose <3.9 mmol/L (70 mg/dL)] was reduced by 50% (−0.70 ± 1.85 h/day; mean ± SD) at 12 months post-baseline compared with baseline (p = 0.0002).
Time in hypoglycemia [glucose <3.1 mmol/L (55 mg/dL)] was reduced by 62% (−0.40 ± 1.09 h/day) at 12 months post-baseline compared with baseline (p = 0.0002).
Time in hypoglycemia [glucose <2.5 mmol/L (45 mg/dL)] was reduced by 67% (−0.23 ± 0.73 h/day) at 12 months post-baseline compared with baseline (p = 0.0013).
Nocturnal hypoglycemia [glucose <3.9 mmol/L (70 mg/dL), 2300–0600 hours] was reduced by 52% (−0.31 ± 0.84 h per 7 h) at 12 months post-baseline compared with baseline (p = 0.0002) (Fig. 3).
Daytime hypoglycemia [glucose <3.9 mmol/L (< 70 mg/dL), 0600–2300 hours] was reduced by 48% (−0.38 ± 1.18 h per 17 h) at 12 months post-baseline compared with baseline [p = 0.0011 (Fig. 3).
The frequency of events with glucose at <3.9 mmol/L (70 mg/dL) was reduced by 41% (−0.27 ± 0.67, mean ± SD) at 12 months compared with baseline (p < 0.0001). The frequency of events with glucose at <3.1 mmol/L (55 mg/dL) was reduced by 56% (−0.20 ± 0.49, p < 0.0001), and that of events with glucose at <2.5 mmol/L (45 mg/dL) by 62% (−0.13 ± 0.35) compared with baseline (p = 0.0002).
A difference for area under the curve of 58% (−12.73 ± 34.53 h/day × mg/dL, mean ± SD) for sensor glucose level of <3.9 mmol/L (70 mg/dL) was observed at 12 months compared to baseline (p = 0.0002). For sensor glucose levels of <3.1 mmol/L (55 mg/dL) and <2.5 mmol/L (45 mg/dL), the area under the curve was reduced by 65% (−4.28 ± 12.76 h/day × mg/dL, p = 0.0007) and by 69% (−1.12 ± 3.67 h/day × mg/dL p = 0.0021), respectively.
At 12 months post-baseline there was no difference in time in hyperglycemia [>10.0 mmol/L (180 mg/dL), >13.3 mmol/L (240 mg/dL), and >16.7 mmol/L (300 mg/dL)] compared to baseline (p = 0.1981, p = 0.9533, and p = 0.8349, respectively, Fig. 2).
There was also no difference in time in glucose range 3.9–10.0 mmol/L (70–180 mg/dL)] between baseline and 12 months post-baseline (p = 0.8519) or change in glycemic variability [p = 0.1324; Table 2 and Fig. 2]. The mean glucose level increased from 9.1 ± 1.8 to 9.4 ± 1.5 mmol/L (p = 0.0409).
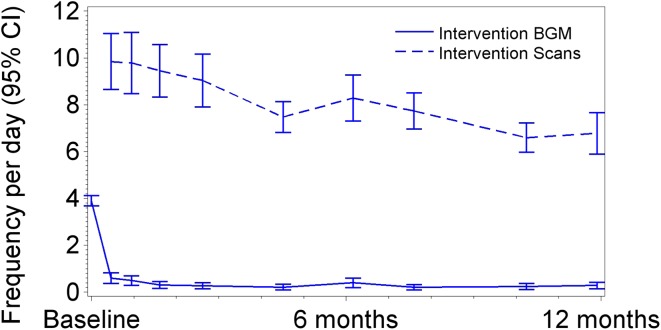
For the participants who continued into the open-access phase, mean SMBG frequency was 3.9 ± 1.2 (SD) tests/day (median 3.9 tests/day) at baseline, falling to a mean of 0.6 ± 1.2 tests/day (median 0.1) when participants first had full access to sensor glucose data (days 15–31, treatment phase). The mean overall blood glucose monitoring rate for the 6-month treatment phase was 0.3 ± 0.7 tests/day (median 0.1), further reducing to 0.2 ± 0.6 tests/day (median 0.0) during the open-access phase (Fig. 4).
