Abstract
Introduction
Patients with diabetes type 1 (DM1) struggle daily to achieve good glucose control. The last decade has seen a rush of research groups working towards an artificial pancreas (AP) through the application of a double subcutaneous approach, i.e., subcutaneous (SC) continuous glucose monitoring (CGM) and continuous subcutaneous insulin infusion. Few have focused on the fundamental limitations of this approach, especially regarding outcome measures beyond time in range.
Methods
Based on insulin physiology, the limitations of CGM, SC insulin absorption, meal challenge, and physical activity in DM1 patients, we discuss the limitations of the double SC approach. Finally, we discuss safety measures and the achievements reported in some recent AP studies that have utilized the double SC approach.
Results
Most studies show that a double SC AP increases the time in range compared to a sensor-augmented insulin pump and shortens the time in hypoglycemia. Despite these achievements, the proportion of time spent in hyperglycemia is still roughly 20–40%, and hypoglycemia is still present 1–4% of the time. The main factors limiting further progress are the latency of SC CGM (at least 5–10 min) and the slow pharmacokinetics of SC-delivered fast-acting insulin. The maximum blood insulin level is reached after 45 min and the maximum glucose-lowering effect is observed after 1.5–2 h, while the glucose-lowering effect lasts for at least 5 h.
Conclusions
Although using a double SC AP leads to significant improvements in glucose control, the SC approach has severe limitations that hamper further progress towards a robust AP.
Keywords: Artificial pancreas, Continuous glucose monitoring, Continuous subcutaneous insulin infusion, Type 1 diabetes
Introduction
Patients with diabetes mellitus type 1 (DM1) are totally dependent on external insulin to adjust their glucose levels to ensure that they are within the safe range that avoids diabetic ketoacidosis or serious hypoglycemia [1, 2]. Due to the adverse effects of chronically elevated glucose levels, patients with DM1 are further advised to aim for glucose levels that approach the physiologically normal range, but this increases the risk of hypoglycemia in most patients [3–5]. While attempting to shift glucose levels to keep them within the therapeutic range, oscillations between the extremes of hyper- and hypoglycemia are common, even in experienced patients, and some patients do not regain control despite spending significant effort to do so [6, 7]. In many patients, this persistent focus on glucose levels and insulin administration has a major impact on their quality of life, with some ending up on sick leave or drawing a disability pension [8–10]. Severe hypoglycemia is a fearful experience as it can have serious—even life-threatening—consequences [11, 12].
To help patients to achieve optimal glucose control, researchers are constantly hunting for new treatment options and accessory devices. The state of the art treatment for DM1 is continuous subcutaneous insulin infusion (CSII) combined with continuous glucose monitoring (CGM), which makes the patient continuously aware of their current glucose levels and allows them to adjust their insulin delivery accordingly [13]. However, it is still not entirely clear whether the use of CGM enables patients to reduce their glucose levels over the long term, and it is a more costly surveillance method than traditional capillary measurements [14]. In recent years, CGM technology has been incorporated into CSII technology, enabling insulin delivery to be suspended during hypoglycemic episodes that occur during unawareness or sleep [15, 16]. However, even when this treatment is used, most patients still have glucose levels that are far into the diabetic range.
The dream of an artificial pancreas (AP) for the fully automatic delivery of insulin in patients with DM1 has persisted for decades, starting in the 1970s [17–19]. When the first device for subcutaneous CGM became commercially available in the year 2000, the dream gained new momentum [20]. At present, at least 20 major research groups around the world are working on making the dream of an AP come true [21–37]. Most of these groups concentrate on what can be termed the double subcutaneous (SC) approach, combining SC CGM and CSII into a single device [38]. A double SC approach has the advantage that off-the-shelf technology can be used for CGM and insulin delivery by an insulin pump. However, it is often a major challenge for the control algorithm to maintain glucose levels within the therapeutic range when using this approach. The most recent development in this field is the FDA approval of a double subcutaneous approach using a proportional-integral-derivative (PID) algorithm, where insulin doses are tailored according to the output of the CGM [39–41]. Ideally, a robust (fully automatic) AP controls the administration of insulin such that glucose levels are maintained in the therapeutic range and hypo- and hyperglycemic episodes are avoided without the need for ongoing user input (i.e., announcements of meals/physical activity). Although AP systems based on the double SC approach represent significant progress, this approach has certain limitations that hamper the performance of these systems to such an extent that, in our view, they (or, at least, not those systems based on the technology available in the foreseeable future) cannot maintain glucose levels permanently in the normal or near-normal range, which is required of a robust, fully automatic AP. This paper discusses those physiological and technological limitations and their consequences for the possibility of realizing a robust double SC AP that normalizes or near-normalizes glucose levels in patients with DM1.
Methods
We performed a PubMed search for recent publications (published between January 2014 and December 31, 2016) regarding closed-loop control in the outpatient setting, using the following search terms: [(Outpatient [Title] or outpatient [Title] or home [Title] or Home [Title] or Free-Living [Title] or free-living [Title] or Free-living [Title] or Camp [Title] or camp [Title] or Safety [Title] or safety [Title]) and (Pancreas [Title] or pancreas [Title] or Closed-Loop [Title] or closed-loop [Title] or Closed-loop [Title]) and (Diabetes [Title] or diabetes [Title] or Insulin [Title] or insulin [Title])]. The results of our search are shown in Tables 1 and 2.
Table 1.
Results of the PubMed search for long-term studies (≥5 days) published between 2014 and 2016 on continuous closed-loop versus open-loop control during the day and night in the outpatient setting
| Study | Included (N) |
HbA1c (%) | Evaluated (N) |
Observation period | Physical activity during CL period | CL period | CGM | Algorithm | Defined glucose range (mmol/L) |
Time in hypoglycaemia (%) | Time in euglycemia (%) |
Time in hyperglycemia (%) |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OL | CL | ||||||||||||
| Leelarathna [35] | 21 adults | 7.6 | 17 | 7 d | 7 d | Advised not to drive or to undertake strenuous physical exercise under CL | 83.0% of the total time |
FreeStyle Navigator; Abbott |
Control to range MPC | [3.9, 10] | 3.7 CL vs 5.0 OL (ns)# | 74.5 CL vs 61.8 OL (s)# | 21.9 CL vs 30.5 OL (s)# |
| Russellab [29] | 20 adults | 7.1 | 20 | 5 d | 5 d | NR | 18–18 | G4 Platinum, Dexcom | Control to target MPC | [3.9, 10] | 4.1 CL vs 7.3 OL (s) | 79.5 CL vs 58.8 OL (s | 16.5 CL vs 33.8 OL (s) |
| Russellab [29] | 32 adolescents | 8.2 | 32 | 5 d | 5 d | NR | 18–18 | G4 Platinum, Dexcom | Control to target MPC | [3.9, 10] | 3.1 CL vs 4.9 OL (ns) | 75.9 CL vs 64.5 OL (s) | 21.0 CL vs 30.6 OL (s) |
| Ly¶ [36] | 21 adults/adolescents | 8.6 | 10 adults/adolescents in CL and 10 adults/adolescents in OL | 6 d | 6 d | NR |
07–07 (93% of total time during day and night) |
4 s sensor CL, Enlite sensor OL, Medtronic | PID | [3.9, 10] | 2.1 CL vs 2.4 OL (ns) | 69.9 CL vs 73.1 OL (ns) | 28.4 CL vs 24.8 OL (ns) |
| Thabit [30] | 33 adults | 8.5 | 32 CL, 33 OL | 12 w | 12 w | CL not used during physical activity during the initial 2 weeks |
00–24 (20.2 h/day#) |
FreeStyle Navigator, Abbott | Control to range MPC | [3.9, 10] | 2.9 CL vs 3.0 OL (s) | 67.7 CL vs 56.8 OL (s) | 29.2 CL vs 38.9 OL (s) |
| De Bock [37] | 8 adults/adolescents | 7.5 | 8 | 5 d | 5 d | NR | 00–24 |
Enlite II, MiniLink REAL-time sensor, Medtronic |
PID | [4.0, 9.9] | NI | 67.6 CL vs 58.7 OL (ns)# | NI |
| Renard [31] | 20 adults | 8.2 | 20 | 1 m | 1 m | NR | 00–24 | G4 Platinum, Dexcom | Control to target MPC | [3.9, 10] | 1.9 CL vs 3.2 OL (s) | 64.7 CL vs 59.7 OL (s) | 33.3 CL vs 37.0 (ns) |
| Bergenstal¶¶ [39] | 124 adults/adolescents | 7.4 | 124 | 2 w | 3 m | NR |
87.2% of the total time |
Enlite III, Medtronic | PID-IFB | (3.9, 10] | 3.3 CL vs 5.9 OL (NS) | 72.2 CL vs 66.7 OL (NS) | 24.5 CL vs 27.4 OL (NS) |
The studies had no restrictions regarding meals
HbA1c values and values for the percentage of time that glucose was within the defined range are reported as mean values unless they are labeled as median values
s significant, p ≤ 0.05
ns nonsignificant, p > 0.05
# Median, d days, w weeks, m months, CGM continuous glucose measurement, CL closed loop, OL open loop, NR no restrictions, NS not specified, MPC model predictive control, PID-IFB proportional integral derivative insulin feedback, NI no information
¶ During the day, research staff accompanied the participant
¶¶ During the CL period, the initial 6 days were spent in a hotel
aInsulin combined with the use of glucagon in CL modus
bIn adults, the OL period was performed in the outpatient setting, while the CL period was performed in an 8-km2 area accompanied by study staff, with the night spent in a hotel, where blood samples were drawn every 30 min. In adolescents, the OL and CL periods were performed in a diabetes camp
Table 2.
