Abstract
Background:
Diabetes mellitus is one of the most common endocrinal disorders and medicinal plants continue to play an important role in the management of this disease. In this study, Rosa canina was investigated for the antioxidant and α-amylase inhibition activities.
Materials and Methods:
Methanolic extract of Rosa canina was investigated for its potential antioxidant activity. The extracts’ total phenolic and flavonoid contents and scavenging capacity for free radicals were evaluated. The α-amylase inhibition assay was also carried.
Results:
Rosa canina extract exhibits a total Phenolic and flavonoid levels respectively (21.918 mg GAE/g and 2.647mg ER/g). The free radical scavenging activity was found to be prominent against DPPH with an IC50 of 0.668 mg/ml and against ABTS with an IC50 of 0.467 mg/ml. Extract showed a significant ferric ion reducing activities with an IC50 of4.962 mg/ml.
Conclusion:
Rosa canina exerted a higher inhibitory activity against α-amylase. The obtained results support the antidiabetic use of rosa canina.
Keywords: Rosa canina, α-amylase, hyperglycemia, antioxidant activity, phytochemical profile
Introduction
Rosa canina L. (Rosaceae) has been widely used in folk confectionery and remedy in Tunisia. Its Arabic name is Nesri. Rosa canina is traditionally used for the prevention and therapy of common cold, flu, gastrointestinal disorders and infections (Wenzig et al., Nadpal et al., 2016).
The fruit is known as the most effective remedy against hemorrhoids and diabetes mellitus in Turkish folk medicine (Orhan et al., 2007). Rose hip, the pseudo-fruit of Rosa canina, is a source of phytonutrients, such as vitamin C, tocopherols, carotenoids, phenolic, organic acids, sugars and essential fatty acids (Barros et al., 2011). Many studies have reported that the rose hips of Rosa canina exhibit a wide range of bioactivities, such as anti-inflammatory, anti-obesity and anti-diabetic activity (Orhan et al., 2007; Wenzig et al., 2008; Barros et al., 2011). However, chemical composition and biological activity of other Rosa species, rose hips, have been poorly investigated (Ercisli 2007; Nadpal et al., 2016).
Diabetes type 2 is an important metabolic disorder described as hyperglycemia due to insulin resistance and insufficient insulin secretion (WHO 2006). The number of excess deaths due to diabetes was 6.8% of global mortality (Roglic and Unwin, 2010). This disease leads to many complications such as retinopathy, nephropathy, neuropathy, coronary heart disease, peripheral vascular disease and hypertension (Brownlee, 2001). Changes in the antioxidant parameters status in diabetes have been reported in different tissues. It is important to evaluate both the antioxidant potential and the hypoglycemic activity of antidiabetic drugs. In recent years, the searches for new antidiabetic agents have been focused on plants used in traditional medicine (Sabu and Kuttan, 2002).
Plants and food ingredients with inhibitory effects on digestive enzymes which affect starch degradation and glucose metabolism are a potential approach to alleviate postprandial glucose increase and subsequent diabetes development (Kuritzky et al., 1999). α-Amylase is involved in starch degradation. It initiates starch hydrolysis into maltose and then α-glucosidase cleaves maltose into glucose which is later transported to the blood stream (Lee et al., 2010). Thus, inhibition of these two digestive enzymes can be used to reduce the postprandial response after consumption of foods containing starch. Based on this rationale, acarbose, voglibose and miglitol three specific inhibitors for the enzymes are used in diabetic therapy by limiting starch hydrolysis and glucose release (Van De Laar et al., 2005). Gastrointestinal problem and abdominal cramps are the most common side effects of acarbose treatment. Therefore, natural food components exhibiting α-amylase and α-glucosidase inhibition will be ideal for management of diabetes (Joshi et al., 2015)
Recent studies have also shown that α-amylase and α-glucosidase can be inhibited by polyphenols from plants. Thus, foods rich in phenolic compounds are promising components of a healthy diet that can help to stabilize fluctuating blood sugar levels after a meal by controlling the rate and extent of glucose regulation (Gulati et al., 2012).
Rosa canina L. has a reputation of usefulness in treating diabetes (Khojasteh et al., 2015). The aims of the present study were to determine total phenolic and flavonoid contents, to evaluate antioxidant activity and α-amylase inhibitory potential of Rosa canina methanolic extract.