Fig. 4.
Blood glucose monitoring tests and sensor scans frequency per day by study period. BGM Blood glucose monitoring
Average sensor-scanning frequency was 7.1 ± 3.5 times/day (median 5.7) during the open-access phase compared to 8.4 ± 4.6 during the 6-month treatment phase (median 6.8 times/day) (Fig. 4). There was no correlation between increased frequency of sensor scanning and reduction in time in hypoglycemia [<3.9 mmol/L (70 mg/dL)] or hyperglycemia [>13.3 mmol/L (240 mg/dL)] between the baseline phase and end of the open-access phase (12 months). Mean device use (defined as the percentage of data collected, assuming continuous device wear) was 83.6% ± 13.8 (median 88.3%) between 6 and 12 months and 88.7 ± 9.2% (median 90.7%) in the treatment phase.
Participants’ total daily insulin doses recorded at the penultimate visit (day 284) were unchanged compared to either baseline (p = 0.4827) or 6-months post-baseline (p = 0.7220).
For those participants aged <65 years (n = 60; 56%) and those aged ≥65 years (n = 48; 44%), significant reductions in time spent in hypoglycemia [<3.9 mmol/L (70 mg/dL), <3.1 mmol/L (55 mg/dL) and <2.5 mmol/L (45 mg/dL)] were observed at the end of the open-access phase (12 months) compared to the baseline phase (Fig. 5).
Fig. 5.
Summaries of glycemic measures for participants aged <65 years (a) and aged ≥65 years (b). Difference from baseline for glycemic measures at 12 months. Rescaled confidence intervals are confidence intervals for the difference from baseline expressed as a percentage of the baseline mean
Safety
The flash sensor-based system was used for an overall duration of 12 months by participants. During the open-access phase three cardiac events were reported, none of which were related to the study device or study procedure as the three participants had a previous history of cardiovascular disease prior to study entry. There were no reports of diabetic ketoacidosis or hyperosmolar hyperglycemic state.
In total, serious adverse or adverse events (n = 135) were experienced by 60 (43%) of 139 participants. There were nine occurrences of a serious adverse event, none of which were related to the device, study procedure, or to hypoglycemia. Nine mild hypoglycemia adverse events were experienced by two participants and were reported by the clinician as not related to the study device at all or to the study procedure. Five participants experienced an adverse event in the open-access phase, leading to withdrawal from the study; two due to death (not associated with the device or study) and three due to sensor insertion/site reaction. Nine participants reported 16 device-related adverse events; four severe, nine moderate and three mild. These were all sensor-adhesive or site reactions, primarily treated with topical preparations and all were resolved.
Anticipated sensor insertion site symptoms refer to those typically expected using a sensor device and equate to symptoms normally experienced with blood glucose finger-stick testing, such as pain, bleeding, bruising. There were 134 anticipated sensor insertion site symptoms observed for 28 (20%) participants. These symptoms were primarily (n = 117; 87%) due to the sensor wear (erythema, itching and rash) and most were resolved without medical intervention; 63 were mild in nature, 67 were moderate and four were severe. Adverse events and anticipated symptoms associated with the insertion of the sensor and sensor wear are summarized in Table 3 and Table S1 in the supplementary material.
Table 3.