Results of the PubMed search for long-term studies (≥5 days) with eligible data published between 2014 and 2016 on continuous closed-loop versus open-loop control during the day
| Study | Included (N) | HbA1c (%) | Evaluated (N) |
Observation period | Physical activity during CL period | CL period | CGM | Algorithm | Defined glucose range (mmol/L) |
Time in hypoglycemia (%) | Time in euglycemia (%) |
Time in hyperglycemia (%) |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OL | CL | ||||||||||||
| Leelarathna [35] | 21 adults | 7.6 | 17 | 7 d | 7 d | Advised not to drive or to undertake strenuous physical exercise under CL, but usual daily activities were allowed |
83.0% of the total time (day and night) |
FreeStyle Navigator; Abbott |
Control to range MPC | [3.9, 10] | – | 72.5 CL vs 65.4 OL (s)# | – |
| Thabit [30] | 33 adults | 8.5 | 32 CL, 33 OL | 12 w | 12 w | CL not used during physical activity during the initial 2 weeks | 08.00–24.00 | FreeStyle Navigator, Abbott | Control to range MPC | [3.9, 10] | 3.0 CL vs 2.7 OL (ns) | 62.9 CL vs 56.2 OL (s) | 33.9 CL vs 39.7 OL (s) |
| De Bock [37] | 8 adults/adolescents | 7.5 | 8 | 5 d | 5 d | NR | NI | Enlite II, MiniLink REAL-time sensor,Medtronic | PID | [4.0, 9.9] | NI | 66.7 CL vs 57.5 OL (ns)# | NI |
| Renard [31] | 20 adults | 8.2 | 20 | 1 m | 1 m | NR | 08.00–20.00 | G4 Platinum, Dexcom | Control to target MPC | [3.9, 10] | 2.3 CL vs 3.4 OL (s) | 64.9 CL vs 60.7 OL (ns) | 32.8 CL vs 35.8 OL (ns) |
The studies had no restrictions regarding meals
s significant, p ≤ 0.05
ns nonsignificant, p > 0.05
HbA1c values and values for the percentage of time that glucose was within the defined range are reported as mean values unless they are labeled as median values
#Median, d days, w weeks, m months, CGM continuous glucose measurement, CL closed loop, OL open loop, NR no restrictions, NS not specified, MPC model predictive control, PID-IFB proportional integral derivative insulin feedback, NI no information
The search retrieved 30 studies, and the articles were screened for whether they contained day-and-night data or day-only data in adolescents/adults with certain glucose ranges during at least five consecutive days of closed-loop control. Secondly, the reference lists of the seven articles containing day and night data were screened to see if we could find any further day and night studies lasting at least five consecutive days in the period of interest, but we found none.
Although this search and screening strategy was far from infallible, we believe that it found most of the studies published during this period that delivered outpatient closed-loop data during the day or both the day and night for at least a five-day period, and we also performed a cross-check with the online database of the Doyle group (http://thedoylegroup.org/apdatabase/).
Compliance with Ethics Guidelines This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by any of the authors.
Progress in the Development of the Double SC Approach
In parallel with the development of an insulin-suspend function, several groups have developed and refined associated algorithms, some of which are now commonly employed for the double subcutaneous AP approach.
One frequently used algorithm is the proportional-integral-derivative (PID) control method, which is commonly used in industrial applications. It takes into account the deviation from a target glucose value (the proportional term), the past values of the deviation (integral term), and the rate of change in the deviation (the derivative term) [36–39].
The most commonly used algorithm is model predictive control (MPC), which predicts the system’s behavior and calculates the optimal insulin infusion (optimal with respect to, for example, the deviation from the target glucose level, the deviation from a target zone, the insulin expenditure, etc.). Some MPC algorithms also take into account factors such as meal/carbohydrate input, variable insulin sensitivity, and recent glucose excursions when tailoring the dose of fast-acting insulin [23, 27–31, 35]. Most MPC algorithms aim to achieve a predefined glucose target, some aim to keep the glucose level within a particular range (“zone MPC”), and some do both.
Most recent studies lasting at least five consecutive days in the outpatient setting have explored overnight glucose control, and most of those studies showed significant improvements in the time spent within either a narrow or wider range of glucose levels (3.9–8.0 or 3.9–10.0 mmol/L, respectively), ranging from 50 to 85%, when using closed-loop control [21, 29–35]; other studies found no difference from open-loop control [36, 37]. In addition, many of these studies showed that less time during the night was spent in hypoglycemia [21, 29–31, 34], while others found that there was no difference in time spent in hypoglycemia from that obtained with open-loop control [30, 32, 33, 36].
In contrast to the many overnight studies that have been performed, only a few studies have explored outpatient closed-loop control during both the day and the night.
Whereas around 85% of the time was spent within the therapeutic range in some of the night-time studies [29, 32], the best achievement in the day-and-night studies was around 75–80% of the time spent in the therapeutic range [29, 35]. Among the seven day-and-night closed-loop control studies included in Table 1, four found that more time was spent within the therapeutic range [29–31, 35], three found that less time was spent in hypoglycemia [29–31], and three found that less time was spent in hyperglycemia [29, 30, 35] than when open-loop control was employed. The longest day-and-night study with closed-loop control lasted 12 weeks [30].
At present, several studies with even longer periods of outpatient double SC closed-loop control are ongoing. While there are many studies which show that double subcutaneous closed-loop control is better at keeping blood glucose within the therapeutic range and is safer (considering the time spent in hypo- and hyperglycemia) than traditional open-loop control, improved average glucose levels are not a uniform finding when double subcutaneous closed-loop control is applied.
The Influence of Latency on Control System Performance
“Latency” and similar terms that are used in the AP literature relate to several dynamic phenomena that are of fundamental importance in a closed-loop control system such as an AP. We therefore offer a brief and pragmatic explanation of the most prominent concepts—namely time delays and time constants—to aid readers who are not familiar with dynamic systems theory [42]. The applicability of time constants in the present context is justified by the fact that the human glucose metabolic system is predominantly linear within its normal operating range and can thus be well approximated by linear models [43].
A time delay τ (cf. Fig. 1b) is typically associated with the transportation of a substance over a certain distance (e.g., the transport of glucose or insulin through the circulatory system or through tissue), and it denotes the time that passes from the application of a stimulus to a system until the first trace of the incident is theoretically detectable in the system’s output.