Materials and Methods
Plant material and preparation of the extract
Rosa canina L pseudo-fruit and flowers were purchased in mars 2015 from the local market of the Zaghouan region in Tunisia. The plant material was dried at ambient temperature and finely ground in liquid nitrogen, and then soaked in the methanol (1:10, ml plant weight: solvent volume) for 24 h, with continual shaking. After filtration through Whatman N° 1 filter paper, the extract was stored in cold until further use.
Phyto analytical Studies
Determination of total phenolic compounds
Total soluble phenolic compounds in the extracts were determined with Folin-Ciocalteu method. 100 μl of extract diluted with methanol was added to 500μl of Folin-Ciocalteu reagent and was incubated at ambient temperature and obscurity. After 5 min, 400μl of aqueous Na2CO3 (7.5 %) was added and then allowed to stand for 90 min with intermittent shaking. The absorbance was measured at 760 nm in a spectrophotometer (Singleton & Slinkard 1977). A standard curve was prepared using [6.25-100 μg/ml] of gallic acid. The total phenolic contents were expressed in terms of gallic acid equivalents (μg/ mg of dry mass).
Assay for total flavonoid content
Total flavonoid content was determined using the Chang et al., method. 500μl of the extract was added to equal volume of Alcl3-6H2O 2% (2g of Alcl3 was dissolved in100 ml of methanol). After mixing, samples were incubated in the obscurity for 15 minutes (Chang et al., 2002). A standard curve was prepared using (12.5-50μg/ml) of rutin. Results were expressed as mg of rutin equivalent per gram of dry mass of the plant extract (mg RE.g-1 of the dried extract).
Antioxidant Activities
Antioxidant activity determined by 1, 1-diphenyl-2-picrylhydrazyl (DPPH) radical inhibition
The DPPH scavenging activity was determined according the method of Sanchez. 300μl of methanolic extract was added to 900 μl de DPPH. The samples were mixed and incubated in the dark at room temperature for 60 minutes. And then the decrease in absorbance was measured at 517 nm. A control sample containing 100 μl of methanol in the DPPH and its absorbance was measured (Sánchez-Moreno, 2002). The experiment was carried out in triplicate. Radical scavenging activity was calculated using the following formula:
% inhibition= [(AC-AE)/AC] x 100
Where (AC) absorbance of the control sample, and (AE) absorbance of the plant extract.
The anti-radical activity expresses the ability of the extract to reduce the DPPH and thus trapping the free radical. This activity is estimated by the percentage of discoloration of DPPH (the percent inhibition of the appearance of the purple color). The DPPH radical-scavenging activity was expressed in terms of IC50 (mg / ml). The value of IC50 is defined as the concentration of the extract which provides 50% of the molecules of DPPH.
The positive control is represented by a standard antioxidant: The Trolox (6-hydroxy-2,5,7,8 tetramethylchroman-2-carboxylic acid) whose absorbance is measured under the same conditions as the samples.
Antioxidant activity determined by 2,2’-azinobis-3-ethylbenzothiazoline-6-sulfonate (ABTS) radical inhibition
Free radical scavenging activity was determined using the ABTS radical cation. Decolorization assay involves on reduction of the ABTS+ radical by plant extracts having antioxidant capacity (Re et al., 1999). ABTS radical was dissolved in deionized water to make a solution of 7 Mm and then 2.45 Mm potassium per sulfate solution was added. The mixture was allowed to stand in dark at room temperature for 12 to 18 hours, to generate the ABTS free radical cation. The free radical solution of ABTS was diluted with ethanol to an absorbance of 0.7 at 734 nm for the assay. 10 μl of plant extract (range 1to1000 μg/ml) was added to 990 μl of ABTS free radical solution, the solution was kept in the dark at ambient temperature for 60 minutes. The absorbance was measured at 734 nm. Finally, the results were compared with the control containing 10 μl of ethanol instead of plant extracts. Positive controls were BHT and ascorbic acid in concentration range 1.56 to 250 μg/ml. Antioxidant activity was expressed as percentage (%) inhibition = {(control absorbance of 734nm – samples absorbance 734nm)/ control absorbance 734nm} x 100. Antiradical power was estimated as IC50.
Ferric reducing power assay
The ferric reducing power assay was carried out as described by Puntel et al., (2005). 300 μl of plant extracts was added to 300 μl of FeSO4 (0.1mM). After 5 min incubation 300 μl of ferrozine (0.25 mM) was additionated and then incubated 10 min at room temperature in the dark. Absorbance was recorded at 562 nm. Chelating power was estimated IC50 (concentration for 50% inhibition of iron chelation).