Adverse events
| Adverse events | Open-access Phase Participants (N = 139) |
|---|---|
| Participants with adverse or serious adverse events | 60 (43.2%) |
| Number of adverse events (excluding serious events) | 126 |
| Participants with serious adverse events | 7 (5.0%) |
| Number of serious adverse events | 9a |
| Participants with hypoglycemic serious adverse events | 0 (0%) |
| Number of hypoglycemic serious adverse events | 0 |
| Participants with hypoglycemic adverse events | 2 (1.4%) |
| Number of hypoglycemic adverse events | 9 |
| Participants with device-related adverse events | 9 (6.5%) |
| Number of device-related adverse events | 16 |
| Participants discontinuing due to adverse events | 5 (3.6%)b |
| Number of adverse events leading to discontinuation | 5 |
Values in table are presented as a number with/without the percentage in parenthesis
aThis number includes seven serious adverse events reported in the 6-month treatment phase results [9]
bIn addition, 2 subjects withdrew during the open-access phase due to adverse events experienced during the 6-month treatment phase
Discussion
The REPLACE study was the first to publish data investigating the use of flash sensor-based glucose technology as a replacement for standard SMBG in individuals with type 2 diabetes treated with multi-dose insulin therapy. The findings from the treatment phase of the study have demonstrated that flash glucose monitoring technology is a safe replacement for blood glucose monitoring and that the use of the technology is associated with reduced time in hypoglycemia, particularly nocturnal hypoglycemia [9]. During the additional 6 month open-access phase, reductions in time in hypoglycemia were maintained across all age groups, with sustained benefit continued during nighttime. Our findings from a further 6 months of using flash glucose sensing technology reinforce those from the 6 months of use in the REPLACE study [9], demonstrating that the device is safe with repetitive, consecutive use over an extended period of 12 month for adults, irrespective of age, and that the benefit of reduced hypoglycemia is sustained. In addition, our data reinforce the significant reductions in hypoglycemia shown by Bolinder et al. in the IMPACT study for adults with well-controlled type 1 diabetes using flash technology [11].
The American Diabetes Association (ADA) has resisted defining hypoglycemia numerically as all abnormally low glucose events are potentially harmful [2, 12]. However, both the ADA and the European Association for the Study of Diabetes (EASD) consider glucose levels below 3 mmol/L (54 mg/dL) as serious and clinically important [13] due to the associated risks of cardiac arrhythmias [14, 15] and mortality in type 2 diabetes [16, 17]. Notably, our findings of significantly less time in overall hypoglycemia included time at the lower glucose thresholds, and this benefit was maintained over 12 months. Furthermore, a 30% reduction in hypoglycemia is considered to be clinically significant [2], and the use of flash technology reduced time in hypoglycemia [3.9 mmol/L (70 mg/dL)] by 50% at 12 months compared to baseline.
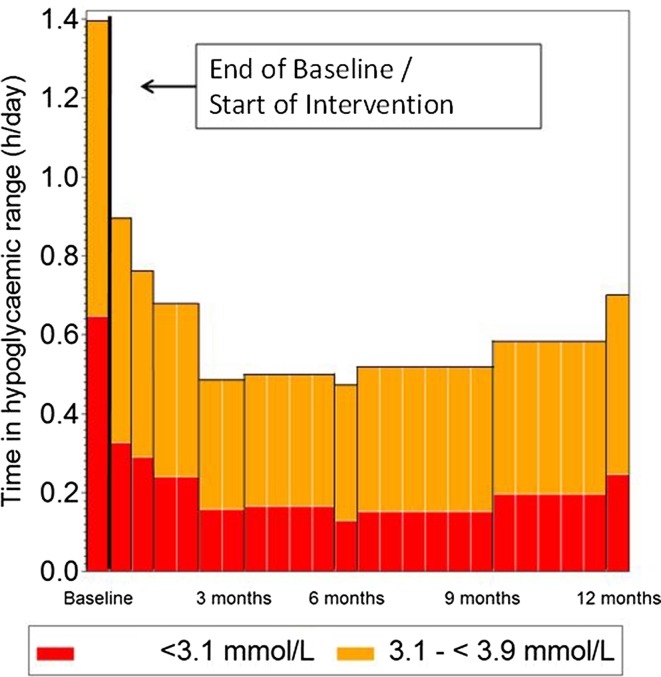
Time in hypoglycemia began to decrease as soon as participants were able to utilize sensor glucose readings for self-management (day 15 of the treatment phase; Fig. 6) and was significantly reduced at 12 months.
Fig. 6.
Significantly reduced time in hypoglycemia is observed as soon as sensor glucose results can be utilized by the participants at the end of the baseline phase and is sustained for 12 months
No change in insulin doses was observed, suggesting that the trend arrow with numerical and graphical sensor glucose information displayed on the reader is of value to support self-management of hypoglycemia detection, prevention and avoidance. The value of continuous monitoring data for self-care modification rather than therapy adjustments has been noted previously for individuals with type 2 diabetes treated with oral therapies [18] and basal insulin [19].