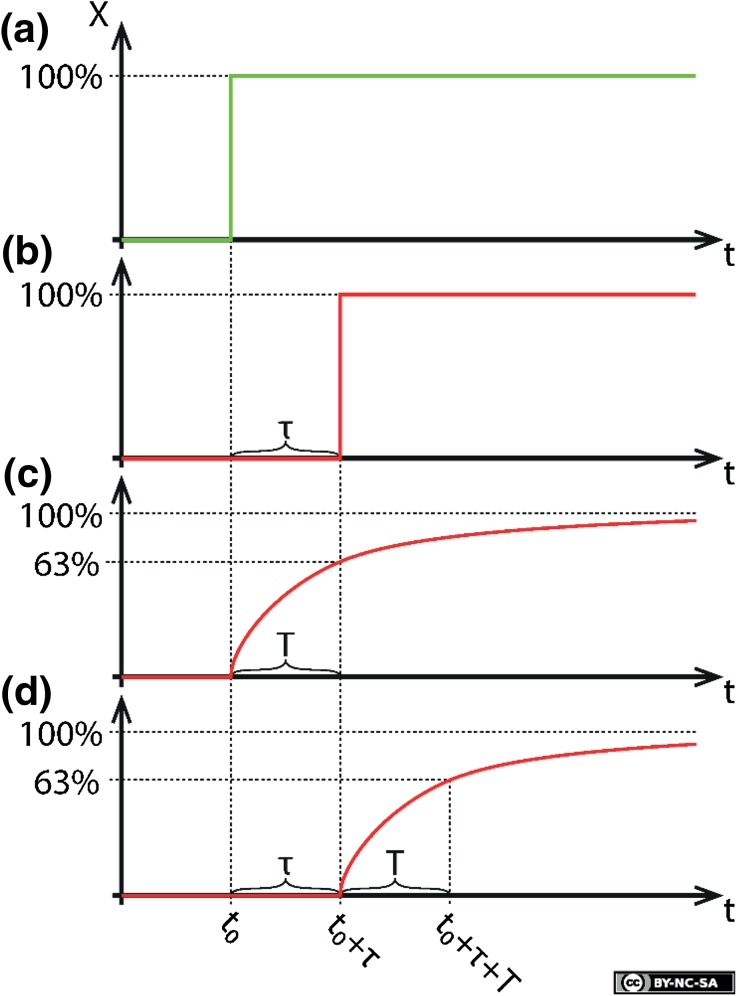
Fig. 1a–d.
Illustration of the concepts of a time delay (τ) and a time constant (T). The system stimulus is shown in a, while the other figures illustrate the output from a system that presents b a time delay, c a time constant, and d both. This figure is licensed for publication under a Creative Commons BY-NC-SA 4.0 license
A time constant T (Fig. 1c) is associated with, for example, the diffusion of a substance across a thin, semipermeable barrier separating two compartments, each of which has a uniform concentration of the substance (e.g., the diffusion of glucose or insulin across cell membranes, or across a protective membrane separating a glucose sensor from the surrounding fluid). A concentration gradient across the barrier will result in the net transport of the substance towards the region of lowest concentration, which gradually reduces the gradient until a dynamic equilibrium is reached. The time constant T associated with this process is the time from the initial establishment of the gradient until the concentration has reached 63% of its asymptotic level.
The effect of a time constant can in part be negated by appropriately filtering the output signal; this is limited only by the signal-to-noise ratio. In contrast, time delays cannot be negated in real time because this would require the ability to predict future events.
When it comes to closed-loop control, both time delays and time constants limit the obtainable bandwidth (i.e., the usable frequency range) of a closed-loop system. In an AP system exhibiting multiple time constants and/or delays (Fig. 1d) in the path from infused insulin to measured glucose, it is the sum of all the time delays and the larger time constant(s) that limit how quickly the AP can respond in order to correct glucose level deviations; larger latencies inevitably lead to larger glucose level fluctuations. This fact is at the core of this paper’s message.
Insulin Physiology
In a healthy nondiabetic person, insulin is released from the β cells in the pancreas 2–3 min after the first increase in circulating glucose [44, 45]. This first phase of insulin response consists of the release of stored insulin and proinsulin from granules within the β cells. After about 10 min, a second and much stronger phase of insulin release commences. This phase consists of freshly synthesized insulin in response to the increased glucose levels sensed by the β cells, and lasts until the glucose levels normalize. At the end of this process, meal-initiated insulin secretion is shut off and insulin secretion is kept at a low level to maintain a steady glucose level. This biphasic pattern is only seen during fast changes in glucose levels as achieved by intravenous (IV) glucose infusions; it is absent during oral glucose loads, where only the second phase of insulin release is observed [45–47].
In the fasting state, insulin is typically released in bursts 4–5 min apart, and the half-life of insulin in the blood is about 11 min. During the fasting state, approximately 70–75% of the insulin is secreted in a pulsatory manner in a canine model [48, 49]. Between these bursts, there is little or no release of insulin from the β cells. It is not known how this release of insulin from β cells is synchronized. However, this pulsatile pattern may be an important factor in the effect of insulin at the cellular level, as has been shown for other hormones with pulsatile release. The pulsatile delivery of insulin to the liver may also be important for increasing hepatic insulin sensitivity and achieving the full effect of insulin [48, 50, 51]. In healthy lean subjects without diabetes, approximately 50% of the insulin appearing in the peripheral circulation is delivered during the basal state in-between meals [52].
Insulin from the pancreatic β cells is released to the portal vein and arrives at the liver at a high concentration. During the passage through the liver, much of the insulin is absorbed in what is called the first-passage effect. Recent data indicate that as much as 80% of the insulin delivered in the bursts is absorbed, while as little as 20% is absorbed between bursts [53, 54]. This means that the exposure of the hepatocytes to insulin probably oscillates to a significantly greater degree than is reflected in the insulin-level oscillations in the portal and systemic circulation. The significance of this phenomenon is unknown. However, it may be important to the inhibition of hepatic glycogenolysis, the stimulation of glucose uptake from the blood arriving from the gastrointestinal tract, and the inhibition of hepatic gluconeogenesis. It has been suggested that the liver adapts its ability to withhold insulin to the levels in the portal circulation and the levels needed to suppress glucose in the systemic circulation [55–57].
After an oral glucose load or a normal meal in a healthy, lean, physically active person, both glucose and insulin will return to their starting levels within 120–150 min [58]. However, in overweight or obese subjects, it may take longer to return to the normal values [52]. In healthy subjects, this means that glucose and insulin will be at, or close to, their steady-state levels during shorter daytime periods and most of the night [52].
One of the important counterregulatory mechanisms to avoid hypoglycemia is the release of glucagon from pancreatic α cells. This mechanism is blunted in patients with long-standing DM1, and although the α cells retain their ability to produce glucagon, the factors inducing the release of glucagon during hypoglycemia seem to be absent [59, 60].
Subcutaneous Continuous Glucose Monitoring
To our knowledge, all commercially available glucose monitoring systems measure glucose in SC tissue using enzymes belonging to the oxidoreductase family. The oxidation of glucose is followed by an electrochemical or optical assay procedure to derive the glucose concentration by measuring the enzymatic products using an amperometric or a chemoluminescent technique, depending on the assay [61, 62].
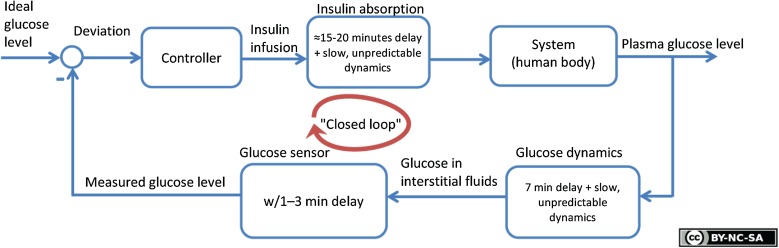
There is a physiologic latency from the point in time when a certain glucose level is present in the blood until the same level is reached in the interstitial fluid (ISF) in SC tissue [63–66] (Fig. 2). This latency is at least 6–7 min, but the physiologic latency between blood and ISF in SC tissue may be 15 min or more in some patients with DM1 [67–69]. Further, there is normally sensor latency, which is the latency between the appearance of glucose in the ISF surrounding the sensor and its consequent appearance in the sensor signal (Fig. 2). This sensor latency may consist of a reaction time (e.g., if glucose needs to react with enzymes or other substances in a typical amperometric sensor), a diffusion time (e.g., the time taken to fill a probe volume in a spectroscopic sensor), or other sources of latency such as the computation time of the sensor’s software. In addition, commercially available SC CGM devices do not perform truly continuous glucose measurements [70]. Most devices provide an average of the glucose level over the last 5 min once every 5 min. This averaging procedure is commonly used to reduce the effect of short-term random noise (which is due to both intrinsic properties of the sensor and extrinsic random factors originating from the sensor’s chemical and physical environment) as well as to save battery capacity. Slowly varying signal variations due to, for example, biofouling are typically taken care of by repeatedly calibrating the sensor during its lifetime. Nevertheless, some recent CGM technology does not require frequent capillary calibrations [71].
Fig. 2.