Amylase inhibition assay
Amylase inhibition screening assay
The α-amylase inhibitory assay was determined by measuring diameter inhibition on petri plates. A medium consisting of 1.5% agar and 1% starch was prepared and placed into petri plates. After cooling the agar medium, three wells are realized. Porcine pancreatic α-amylase solution (EC 3.2.1.1) equivalent to 2 U/ ml in phosphate buffer (pH 6.9) was prepared and placed in the first well (control). The two-other containing the plant extract with different dilutions were mixed with the enzyme. Plates were allowed to stand for 3 days at 25°C then stained with iodine and allowed to stand for 15 min. Clear zone diameter was measured and used to calculate the amylase inhibitory activity (Gulati et al., 2012). Results were expressed as percentage (%) amylase inhibition = {(diameter of control – diameter of sample)/diameter of the control} x 100
Amylase inhibition assay by quantitative starch hydrolysis
The α-amylase inhibitory potential was established according the method used by Hasenah et al., (2006) with some modifications. Porcine pancreatic α-amylase solution (EC 3.2.1.1) was purchased from Sigma–Aldrich. 50 μl of plant extract was mixed with 50 μl of α-amylase solution (1 UI/ml in 0.02 M Sodium phosphate buffer (PBS) pH=6.9) and 10 μl of PBS. The reaction mixture was pre-incubated for 10 minutes at 37°C. After pre-incubation, 90 μl of 0.5% (w/v) starch solution were added. Then, the reaction mixture was incubated for 20 minutes at 37°C. The reaction was completed by adding 100 μl of 3, 5-dinitro salicylic acid reagent. The tubes were placed in boiling water bath for 15 minutes and cooled at ambient temperature. 900 μl of distilled water were added to the reaction mixture. The absorbance of the resulting supernatant at 540 nm was measured using a UV-visible spectrophotometer. The α-amylase inhibitory activity was calculated as follows:
Inhibitory activity (%) = [(Ac+ - Ac-) - (As -Ab)] / (Ac+ - Ac-) × 100
Ac+ : absorbance of 100% enzyme activity (only the solvent with the enzyme), Ac- : 0% enzyme activity (only the solvent without the enzyme), As: the test sample (with the enzyme) and Ab: the blank (test sample without the enzyme).
Statistical analysis
Experiments were performed in triplicate. The results are expressed as mean ± standard error. The concentration response curve was obtained by plotting inhibition percentages versus concentrations. IC50 value (half-maximal inhibitory concentration) of sample was obtained graphically by an inhibition curve. Statistical calculations were carried out by SPSS software version19.
Results and Discussion
The present study was designed to investigate the bioactive properties of Rosa canina relevant to the management of hyperglycemia. These properties included the total phenolic and total flavonoid contents, the antioxidant potential and the inhibition of α-amylase enzyme.
Total Phenolic and Flavonoids Content
Phenolic compounds are considered as a major group of compounds that contribute to the antioxidant activities of botanical materials because of their scavenging ability on free radicals due to their hydroxyl groups (Wenzig et al., 2008). The antioxidant capacity of phenolic compounds is mainly due to their redox properties, which allow them to act as reducing agents, hydrogen donors, singlet oxygen quenchers or metal chelators (Balasundram et al., 2006). Phenolics are believed to be the major phytochemicals responsible for antioxidant activity of plant materials (Fattahi et al., 2012).
Our results demonstrate that the Rosa canina L methanolic extract show a total phenolic amount of GAE 21,918 mg/g fruit and a level of flavonoids of 2.65 mg RE/g.
Fattahi et al., in 2012 tested the fruit of Rosa canina from the Azerbaijan province in Iran. They demonstrated that the extracts’ total phenolic and flavonoid contents were respectively 176.48 ± 2.71 mg gallic acid equivalents /100 g methanolic and 0.41± 0.02 mg quercetin/100 g methanolic extract (Fattahi et al., 2012).
Flavonoids are regarded as one of the most widespread groups of natural constituents found in plants (Fattahi et al., 2012). Our findings are also in agreement with other reports; total flavonoids content for Rosa canina extract reported by Daels-Rakotoarison et al., (2002) was 14.71 mg/100 g extract. Ghazghazi et al., (2010) demonstrated that the flavonoids content of Rosa canina was 0.33 ± 0.01 mg RE/ml. An Iranian study reported that the average of flavonoids content of Rosa canina was 23.6 ± 4.2 mg quercetin/g extract (Montazeri et al., 2011)
Total phenolic content for Rosa canina extract reported by Ercisli, (2007) was 96 mg GAE/g extract. In previous studies, the total phenolic content of rose species were found to range from 55 to 122 mg GAE/g DW which is in accordance with our findings (Gao et al., 2000).