High deployment of the device continued with a utility rate of 84%, and sensor scanning frequency averaged seven times daily with virtually no recourse to blood glucose testing. There was no difference in device use or scanning rate for those younger or older than 65 years, demonstrating confident use of the technology across all adult age groups, which supports current recommendations that those over 65 years should have access to continuous monitoring technology [1].
At the end of the treatment phase, all of the intervention subjects opted to continue into the open-access phase and highly concordant use of the sensor continued. This suggests that flash glucose technology is acceptable as a method of glucose monitoring and that it does not appear to have the same nuisance [20, 21] and variable concordance issues that can be experienced with longer term CGM use [1].
There was no difference in change for time in hyperglycemia compared to baseline at the end of the open-access phase. Of interest, time in hyperglycemia had risen during the treatment phase and subsequently dropped back to baseline values at 12 months. Mean glucose had also risen during the treatment phase and dropped back towards baseline values at 12 months. These apparent rises during the treatment phase were not statistically significantly different to the control group at 6 months. Highly speculative reasoning for this is that previously undetected hypoglycemia, particularly nocturnal, may affect a retrospective fear of hypoglycemia reoccurrence, prompting a resistance to treatment intensification to address hyperglycemia. Although there was no significant change in fear of hypoglycemia in the intervention group compared to the control group [9], any hypoglycemia and especially nocturnal is feared by those with type 2 diabetes [3] and this may partially explain why there was no change in time in hyperglycemia as it was not actively addressed with therapy adjustments.
Similar to the treatment phase, there were no safety concerns during the 12-month-long open-access phase. Skin reactions were reported for nine (6.5%) participants during the open-access phase and six participants (4.0%) in the treatment phase (preceding 6 months). With any medical device that is attached to the body, skin reactions will be experienced by some individuals. Longer duration of sensor wear likely contributes to this [22]. There is little published data on using a device attached to the body with medical grade adhesive; the type of events in our study are similar to those reported for use of flash technology in adults with type 1 diabetes over 6 months [11] and for other systems with on-body sensor use [20, 21].
The original randomized, controlled trial conducted over 6 months included intervention and control participants; only intervention participants continued into the open-access phase for a further 6 months. This is a limitation to our study; however, the primary endpoint of the open-access phase was to assess safety over an extended period of use. A final HbA1c measurement, although of clinical interest, has little value when evaluating safety or the overall effectiveness of flash technology. Similarly, quality of life and patient-reported outcome questionnaires could also have been completed at 12 months. In all phases of the study, our aim was to test the new technology in “real world” conditions. Having restrictive protocols for treatment changes would have made general applicability of our data uncertain. Therefore, this work is limited by the modification of insulin therapy according to local practice rather than using a treatment algorithm. Our inclusion of only adults with type 2 diabetes treated with intensive insulin therapy who performed regular glucose testing means future studies are needed to assess the effectiveness of flash glucose-sensing technology in younger, less concordant, individuals for modifying insulin therapy.
Conclusion
In summary, the use of sensor glucose readings over a 12- month period for glucose self-management by individuals with type 2 diabetes treated with intensive insulin therapy was associated with significant and sustained reductions in hypoglycemic measures across all age groups with no safety concerns. Our findings confirm that longer term use of the convenient flash glucose sensing technology is safe and effective and eliminates the need for standard SMBG for glycemic management of type 2 diabetes treated by intensive insulin therapy with multiple daily injections or CSII.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Anticipated sensor insertion site symptoms. (PDF 189 kb)
Acknowledgements
Sponsorship for this study, provision of study devices, all study materials and article processing charges were funded by Abbott Diabetes Care, Witney, UK. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript and take responsibility for the integrity of the work as a whole. Professor Thomas Haak wrote the first draft of the manuscript and together with all the co-authors worked collaboratively to write, discuss and review this manuscript which was revised and edited by Dr. Ramzi Ajjan. All named authors collectively took the decision to submit it for publication and have given final approval to the version to be published.