Illustration of an artificial pancreas based on the double subcutaneous approach, including sources of delay. Reproduced with permission from [101]
The overall latency from physiologic and sensor-based sources may range from at least 8–10 min to above 20 min, which is far longer than the timescale required for real-time sensing of circulating glucose excursions experienced by healthy β cells. Some of these dynamics may be compensated for by the algorithm used to transform the raw sensor signal into an estimated glucose concentration. However, none of the companies in the CGM field publish their algorithms, so an external independent evaluation of the potential and limitations of this approach is not possible. The precision and accuracy of CGM have steadily improved, but such measurements still present limitations [72]. Although certain anthropometric measures (height, weight, skin thickness) seem to have little influence on accuracy, the impact of the foreign body response potentially limits the longevity of transcutaneous sensors [62, 70]. Further, although improved in the latest versions of CGM devices, the local ISF glucose level and thus the observed SC CGM sensor accuracy relative to blood glucose may be significantly influenced by local effects such as skin temperature, movements, the foreign body reaction, and pressure [73]. SC CGM devices typically show reduced accuracy for the first couple of days after insertion [62, 74]. This limitation should not be overlooked for a sensor with a usable lifetime of seven days. Certain drugs are also known to interfere with the measurements; for instance, the commonly used drug acetaminophen disturbs the enzyme-based CGM such that it leads to the overestimation of glucose levels in the ISF [75–77]. In addition, the mean average relative difference (MARD) between the measured and true glucose levels will be larger during periods of rapidly changing blood glucose than during periods of more slowly changing glucose levels [78]. An important safety issue is that current CGMs show their lowest concordance when capillary glucose is measured in the lower or hypoglycemic range. Although evaluations of the hypoglycemic range are often missing from reports evaluating CGM, there are examples which demonstrate that deviations from capillary measurements are highest when measurements are in the hypoglycemic range as compared to other ranges [79–82]. In other words, improvements to CGM are needed before it truly reflects blood glucose levels in a robust and reliable way.
One aspect of SC CGM that has hardly been discussed is the inflammatory reaction that is initiated whenever a foreign body is introduced into SC tissue [62, 83]. Patients experience this as a drift in sensor readings during the period in which they wear the sensor, and this partly explains why sensors need to be calibrated regularly. After decades of SC insulin infusion, most patients with DM1 have varying degrees of SC fibrous tissue formation at insulin injection sites. The fibrous-tissue-initiating effect of decades of SC CGM use adds to the fibrous tissue formation induced by SC insulin infusion. Most patients that are using SC CGM have used it for less than a decade. Accordingly, one might expect less predictable SC insulin absorption in patients with DM1 when they have been using this new technology in combination with SC insulin infusion for decades.
One challenge when designing an AP is the need for redundancy in glucose sensing. Ideally, this should be achieved by sensing glucose via two completely different techniques in two different tissues. Some AP groups have introduced redundancy by using two identical CGMs concomitantly [22, 23, 26–28]. A downside of this is that it is a weak kind of redundancy and a probable cause of increasing long-term fibrous tissue formation.
Subcutaneous Insulin Delivery
State of the art insulin delivery in patients with DM1 is continuous injection from an insulin pump via a flexible plastic cannula inserted into subcutaneous tissue [84] (“CSII”). The insertion of a foreign body into subcutaneous tissue leads to a foreign body reaction. This effect can be reduced if the cannula is substituted and inserted at a different site after a few days. Local skin complaints (pain, lipohypertrophy) at insertion sites are quite common among insulin-treated patients, where lipohypertrophy is a consequence of the ability of insulin to stimulate local growth. A more important effect is that the pharmacokinetics of insulin uptake are probably altered in these patients, although the results of the few studies that address this issue are conflicting [85]. In the clinical setting, patients who have had DM1 for decades often report that their meal bolus expires earlier, or the opposite—that it has a delaying effect [86].
Importantly, fibrous tissue formation may cause varying and unpredictable absorption of insulin, and it is a major cause of insufficient blood glucose control and unpredictable hypoglycemic episodes. Varying insulin absorption can also occur independent of any foreign body reaction or fibrous tissue formation [86]. After a single injection of the fastest-acting “meal” insulin, the maximum insulin concentration in the blood is reached after 45 min, and the insulin level in the blood is still markedly increased after 3 h [87]. More importantly, the glucose-lowering effect is at its maximum between 90 and 120 min after injection, and this effect is still present after more than 5 h.
Although rapid-acting insulin yields more stable results when delivered by CSII as compared to intermittent SC bolus deposits, considerable day-to-day variation is still observed [88]. This intra-individual effect may vary from hour to hour, and may depend on the local skin temperature (which affects subcutaneous blood flow and hence insulin absorption [89]). The same may be true of physical activity, as it results in increased SC blood flow (to get rid of heat) and induces motion due to the vigorous use of the underlying muscles. The accumulation of SC fibrous tissue after years or decades of insulin injections may markedly increase insulin absorption variability. There is also interindividual variation, which may increase the need for individually adjusted algorithms in APs.
When using CSII, there is also the challenge of transporting the insulin from the ampulla in the device to the SC injection site. This is prone to several errors, which could be down to the user or due to equipment failure [90].
Physical Activity
As with all patients, physical activity increases insulin sensitivity in patients with DM1 [91]. This is frequently observed in DM1 patients who regularly exercise in the evening. Hypoglycemic episodes often occur in such patients later in the evening or during the night. However, “unexplained” hypoglycemic episodes are also often experienced the next morning or later the next day. This may even happen in cases where the patient has increased food intake and/or reduced insulin doses due to the exercise.
There is no official recommendation regarding the amount or type of carbohydrate patients with DM1 should ingest to avoid hypoglycemia during exercise. A clamp study found IV glucose demand changed as the level of physical exercise was increased consecutively. The glucose infusion rate was increased at a moderately intense level of exercise, while the need for IV glucose disappeared during high-intensity exercise [92]. The latter illustrates the complexity involved in attempting to take physical activity into account, as the need for insulin can vary during exercise in ways that are difficult to predict.
It was recently reported that the effects of physical exercise on blood glucose depend on the nature of the physical exercise; i.e., its duration, degree of vigorousness, and the type of physical exercise performed [93]. It seems that the glucose level initially drops more quickly during aerobic activity than resistance activity, but resistance activity leads to a longer-lasting lowering of the glucose after the activity is finished [94]. Research groups who have accounted for physical activity in their algorithms have achieved lower hypoglycemic episode rates during physical activity, but long-term studies are needed to check that these lowered rates do not come at the cost of more time spent in hyperglycemia [95–97].
Any AP with a feedback controller exclusively based on blood glucose levels would need to accept markedly less satisfactory glucose control in order to avoid both short- and medium-term hypoglycemic episodes due to physical activity. In particular, this problem would become more pressing if unpredicted major exercise was to be performed within the first few hours after a meal, as fast-acting “meal” insulin lowers glucose even 5 h after administration. Due to the relatively long half-life of SC-delivered insulin, this issue is difficult to resolve unless a compensating factor such as glucagon is applied [22, 29, 98].
Meals
Most studies of AP have used meal announcement, which involves the patient either deciding the amount of insulin given with a meal or estimating the amount of carbohydrates and leaving it to the algorithm of the AP to suggest the insulin to be given with the meal [21, 23–35]. Meal-related insulin administration is a major challenge for both an AP and patients with DM1.
Only two smaller studies by a Dutch group have tested a bihormonal (insulin and glucagon) AP without meal announcements and user-dependent insulin determination [22, 98]. In the first study, an AP was used for only two days in a limited number of highly surveyed patients [22]. Interestingly, mean glucose levels did not improve compared to the those obtained with the usual open-loop treatment, while night-time glucose levels improved.
In healthy nondiabetic individuals, the first phase of insulin release (see the “Insulin Physiology” section) is probably important for restricting the total meal-induced increase in glucose. The larger release of insulin seen after about 10 min (the second phase) is essential for handling the increase in glucose within a couple of hours. Thus, it is essential for an AP to be able to identify a meal as soon as possible after the meal has been initiated if it is to deliver insulin early enough to achieve proper control of meal-induced glucose excursions. The time until automated meal detection is significantly influenced by the latency of the SC CGM if the detection algorithm is based on these commonly available measurements.
Measures Implemented to Overcome Safety Issues
Several studies of double SC AP have used two SC glucose sensors, where the second sensor signal is not actively used until the first sensor fails [23, 26–28]. One study did apply a rule where an alarm was triggered if the difference between the two sensors exceeded >20% when the glucose level was lower than 8 mmol/L, or if the difference between the sensors exceeded >1.5 mmol/L when the glucose level was above 8 mmol/L [22].