Total phenolic contents of Turkish Rosa canina was 31.08 gallic acid/g of dry weight (Demir et al., 2014).
Previously, Wenzig et al., demonstrated that total phenolic content of the rose hip methanolic extract containing fruits was 82.2±1.4 (mg GAE/mg dry extract) (Wenzig et al., 2008)
Difference found between our results and those reported by the literature can be explained by diverse factors that affect total phenolic content in fruit like; plant genotype, cultivation area and extraction technique, as well as differences in fruit ripeness (Demir et al., 2014).
Ghazghazi et al., who studied the phenol content of methanolic extracts of Rosa canina samples growing in different localities and expressed in gallic acid equivalents/mL (GAE/mL). The level of total phenols is more heterogeneous in Aindrahan’s samples since the concentrations ranged from 5.42 ± 0.10 mg/mL, at Guoirya, to 9.21 ± 0.10 mg/mL, at Ain Zena (Ghazghazi et al., 2010). Several factors seemed to be responsible for such variability, including altitude, developmental stage and climate, among others. Beyond the importance of these factors on the level of phenols they are also important on the type of phenol formed (Fratianni et al., 2007).
Plants which contain elevated levels of phenolic are considered a good source of antioxidants and therefore it is important to quantify phenolic and flavonoids contents in plant extracts as they might have many advantageous effects on health (Gulati et al., 2012)
Evaluation of the Antioxidant power
Radical Scavenging activity DPPH (2,2-diphenyl-1-picrylhydrazyl)
DPPH is a radical, which is reduced to the diphenylpicrylhydrazine by plant extracts. This antioxidant assay consisted on the reduction of alcoholic DPPH in the presence of hydrogen-donating antioxidant due to the formation of the DPPH-H non-radical form (Gulati et al., 2012).
The ability of Rosa canina methanolic extract to donate hydrogen was checked by using the staple free radical DPPH •. The amount of sample needed to decrease the initial DPPH-concentration (EC50) by 50% is a parameter widely used to measure the antioxidant activity. The lower the EC50, the higher is the antioxidant power.
As revealed in table 1, the DPPH radical scavenging activities of Rosa canina extract shows an IC50 of 0.668±0.0017 mg/ml.
Table 1.
Total Phenolic and Flavonoids contents and antioxidant power of Rosa canina extract
| Total Phenolic (mgGAE/gDW) | 21.918±1.784 |
| Flavonoids (mgER/gDW) | 2.647±0.040 |
| Antioxidant activity (mg/ml) | |
| IC50 /DPPH | 0.668±0.002 |
| IC50/ABTS | 0.467±0.034 |
| IC50/ferrozin | 4.962±0.166 |
Data are expressed as the mean of triplicate (± SD)
DW: dry weight, GAE: Gallic acid equivalents, RE: Rutin equivalent
The DPPH radical scavenging percentage of Rosa canina fruits from West Azerbaijan were 87.78 ± 0.10 % for Rosa canina from Takab, and 79.16 ± 0.61% for fruits from Urmia (Fattahi et al., 2012).
Extracts of rose hip containing fruits show a DPPH radical scavenging activity ranging from EC50 values of 25.0 mg/ml for the methanolic extract to 988 mg/ml for the aqueous extract (Wenzig et al., 2008).
Anti-radical activity: ABTS test
The antioxidant assay consisted on the reduction of ABTS solution by plant extracts. Table 1 illustrated that the methanolic extracts of Rosa canina having an activity against the radical ABTS with an IC50 of 0.467 ±0.033 (mg / ml).
Ghazghazi et al, have studied different samples of Rosa canina leaves methanolic extracts from different locality in the region of Feija and Aindraham in Tunisia. They reported a lower capacity for scavenging the free radicals ABTS with an IC50 ranged between 0.0061 ± 0.0004 and 0.0125 ± 0.0004 (mg/ml) (Ghazghazi et al., 2010).
Ferric Reducing Potential
Antioxidant activities of our plant extracts were determined through their ability to reduce the Fe3 +/ferricyanide complex to the ferrous (Fe2+). Result of ferric reducing capacities of Rosa canina fruits extract possessed a ferric capacity with an IC50 of 4.962 mg / ml.