The authors thank the participants for their involvement in the study, are grateful to those who contributed to the collection of data at the REPLACE study sites and to Zoe Welsh (Abbott Diabetes Care) for statistical support.
Disclosures
Thomas Haak reports personal fees from Abbott Diabetes Care outside the submitted work; Gerry Rayman reports personal fees from Abbott Diabetes Care outside the submitted work; Hélène Hanaire reports personal fees from Abbott Diabetes Care and Medtronic, and grants from Johnson and Johnson outside the submitted work; Ramzi Ajjan reports other funding from Abbott Diabetes Care during the conduct of the study and personal fees from Abbott Diabetes Care outside the submitted work; Norbert Hermanns reports grants and personal fees from Abbott Diabetes Care Germany, grants from Dexcom, grants and personal fees from Berlin-Chemie, grants from Ypsomed, personal fees and non-financial support from Novo Nordisk, and grants from Lilly International, outside the submitted work; and Jean-Pierre Riveline reports grants outside the submitted work.
Compliance with Ethics Guidelines
Approval was given by the appropriate competent authorities in each country. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. Informed consent was obtained from all patients for being included in the study.
Data Availability
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced content To view enhanced content for this article go to http://www.medengine.com/Redeem/99F7F06029979785.
References
- 1.American Diabetes Association Standards of medical care in diabetes 2017. Diabetes Care. 2017;40(Supple 1):S52. [Google Scholar]
- 2.Workgroup on hypoglycemia, American Diabetes Association Defining and reporting hypoglycemia in diabetes: a report from the American Diabetes Association Workgroup on Hypoglycemia. Diabetes Care. 2005;28:1245–1249. doi: 10.2337/diacare.28.5.1245. [DOI] [PubMed] [Google Scholar]
- 3.Khunti K, Alsifri S, Aronson R, Cigrovski Berkovic M, Enters-Weijnen C, Forsen T, Galstyan G, Geelhoed-Duijvestijn P, Goldfracht M, Gydesen H, Kapur R, Lalic N, Ludvik B, Moberg E, Pedersen-Bjergaard U, Ramchandran A (the HAT Investigator Group) Rates and predictors of hypoglycemia in 27,585 people from 24 countries with insulin-treated type 1 and type 2 diabetes: the global HAT study. Diabetes Obes Metab. 2016;18:907–915. doi: 10.1111/dom.12689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holman RR, (4-T Study Group) et al. Three-year efficacy of complex insulin regimens in type 2 diabetes. N Engl J Med. 2009;361(18):1736–1747. doi: 10.1056/NEJMoa0905479. [DOI] [PubMed] [Google Scholar]
- 5.McCoy RG, Lipska KJ, Yao X, Ross JS, Montori VM, Shah ND. Intensive treatment and severe hypoglycemia among adults with type 2 diabetes. JAMA Intern Med. 2016;176(7):969–978. doi: 10.1001/jamainternmed.2016.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Toschi E, Wolpert H. Utility of continuous glucose monitoring in type and type 2 diabetes. Endocrinol Metab Clin. 2016;45(4):895–904. doi: 10.1016/j.ecl.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 7.Peters AL, Ahmann AJ, Battelino T, Evert A, Hirsch IB, Hassan Murad M, Winter WE, Wolpert H. Diabetes technology—continuous subcutaneous insulin infusion and continuous glucose monitoring in adults: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2016;101(11):3922–3937. doi: 10.1210/jc.2016-2534. [DOI] [PubMed] [Google Scholar]
- 8.Zick R, Petersen B, Richter M, Haug C, (The SAFIR Study Group) Comparison of continuous blood glucose measurement with conventional documentation of hypoglycemia in patients with type 2 diabetes on multiple daily insulin injection therapy. Diabetes Technol Therap. 2007;9(6):483–492. doi: 10.1089/dia.2007.0230. [DOI] [PubMed] [Google Scholar]
- 9.Haak T, Hanaire H, Ajjan RA, Hermanns N, Riveline J-P, Rayman G. Flash glucose-sensing technology as a replacement for blood glucose monitoring for the management of insulin-treated type 2 diabetes: a multicenter, open-label randomized controlled trial. Diabetes Therapy. 2017;8:55–73. [DOI] [PMC free article] [PubMed]
- 10.FreeStyle Libre Software. www.FreeStyleLibre.com. Accessed 11 Dec 2016.