Some control methods follow certain safety rules; for instance, only a partial (pre-meal) bolus is given at the start of the meal in order to avoid postprandial hypoglycemia, and an extra post-meal bolus is delivered if hyperglycemia becomes imminent [26, 28]. Other efforts to avoid hypoglycemia allow the algorithm to adapt its insulin sensitivity factor based on the rate of change in postprandial glucose excursions, and/or to use a “withhold action threshold” by defining an upper limit for the rate of change in insulin delivery as compared to baseline insulin delivery [22, 28]. Some control methods also try to achieve safe insulin delivery at decreasing glucose values by, for instance, implementing a time-limited suspend at a defined hypoglycemic limit when hypoglycemia is present or imminent, and/or by simply alerting the user to the need to ingest carbohydrates at low glucose values [25, 26]. Some groups apply glucagon, typically by giving increasing pulses of glucagon at certain glucose “action thresholds” defined by glucose levels or defined changes in the rate of decrease in glucose [22, 29]. Other control methods also account for the potential “stacking” of previously delivered insulin [25–27, 29].
Discussion
Most of the studies published in this field have used an AP based on the double SC approach, and most of those studies were designed to evaluate performance during the night; there have been relatively few day-time studies (although some of the night-time studies included a short post-breakfast period) [21, 23–41]. Long-term studies (≥5 days) show that time in the euglycemic state (defined as glucose levels between 3.9 and 8–10 mmol/L) is increased when double SC AP is applied rather than a control period with sensor-augmented pumps (SAP) (Tables 1, 2 and 3). Some (but not all) studies show that time spent in hypoglycemia is decreased by the use of double SC AP. In addition, most studies show a significant reduction in the time spent in hyperglycemia during the use of double SC AP, but the time spent in hyperglycemia was still around 20–30% in most of the long-term studies shown in Tables 1 and 2. Interestingly, when an extended long-term study compared the benefit of one month of 24-h double SC CL with the results obtained in a previous trial (six weeks of nightly CL versus SAP), no additional benefit of 24-h CL as compared to night-time double SC CL was found [31].
Table 3.
Advantages of the double subcutaneous approach
| Anticipation of changes in insulin demand during the day and night (in contrast to sensor-augmented CSII) |
| More time spent in the therapeutic range (i.e., less time spent in the hypoglycemic and hyperglycemic ranges) as compared to sensor-augmented CSII |
| Easily managed by the user on a daily basis |
| Based on available off the shelf technology |
An approach utilizing glucagon to counteract the progression of glucose lowering towards hypoglycemia achieved a notable increase in euglycemia time during day and night, but this approach required increased consumption of insulin [29].
There are two major limitations of the double SC approach that hamper progression towards the ideal AP (Table 4 and 5). First, there are the latencies associated with SC CGM based on presently available technology. The combined physiological and sensor-based latency is at least 10 min, but may be more in some patients. Combined with the fact that the accuracy and robustness of CGM are still not optimal, this means that it takes time for the AP to identify a meal-induced increase in glucose levels and initiate a meal dose of insulin. In any case, this will be too late to mimic both the first phase and the early part of the second phase of the physiological meal-induced insulin increase seen in healthy nondiabetic people. Perhaps equally important is the fact that it takes even more time to supply the liver with blood saturated with the quantity of insulin tailored to stimulate glucose uptake and suppress gluconeogenesis.
Table 4.
Limitations of an artificial pancreas based on the double subcutaneous approach
| Low accuracy of glucose measurements, especially at lower glucose levels |
| User-dependent calibrations of continuous glucose measurement |
| Delayed insulin absorption (results in a delay in the onset of the effect of insulin when demanded) |
| Unpredictable variations in subcutaneous insulin absorption |
| Ongoing effect (“tail”) of previously delivered insulin when glucose approaches hypoglycemic levels |
| Still requires user input regarding meal bolus delivery and estimation of carbohydrate and fat contents of meals |
| Varying improvement in average glucose levels |
Table 5.
Possible ways to improve the double subcutaneous approach
| Use faster and more accurate ways of measuring glucose |
| Employ several identical sensors or a mixture of several different sensor technologies (“sensor fusion”) |
| Use sensors that require less frequent calibration |
| Use sensors with longer durations/lifespans |
| Employ faster-acting insulins |
| Provide meal detection tools |
| Tools for detecting physical activity |
Secondly, and probably more importantly, the slow absorption of insulin from SC tissue and the substantially delayed glucose-lowering effect is still present 5 h after the SC delivery of insulin. This means that any insulin dose that will regularly normalize glucose levels within a couple of hours after a meal (to the same degree as in healthy people) will induce serious late postprandial hypoglycemia if the patient does not ingest more food in the postprandial period. In fact, the need for many patients with DM1 to eat between meals to avoid hypoglycemia probably contributes to the increased body weight of DM1 patients compared to the nondiabetic population [99].
Mealtime excursions remain a challenge during the use of double SC AP. To our knowledge, only one study has been able to maintain daytime glucose control with the aid of double SC AP without including announcements of meals and physical activity [22]. That study, however, had several weaknesses, as it was small-sized, of short duration, suffered from technical problems in 30% of the participants, and had a rather high glucose threshold of 6.5 mmol/L before insulin delivery was started [22]. Such a high threshold for glucose delivery can act as a safety buffer to avoid hypoglycemia, compensating for the slow pharmacokinetics of SC-delivered insulin and the latency of glucose feedback, but it also implies that more time is spent in the hyperglycemic range.
These physiologic realities are reflected in the fact that most studies of AP report improved night-time glucose control (i.e., improvement in the near-fasting and fasting state). Data from daytime studies are often reported not as daytime-only data but in combined day-and-night datasets, which makes it difficult for the reader to distinguish the daytime effect from the night-time effect [29, 36, 39]. Time in hyperglycemia during the daytime in the few studies which explicitly report daytime data appears to be higher than the time in hyperglycemia reported for 24-h data (Table 2) [30, 31].
Lastly, but importantly, there is another challenge associated with the use of new invasive technologies such as the double SC approach which require user maintenance: user fatigue, which can increase with the number of systems (e.g., sensors) that must be maintained [100].
Conclusions
A fully automated robust AP that normalizes or near-normalizes glucose levels in patients with DM1 without risking serious hypoglycemia is difficult to achieve using the double SC approach (i.e., by measuring glucose and delivering insulin into subcutaneous tissue). The major obstacle is the slow and sometimes unpredictable absorption and thus the delayed glucose-lowering effect of SC-delivered insulin. However, SC glucose sensing also has an inherent and probably unavoidable physiologic latency, adding to the delay in SC insulin absorption.
The major challenge is that the present off-the-shelf technology used for the double SC approach cannot handle meal-induced glucose excursions without incurring a substantial risk of serious late postprandial hypoglycemia. Accordingly, patients, healthcare professionals, and insurance companies should not be given the impression that a robust AP that can normalize or near-normalize glucose levels by applying the double SC approach is within reach using current technology.
Acknowledgements
This review was supported by grant number 248872 from the Research Council of Norway. Funding for the article processing charges were provided by our employer, the Norwegian University of Science and Technology. All the authors are members of the Artificial Pancreas Trondheim (APT) research group (http://www.apt-norway.com/), which focuses on the double intraperitoneal approach for an artificial pancreas. All of the named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and gave final approval to the version to be published.
Disclosures
Anders Lyngvi Fougner has received funding from the Liaison Committee for Central Norway Regional Health Authority (RHA), grant numbers 46075401 and 46075403, respectively. Konstanze Kölle has also received funding from the Liaison Committee for Central Norway Regional Health Authority (RHA), grant numbers 46075401 and 46075403, respectively. APT cooperates with Prediktor Medical AS, a company in the field of glucose monitoring. Sven M. Carlsen is an advisory board member of Prediktor Medical AS. Reinold Ellingsen is a shareholder and board member of GlucoSet, another company in the field of glucose monitoring. Sverre Christian Christiansen and Øyvind Stavdahl have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies, and does not involve any new studies of human or animal subjects performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced Content
To view enhanced content for this article go to www.medengine.com/Redeem/E818F0605766600C.