Some previous studies reported a considerable antioxidant activity of the Rosa canina fruits, which was stronger than the activity of some other wild fruits extensively consumed in the diet like strawberry tree and wild blackberry (Barros et al., 2010, 2011). In addition, other clinical trial reported that the antioxidant activity could contribute to the pharmaceutical effect of Rosa canina rose hips preparation (Chrubasik et al., 2006, 2008).
Test of the inhibitory activity of alphα-amylase
The α-amylase inhibitors are among the drugs that reduce hyperpostprandial blood glucose by inhibiting the hydrolysis of the starch (Gulati et al., 2012). Inhibition of α-amylase contributes to improve symptoms of type 2 diabetes by delaying or interrupting glucose absorption as a result of slowing starch digestion. Although the main purpose of α-amylase inhibition is to slow down maltose and glucose production, it can also slow α-glucosidase function by eliminating the substrate of this enzyme (Golay et al., 1991; Joshi et al., 2015). Several medicinal plants have been reported for their antidiabetic power throughout the world (Hasenah et al., 2006).
In the present study, Results of the preliminary agar diffusion of amylase inhibition assays indicated that Rosa canina methanolic extract exhibit a complete inhibition of α-amylase enzyme such that no hydrolysis of starch was evident. The percentage inhibition displayed by each extract is shown in table 2.
Table 2.
Screening assay of α-amylase inhibitory activity of different concentrations of Rosa canina extract
| Concentrations (mg/ml) | % d’inhibition | |
|---|---|---|
| Rosa canina extract | 100 | 100 |
| 50 | 100 | |
| 25 | 100 | |
| 12.5 | 100 | |
| Control Enzyme | (2U/ml) | 0 |
This result was confirmed by the α-amylase inhibition quantitative assay, which demonstrate that Rosa canina extract exhibit a very interesting inhibitory activity with 100% inhibition for extract concentration of 5.5 mg/mL. An activity which varies from 88.46% to 40.33% for the respective concentrations 0.011 and 0.0022 mg /mL. In addition, values of IC50 of extract (0.003 mg/mL) (Fig 1).
Figure 1.
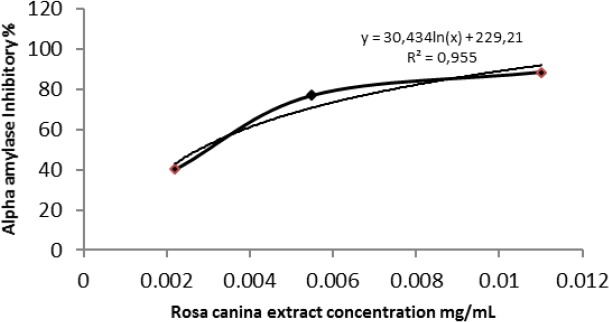
α-amylase inhibitory activity of different concentrations of Rosa canina extract
The scientific knowledge on the carbohydrate hydrolyzing enzymes inhibitory effect of Rosa canina L are missing. Recently, an Iranian study revealed that the methanolic and acetonic extract of Rosa canina fruit have a significant inhibitory activity of α-glucosidase (Asghari et al., 2015).
The mechanism of a amylase inhibition differs somewhat depending on inhibitor type. Suggested inhibition mechanism was complex formation with large a molecular weight macromolecule as polyphenol and polysaccharide.
The antidiabetic effect of Rosa canina was tested in diabetic male rats by Khojasteh et al., (2015). They reported a significant decrease in glycemia level after administration of ethanolic extract of Rosa canina fruits.
Recent study was conducted to evaluate the efficacy of Rosa canina fruit aqueous extract in type 2 diabetic patients. They demonstrated that fasting blood glucose level decreased significantly (p = 0.002) in Rosa canina treated group after 3 months compared to the baseline (Hashem Dabaghian et al., 2015).
Plant polyphenols and flavonoids are some of the naturally occurring antidiabetic agents which are known to show an inhibitory effect on carbohydrate hydrolyzing enzyme inhibition, by virtue of their capability to bind with proteins (Ganeshpurkar et al., 2013; Bejaoui et al., 2016).
Apart polyphenols and flavonoids, the extract of rosa canina is rich in vitamin C. Previously, ascorbic acid has been evaluated as inhibitors of pancreatic and salivary a amylases. The study reported that the enediol moiety of ascorbic acid is essential for a amylase inhibition (Abell et al., 1998).
Rose hips are considered to be an extremely valuable natural source of ascorbic acid. Additionally, it is regarded that flavonoids and organic acid in rosa canina prevent oxidation of vitamin C, which moreover increases its stability and bioavailability (Adamczak et al., 2012).