- 11.Bolinder J, Antuna R, Geelhoed-Duijvestijn, Kröger J, Weitgasser R. Novel glucose-sensing technology and hypoglycemia in type 1 diabetes: a multicentre, non-masked, randomised controlled trial. Lancet. 2016;388:2254–2263. doi: 10.1016/S0140-6736(16)31535-5. [DOI] [PubMed] [Google Scholar]
- 12.Seaquist ER, Anderson J, Childs B, Cryer P, Dagogo-Jack S, Fish L, Heller SR, Rodriguez H, Rosenzweig J, Vigersky R. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care. 2013;36(5):1384–1395. doi: 10.2337/dc12-2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.American Diabetes Association and the European Association for the Study of Diabetes Glucose concentrations of less than 3.0 mmol/l (54 mg/dl) should be reported in clinical trials: a joint position statement. Diabetalogia. 2017;60:3–6. doi: 10.1007/s00125-016-4146-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chow E, Bernjak A, Williams S, Elaine, Fawdry RA, Hibbert S, Freeman J, Sheridan PJ, Heller SR. Risk of cardiac arrhythmias during hypoglycemia in patients with type2 diabetes and cardiovascular risk. Diabetes. 2014;63:1738–1747. doi: 10.2337/db13-0468. [DOI] [PubMed] [Google Scholar]
- 15.Pistrosch F, Ganfacz X, Bornstein SR, Birkenfeld AL, Henkel E, Hanefeld M. Risk of and risk factors for hypoglycemia and associated arrhythmias in patients with type 2 diabetes and cardiovascular disease; a cohort study under real world conditions. Acta Diabetol. 2015;52:889–895. doi: 10.1007/s00592-015-0727-y. [DOI] [PubMed] [Google Scholar]
- 16.Bonds DE, Miller ME, Bergenstal RM, Buse JB, Byington RP, Cutler JA, Dudl RJ, Ismail-Beigi F, Kimel AR, Hoogwerf B, Horowitz KR, Savage PJ, Seaquist ER, Simmons DL, Sivitz WI, Speril-Hillen JM, Sweeney ME. The association between symptomatic, severe hypoglycemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ. 2010;340:b4909. doi: 10.1136/bmj.b4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Outcomes Reduction with an Initial Glargine Intervention (ORIGIN) Investigators Does hypoglycemia increase the risk of cardiovascular events? A report from the ORIGIN trial. Euro Heart J. 2013;34:3137–3144. doi: 10.1093/eurheartj/eht332. [DOI] [PubMed] [Google Scholar]
- 18.Ehrhardt NM, Chellappa M, Walker SM, Fonda SJ, Vigersky RA. The effect of real-time continuous glucose monitoring on glycemic control in patients with type 2 diabetes mellitus. J Diabetes Sci Technol. 2011;5(3):668–675. doi: 10.1177/193229681100500320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vigersky RA, Fonda SJ, Chellappa M, Walker SM, Ehrhardt NM. Short- and long-term effects of real-time continuous glucose monitoring in patients with type 2 diabetes. Diabetes Care. 2012;35:32–38. doi: 10.2337/dc11-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramchandani N, Arya S, Ten S, Bhandari S. Real-life utilization of real-time continuous glucose monitoring: the complete picture. J Diabetes Sci Technol. 2011;5:860–867. doi: 10.1177/193229681100500407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wong JC, Foster NC, Maahs DM, et al. Real-time continuous glucose monitoring among participants in the T1D exchange registry. Diabetes Care. 2014;37:2702–2709. doi: 10.2337/dc14-0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heinemann L, Kamann S. Adhesives used for diabetes medical devices: a neglected risk with serious consequences? J Diabetes Sci Technol. 2016;10:1211–1215. doi: 10.1177/1932296816662949. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Anticipated sensor insertion site symptoms. (PDF 189 kb)
Data Availability Statement
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.