References
- 1.Sovik O, Thordarson H. Dead-in-bed syndrome in young diabetic patients. Diabetes Care. 1999;22(Suppl 2):B40–B42. [PubMed] [Google Scholar]
- 2.Basu A, Close CF, Jenkins D, et al. Persisting mortality in diabetic ketoacidosis. Diabet Med. 1993;10:282–284. doi: 10.1111/j.1464-5491.1993.tb00060.x. [DOI] [PubMed] [Google Scholar]
- 3.The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 4.Skyler JS. Diabetic complications. The importance of glucose control. Endocrinol Metab Clin North Am. 1996;25:243–254. doi: 10.1016/S0889-8529(05)70323-6. [DOI] [PubMed] [Google Scholar]
- 5.The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group Retinopathy and nephropathy in patients with type 1 diabetes 4 years after a trial of intensive therapy. N Engl J Med. 2000;342:381–389. doi: 10.1056/NEJM200002103420603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Le Floch JP, Kessler L. Glucose variability: comparison of different indices during continuous glucose monitoring in diabetic patients. J Diabetes Sci Technol. 2016;10:885–891. doi: 10.1177/1932296816632003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kohnert KD, Freyse EJ, Salzsieder E. Glycemic variability and pancreatic beta-cell dysfunction. Curr Diabetes Rev. 2012;8:345–354. doi: 10.2174/157339912802083513. [DOI] [PubMed] [Google Scholar]
- 8.Wandell PE. Quality of life of patients with diabetes mellitus. An overview of research in primary health care in the Nordic countries. Scand J Prim Health Care. 2005;23:68–74. doi: 10.1080/02813430510015296. [DOI] [PubMed] [Google Scholar]
- 9.Goldney RD, Phillips PJ, Fisher LJ, et al. Diabetes, depression, and quality of life: a population study. Diabetes Care. 2004;27:1066–1070. doi: 10.2337/diacare.27.5.1066. [DOI] [PubMed] [Google Scholar]
- 10.Davis RE, Morrissey M, Peters JR, et al. Impact of hypoglycemia on quality of life and productivity in type 1 and type 2 diabetes. Curr Med Res Opin. 2005;21:1477–1483. doi: 10.1185/030079905X61929. [DOI] [PubMed] [Google Scholar]
- 11.Barendse S, Singh H, Frier BM, et al. The impact of hypoglycemia on quality of life and related patient-reported outcomes in type 2 diabetes: a narrative review. Diabetes Med. 2012;29:293–302. [DOI] [PubMed]
- 12.Zoungas S, Patel A, Chalmers J, et al. Severe hypoglycemia and risks of vascular events and death. N Engl J Med. 2010;363:1410–1418. doi: 10.1056/NEJMoa1003795. [DOI] [PubMed] [Google Scholar]
- 13.Battelino T, Phillip M, Bratina N, et al. Effect of continuous glucose monitoring on hypoglycemia in type 1 diabetes. Diabetes Care. 2011;34:795–800. doi: 10.2337/dc10-1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodbard D. Continuous glucose monitoring: a review of successes, challenges, and opportunities. Diabetes Technol Ther. 2016;18(Suppl 2):S23–S213. doi: 10.1089/dia.2015.0417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bergenstal RM, Klonoff DC, Garg SK, et al. Threshold-based insulin-pump interruption for reduction of hypoglycemia. N Engl J Med. 2013;369:224–232. doi: 10.1056/NEJMoa1303576. [DOI] [PubMed] [Google Scholar]
- 16.Ly TT, Nicholas JA, Retterath A, et al. Effect of sensor-augmented insulin pump therapy and automated insulin suspension vs standard insulin pump therapy on hypoglycemia in patients with type 1 diabetes: a randomized clinical trial. JAMA. 2013;310:1240–1247. doi: 10.1001/jama.2013.277818. [DOI] [PubMed] [Google Scholar]
- 17.Marliss EB, Murray FT, Stokes EF, et al. Normalization of glycemia in diabetics during meals with insulin and glucagon delivery by the artificial pancreas. Diabetes. 1977;26:663–672. doi: 10.2337/diab.26.7.663. [DOI] [PubMed] [Google Scholar]
- 18.Mirouze J, Selam JL, Pham TC, et al. Evaluation of exogenous insulin homoeostasis by the artificial pancreas in insulin-dependent diabetes. Diabetologia. 1977;13:273–278. doi: 10.1007/BF01219712. [DOI] [PubMed] [Google Scholar]
- 19.Albisser AM, Leibel BS, Ewart TG, et al. Clinical control of diabetes by the artificial pancreas. Diabetes. 1974;23:397–404. doi: 10.2337/diab.23.5.397. [DOI] [PubMed] [Google Scholar]
- 20.Gross TM, Bode BW, Einhorn D, et al. Performance evaluation of the MiniMed continuous glucose monitoring system during patient home use. Diabetes Technol Ther. 2000;2:49–56. doi: 10.1089/152091500316737. [DOI] [PubMed] [Google Scholar]
- 21.Nimri R, Muller I, Atlas E, et al. MD-Logic overnight control for 6 weeks of home use in patients with type 1 diabetes: randomized crossover trial. Diabetes Care. 2014;37:3025–3032. doi: 10.2337/dc14-0835. [DOI] [PubMed] [Google Scholar]
- 22.van Bon AC, Luijf YM, Koebrugge R, et al. Feasibility of a portable bihormonal closed-loop system to control glucose excursions at home under free-living conditions for 48 hours. Diabetes Technol Ther. 2014;16:131–136. doi: 10.1089/dia.2013.0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Del Favero S, Place J, Kropff J, et al. Multicenter outpatient dinner/overnight reduction of hypoglycemia and increased time of glucose in target with a wearable artificial pancreas using modular model predictive control in adults with type 1 diabetes. Diabetes Obes Metab. 2015;17:468–476. doi: 10.1111/dom.12440. [DOI] [PubMed] [Google Scholar]
- 24.Phillip M, Battelino T, Atlas E, et al. Nocturnal glucose control with an artificial pancreas at a diabetes camp. N Engl J Med. 2013;368:824–833. doi: 10.1056/NEJMoa1206881. [DOI] [PubMed] [Google Scholar]
- 25.Reddy M, Herrero P, El Sharkawy M, et al. Feasibility study of a bio-inspired artificial pancreas in adults with type 1 diabetes. Diabetes Technol Ther. 2014;16:550–557. doi: 10.1089/dia.2014.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Capel I, Rigla M, Garcia-Saez G, et al. Artificial pancreas using a personalized rule-based controller achieves overnight normoglycemia in patients with type 1 diabetes. Diabetes Technol Ther. 2014;16:172–179. doi: 10.1089/dia.2013.0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmidt S, Boiroux D, Duun-Henriksen AK, et al. Model-based closed-loop glucose control in type 1 diabetes: the DiaCon experience. J Diabetes Sci Technol. 2013;7:1255–1264. doi: 10.1177/193229681300700515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dassau E, Brown SA, Basu A, et al. Adjustment of open-loop settings to improve closed-loop results in type 1 diabetes: a multicenter randomized trial. J Clin Endocrinol Metab. 2015;100:3878–3886. doi: 10.1210/jc.2015-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Russell SJ, El-Khatib FH, Sinha M, et al. Outpatient glycemic control with a bionic pancreas in type 1 diabetes. N Engl J Med. 2014;371:313–325. doi: 10.1056/NEJMoa1314474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thabit H, Tauschmann M, Allen JM, et al. Home use of an artificial beta cell in type 1 diabetes. N Engl J Med. 2015;373:2129–2140. doi: 10.1056/NEJMoa1509351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Renard E, Farret A, Kropff J, et al. Day-and-night closed-loop glucose control in patients with type 1 diabetes under free-living conditions: results of a single-arm 1-month experience compared with a previously reported feasibility study of evening and night at home. Diabetes Care. 2016;39:1151–1160. doi: 10.2337/dc16-0008. [DOI] [PubMed] [Google Scholar]
- 32.Hovorka R, Elleri D, Thabit H, et al. Overnight closed-loop insulin delivery in young people with type 1 diabetes: a free-living, randomized clinical trial. Diabetes Care. 2014;37:1204–1211. doi: 10.2337/dc13-2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thabit H, Lubina-Solomon A, Stadler M, et al. Home use of closed-loop insulin delivery for overnight glucose control in adults with type 1 diabetes: a 4-week, multicentre, randomised crossover study. Lancet Diabetes Endocrinol. 2014;2:701–709. doi: 10.1016/S2213-8587(14)70114-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kropff J, Del Favero S, Place J, et al. 2 month evening and night closed-loop glucose control in patients with type 1 diabetes under free-living conditions: a randomised crossover trial. Lancet Diabetes Endocrinol. 2015;3:939–947. doi: 10.1016/S2213-8587(15)00335-6. [DOI] [PubMed] [Google Scholar]
- 35.Leelarathna L, Dellweg S, Mader JK, et al. Day and night home closed-loop insulin delivery in adults with type 1 diabetes: three-center randomized crossover study. Diabetes Care. 2014;37:1931–1937. doi: 10.2337/dc13-2911. [DOI] [PubMed] [Google Scholar]
- 36.Ly TT, Roy A, Grosman B, et al. Day and night closed-loop control using the integrated Medtronic hybrid closed-loop system in type 1 diabetes at diabetes camp. Diabetes Care. 2015;38:1205–11. doi: 10.2337/dc14-3073. [DOI] [PubMed] [Google Scholar]
- 37.de Bock MI, Roy A, Cooper MN, et al. Feasibility of outpatient 24-hour closed-loop insulin delivery. Diabetes Care. 2015;38:e186–e187. doi: 10.2337/dc15-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peyser T, Dassau E, Breton M, et al. The artificial pancreas: current status and future prospects in the management of diabetes. Ann N Y Acad Sci. 2014;1311:102–123. doi: 10.1111/nyas.12431. [DOI] [PubMed] [Google Scholar]
- 39.Bergenstal RM, Garg S, Weinzimer SA, et al. Safety of a hybrid closed-loop insulin delivery system in patients with type 1 diabetes. JAMA. 2016;316:1407–1408. doi: 10.1001/jama.2016.11708. [DOI] [PubMed] [Google Scholar]
- 40.de Bock M, Dart J, Roy A et al. Exploration of the performance of a hybrid closed loop insulin delivery algorithm that includes insulin delivery limits designed to protect against hypoglycemia. J Diabetes Sci Technol. 2017;11:68–73. [DOI] [PMC free article] [PubMed]
- 41.Grosman B, Ilany J, Roy A, et al. Hybrid closed-loop insulin delivery in type 1 diabetes during supervised outpatient conditions. J Diabetes Sci Technol. 2016;10:708–713. doi: 10.1177/1932296816631568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Åstrøm KJ, Murray RM. Feedback systems: an introduction for scientists and engineers. Princeton: Princeton University Press; 2014.
- 43.Chee F, Fernando T. Closed-loop control of blood glucose. Berlin: Springer; 2007.
- 44.Kruszynska YT, Home PD, Hanning I, et al. Basal and 24-h C-peptide and insulin secretion rate in normal man. Diabetologia. 1987;30:16–21. doi: 10.1007/BF01788901. [DOI] [PubMed] [Google Scholar]
- 45.Caumo A, Luzi L. First-phase insulin secretion: does it exist in real life? Considerations on shape and function. Am J Physiol Endocrinol Metab. 2004;287:E371–E385. doi: 10.1152/ajpendo.00139.2003. [DOI] [PubMed] [Google Scholar]
- 46.Grodsky GM. A threshold distribution hypothesis for packet storage of insulin and its mathematical modeling. J Clin Invest. 1972;51:2047–59. [DOI] [PMC free article] [PubMed]
- 47.Sorenson RL, Lindell DV, Elde RP. Glucose stimulation of somatostatin and insulin release from the isolated, perfused rat pancreas. Diabetes. 1980;29:747–751. doi: 10.2337/diab.29.9.747. [DOI] [PubMed] [Google Scholar]
- 48.Porksen N, Munn S, Steers J, et al. Pulsatile insulin secretion accounts for 70% of total insulin secretion during fasting. Am J Physiol. 1995;269:E478–E488. doi: 10.1152/ajpendo.1995.269.3.E478. [DOI] [PubMed] [Google Scholar]
- 49.Porksen N, Nyholm B, Veldhuis JD, et al. In humans at least 75% of insulin secretion arises from punctuated insulin secretory bursts. Am J Physiol. 1997;273:E908–E914. doi: 10.1152/ajpendo.1997.273.5.E908. [DOI] [PubMed] [Google Scholar]
- 50.Paolisso G, Scheen AJ, Giugliano D, et al. Pulsatile insulin delivery has greater metabolic effects than continuous hormone administration in man: importance of pulse frequency. J Clin Endocrinol Metab. 1991;72:607–615. doi: 10.1210/jcem-72-3-607. [DOI] [PubMed] [Google Scholar]
- 51.Matthews DR, Naylor BA, Jones RG, et al. Pulsatile insulin has greater hypoglycemic effect than continuous delivery. Diabetes. 1983;32:617–621. doi: 10.2337/diab.32.7.617. [DOI] [PubMed] [Google Scholar]
- 52.Polonsky KS, Given BD, Van CE. Twenty-four-hour profiles and pulsatile patterns of insulin secretion in normal and obese subjects. J Clin Investig. 1988;81:442–448. doi: 10.1172/JCI113339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Navalesi R, Pilo A, Ferrannini E. Insulin kinetics after portal and peripheral injection of [125I] insulin: II. Experiments in the intact dog. Am J Physiol. 1976;230:1630–1636. doi: 10.1152/ajplegacy.1976.230.6.1630. [DOI] [PubMed] [Google Scholar]
- 54.Meier JJ, Veldhuis JD, Butler PC. Pulsatile insulin secretion dictates systemic insulin delivery by regulating hepatic insulin extraction in humans. Diabetes. 2005;54:1649–1656. doi: 10.2337/diabetes.54.6.1649. [DOI] [PubMed] [Google Scholar]
- 55.De Vos P, De Haan BJ, Vegter D, et al. Insulin levels after portal and systemic insulin infusion differ in a dose-dependent fashion. Horm Metab Res. 1998;30:721–725. doi: 10.1055/s-2007-978966. [DOI] [PubMed] [Google Scholar]
- 56.Eaton RP, Allen RC, Schade DS. Hepatic removal of insulin in normal man: dose response to endogenous insulin secretion. J Clin Endocrinol Metab. 1983;56:1294–1300. doi: 10.1210/jcem-56-6-1294. [DOI] [PubMed] [Google Scholar]
- 57.Porksen N, Munn SR, Steers JL, et al. Effects of somatostatin on pulsatile insulin secretion: elective inhibition of insulin burst mass. Am J Physiol. 1996;270:E1043–E1049. doi: 10.1152/ajpendo.1996.270.6.E1043. [DOI] [PubMed] [Google Scholar]
- 58.Geidenstam N, Spegel P, Mulder H, et al. Metabolite profile deviations in an oral glucose tolerance test—a comparison between lean and obese individuals. Obesity (Silver Spring). 2014;22:2388–95. [DOI] [PubMed]
- 59.Gerich JE, Langlois M, Noacco C, et al. Lack of glucagon response to hypoglycemia in diabetes: evidence for an intrinsic pancreatic alpha cell defect. Science. 1973;182:171–173. doi: 10.1126/science.182.4108.171. [DOI] [PubMed] [Google Scholar]
- 60.Boden G, Reichard GA, Jr, Hoeldtke RD, et al. Severe insulin-induced hypoglycemia associated with deficiencies in the release of counterregulatory hormones. N Engl J Med. 1981;305:1200–1205. doi: 10.1056/NEJM198111123052007. [DOI] [PubMed] [Google Scholar]
- 61.Ferri S, Kojima K, Sode K. Review of glucose oxidases and glucose dehydrogenases: a bird’s eye view of glucose sensing enzymes. J Diabetes Sci Technol. 2011;5:1068–1076. doi: 10.1177/193229681100500507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vaddiraju S, Burgess DJ, Tomazos I, et al. Technologies for continuous glucose monitoring: current problems and future promises. J Diabetes Sci Technol. 2010;4:1540–1562. doi: 10.1177/193229681000400632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Burnett DR, Huyett LM, Zisser HC, et al. Glucose sensing in the peritoneal space offers faster kinetics than sensing in the subcutaneous space. Diabetes. 2014;63:2498–2505. doi: 10.2337/db13-1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Basu A, Dube S, Veettil S, et al. Time lag of glucose from intravascular to interstitial compartment in type 1 diabetes. J Diabetes Sci Technol. 2015;9:63–68. doi: 10.1177/1932296814554797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Basu A, Dube S, Slama M, et al. Time lag of glucose from intravascular to interstitial compartment in humans. Diabetes. 2013;62:4083–4087. doi: 10.2337/db13-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stavdahl Ø, Fougner AL, Kölle K, et al. The artificial pancreas: a dynamic challenge. IFAC-PapersOnLine. 2016;49:765–72. doi: 10.1016/j.ifacol.2016.07.280. [DOI] [Google Scholar]
- 67.Blevins TC, Bode BW, Garg SK, et al. Statement by the American Association of Clinical Endocrinologists Consensus Panel on Continuous Glucose Monitoring. Endocr Pract. 2010;16:730–45. [DOI] [PubMed]
- 68.Facchinetti A, Sparacino G, Guerra S, et al. Real-time improvement of continuous glucose monitoring accuracy: the smart sensor concept. Diabetes Care. 2013;36:793–800. doi: 10.2337/dc12-0736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cobelli C, Schiavon M, Dalla MC, et al. Interstitial fluid glucose is not just a shifted-in-time but a distorted mirror of blood glucose: insight from an in silico study. Diabetes Technol Ther. 2016;18:505–511. doi: 10.1089/dia.2016.0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schmelzeisen-Redeker G, Schoemaker M, Kirchsteiger H, et al. Time delay of CGM sensors: relevance, causes, and countermeasures. J Diabetes Sci Technol. 2015;9:1006–1015. doi: 10.1177/1932296815590154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bailey T, Bode BW, Christiansen MP, et al. The performance and usability of a factory-calibrated flash glucose monitoring system. Diabetes Technol Ther. 2015;17:787–794. doi: 10.1089/dia.2014.0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Damiano ER, McKeon K, El-Khatib FH, et al. A comparative effectiveness analysis of three continuous glucose monitors: the Navigator, G4 Platinum, and Enlite. J Diabetes Sci Technol. 2014;8:699–708. [DOI] [PMC free article] [PubMed]
- 73.Helton KL, Ratner BD, Wisniewski NA. Biomechanics of the sensor–tissue interface—effects of motion, pressure, and design on sensor performance and foreign body response—part II: examples and application. J Diabetes Sci Technol. 2011;5:647–56. [DOI] [PMC free article] [PubMed]
- 74.Lodwig V, Kulzer B, Schnell O, et al. What are the next steps in continuous glucose monitoring? J Diabetes Sci Technol. 2014;8:397–402. doi: 10.1177/1932296814525825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Basu A, Veettil S, Dyer R, et al. Direct evidence of acetaminophen interference with subcutaneous glucose sensing in humans: a pilot study. Diabetes Technol Ther. 2016;18(Suppl 2):S243–S247. doi: 10.1089/dia.2015.0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Maahs DM, DeSalvo D, Pyle L, et al. Effect of acetaminophen on CGM glucose in an outpatient setting. Diabetes Care. 2015;38:e158–e159. doi: 10.2337/dc15-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Slama M, Veettil S, Norby B et al. Medication interference with continuous glucose monitoring devices: implications for the artificial endocrine pancreas (abstract for poster 907-P-2016). In: American Diabetes Association, editor. American Diabetes Association 76th Scientific Sessions; 2016 June 10–14; New Orleans, LA, USA. Rochester: ADA; 2016. p. 907. https://ada.scientificposters.com/epsAbstractADA.cfm?id=1.
- 78.Pleus S, Schoemaker M, Morgenstern K, et al. Rate-of-change dependence of the performance of two CGM systems during induced glucose swings. J Diabetes Sci Technol. 2015;9:801–807. doi: 10.1177/1932296815578716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zijlstra E, Heise T, Nosek L, et al. Continuous glucose monitoring: quality of hypoglycaemia detection. Diabetes Obes Metab. 2013;15:130–135. doi: 10.1111/dom.12001. [DOI] [PubMed] [Google Scholar]
- 80.Zschornack E, Schmid C, Pleus S, et al. Evaluation of the performance of a novel system for continuous glucose monitoring. J Diabetes Sci Technol. 2013;7:815–823. doi: 10.1177/193229681300700403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kropff J, Bruttomesso D, Doll W, et al. Accuracy of two continuous glucose monitoring systems: a head-to-head comparison under clinical research centre and daily life conditions. Diabetes Obes Metab. 2015;17:343–349. doi: 10.1111/dom.12378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mader JK, Hajnsek M, Aberer F et al. Standardized evaluation of three continuous glucose monitoring systems under routine clinical conditions (abstract for poster 870-P-2016). In: American Diabetes Association, editor. American Diabetes Association 76th Scientific Sessions; 2016 June 10–14; New Orleans, LA, USA. Rochester: ADA; 2016. p. 870. https://ada.scientificposters.com/epsAbstractADA.cfm?id=1.
- 83.Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol. 2008;20:86–100. doi: 10.1016/j.smim.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pickup JC. Insulin-pump therapy for type 1 diabetes mellitus. N Engl J Med. 2012;366:1616–1624. doi: 10.1056/NEJMct1113948. [DOI] [PubMed] [Google Scholar]
- 85.Heinemann L, Krinelke L. Insulin infusion set: the Achilles heel of continuous subcutaneous insulin infusion. J Diabetes Sci Technol. 2012;6:954–964. doi: 10.1177/193229681200600429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Guerci B, Sauvanet JP. Subcutaneous insulin: pharmacokinetic variability and glycemic variability. Diabetes Metab. 2005;31:4S7–4S24. doi: 10.1016/S1262-3636(07)70166-0. [DOI] [PubMed] [Google Scholar]
- 87.Heise T, Hovelmann U, Brondsted L, et al. Faster-acting insulin aspart: earlier onset of appearance and greater early pharmacokinetic and pharmacodynamic effects than insulin aspart. Diabetes Obes Metab. 2015;17:682–688. doi: 10.1111/dom.12468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.De Vries JH, Snoek FJ, Kostense PJ, et al. A randomized trial of continuous subcutaneous insulin infusion and intensive injection therapy in type 1 diabetes for patients with long-standing poor glycemic control. Diabetes Care. 2002;25:2074–80. doi: 10.2337/diacare.25.7.1259. [DOI] [PubMed] [Google Scholar]
- 89.Hildebrandt P, Sejrsen P, Nielsen SL, et al. Diffusion and polymerization determines the insulin absorption from subcutaneous tissue in diabetic patients. Scand J Clin Lab Invest. 1985;45:685–90. [DOI] [PubMed]
- 90.Deiss D, Adolfsson P, Alkemade-van Zomeren M, et al. Insulin infusion set use: European perspectives and recommendations. Diabetes Technol Ther. 2016;18:517–524. doi: 10.1089/dia.2016.07281.sf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zinman B, Ruderman N, Campaigne BN, et al. Physical activity/exercise and diabetes mellitus. Diabetes Care. 2003;26(Suppl 1):S73–S77. doi: 10.2337/diacare.26.2007.s73. [DOI] [PubMed] [Google Scholar]
- 92.Shetty VB, Fournier PA, Davey RJ, et al. Effect of exercise intensity on glucose requirements to maintain euglycemia during exercise in type 1 diabetes. J Clin Endocrinol Metab. 2016;101:972–980. doi: 10.1210/jc.2015-4026. [DOI] [PubMed] [Google Scholar]
- 93.Tonoli C, Heyman E, Roelands B, et al. Effects of different types of acute and chronic (training) exercise on glycaemic control in type 1 diabetes mellitus: a meta-analysis. Sports Med. 2012;42:1059–1080. doi: 10.1007/BF03262312. [DOI] [PubMed] [Google Scholar]
- 94.Yardley JE, Kenny GP, Perkins BA, et al. Resistance versus aerobic exercise: acute effects on glycemia in type 1 diabetes. Diabetes Care. 2013;36:537–542. doi: 10.2337/dc12-0963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Stenerson M, Cameron F, Wilson DM, et al. The impact of accelerometer and heart rate data on hypoglycemia mitigation in type 1 diabetes. J Diabetes Sci Technol. 2014;8:64–69. doi: 10.1177/1932296813516208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Breton MD, Brown SA, Karvetski CH, et al. Adding heart rate signal to a control-to-range artificial pancreas system improves the protection against hypoglycemia during exercise in type 1 diabetes. Diabetes Technol Ther. 2014;16:506–511. doi: 10.1089/dia.2013.0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Turksoy K, Quinn LT, Littlejohn E, et al. An integrated multivariable artificial pancreas control system. J Diabetes Sci Technol. 2014;8:498–507. doi: 10.1177/1932296814524862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Blauw H, van Bon AC, Koops R, et al. Performance and safety of an integrated bihormonal artificial pancreas for fully automated glucose control at home. Diabetes Obes Metab. 2016;18:671–677. doi: 10.1111/dom.12663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fullerton B, Jeitler K, Seitz M, et al. Intensive glucose control versus conventional glucose control for type 1 diabetes mellitus. Cochrane Database Syst Rev. 2014;2014:CD009122. doi: 10.1002/14651858.CD009122.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wong JC, Neinstein AB, Spindler M, et al. A minority of patients with type 1 diabetes routinely downloads and retrospectively reviews device data. Diabetes Technol Ther. 2015;17:555–562. doi: 10.1089/dia.2014.0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Christiansen SC, Carlsen SM. Kunstig pankreas – drøm eller virkelighet? Indremedisineren. 2017;1:30–3. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study