Furthermore, it was reported that rose hips of rosa canina contain the highest vitamin C content, which was six times higher than that in orange (Nojavan et al., 2008).
In the present work, we can suggest that vitamin C of rosa canina extracts was found to inhibit, in a concentration dependent manner, the α-amylase activity.
Conclusion
Methanolic extract of Rosa canina exhibit an important total phenol and flavonoid contents. Apart from being a source of antioxidant, Rosa canina was also able to detain starch digestion via digestive enzyme inhibition. Results demonstrated that methanolic extract of rosa canina was very potent in inhibiting α-amylase with a higher degree of inhibition. This inhibitory capability suggested that rosa canina could potentially delay starch digestion following carbohydrate dense meal consumption. Hence, habitual drinking of rosa canina infusion could be beneficial for controlling diabetes. Therefore, further research concerning the principles bioactive compounds and the clinical effectiveness of rose hip will be necessary in order to allow appropriate standardization and a rational use of rose hip preparations.
Acknowledgments
The authors thank Mss Sonia Zaier, English teacher at the High School of Sciences and Technologies of Health of Tunis for having proofread this paper.
Footnotes
Declaration of conflicting interests:
The authors declare that there is no conflict of interest.
References
- 1.Abell AD, Ratclffe M.J, Tb JG. Ascorbic acid-based inhibitors of α-amylases. 1998;98:4–7. doi: 10.1016/s0960-894x(98)00298-4. [DOI] [PubMed] [Google Scholar]
- 2.Adamczak A, Buchwald W, Zielinski J, Mielcarek S. flavonoid and organic acid content in rose hips (rosa l, sect. Caninae dc. Em. Christ.) Acta biologica cracoviensia series botanica. 2012;54(1):105–112. [Google Scholar]
- 3.Asghari B, Salehi P, Moridi Farimani M, Ebrahimi SN. A-glucosidase inhibitors from fruits of rosa canina l. Records of natural products. 2015;9(3):276–283. [Google Scholar]
- 4.Balasundram n, sundram k, samman s. phenolic compounds in plants and agri-industrial by-products: antioxidant activity, occurrence, and potential uses. Food chemistry. 2006;99(1):191–203. [Google Scholar]
- 5.Barros L, Carvalho NM, Sa Morais J, Ferreira CFRI. Strawberry-tree, blackthorn and rose fruits: detailed characterisation in nutrients and phytochemicals with antioxidant properties. Food chemistry. 2010;120(1):247–254. [Google Scholar]
- 6.Barros L, Carvalho A.M, Ferreira ICF.R. Exotic fruits as a source of important phytochemicals: improving the traditional use of rosa canina fruits in portugal. Food research international. 2011;44(7):2233–2236. [Google Scholar]
- 7.Bejaoui A, Boulila A, Sanaa A, Boussaid M, Fernandez X. Antioxidant activity and a -amylase inhibitory effect of polyphenolic-rich extract from origanum glandulosum desf. Journal of food biochemistry in press 2016 [Google Scholar]
- 8.Brownlee M. Biochemicstry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 9.Chang CC, Yang MH, Wen HM, Chern JC. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. Journal of food and drug analysis. 2002;10(3):178–182. [Google Scholar]
- 10.Chrubasik C, Roufogalis BD, Muller-Ladner U, Chrubasik S. A systematic review on the rosa canina effect and efficacy profiles. Phytotherapy research. 2008;22:725–733. doi: 10.1002/ptr.2400. [DOI] [PubMed] [Google Scholar]
- 11.Chrubasik C, Duke R.K, Chrubasik S. The evidence for clinical efficacy of rose hip and seed: a systematic review. Phytotherapy research. 2006;20(1):1–3. doi: 10.1002/ptr.1729. [DOI] [PubMed] [Google Scholar]
- 12.Daels-Rakotoarison DA, Gressier B, Trotin F, Brunet C, Luyckx M, Dine T, Bailleul F, Cazin M, Cazin J-C. Effects of rosa canina fruit extract on neutrophil respiratory burst. Phytotherapy research. 2002;16(2):157–161. doi: 10.1002/ptr.985. [DOI] [PubMed] [Google Scholar]
- 13.Demir N, Yildiz O, Alpaslan M, Hayaloglu A. A. evaluation of volatiles, phenolic compounds and antioxidant activities of rose hip (rosa l.) Fruits in turkey. Lwt - food science and technology. 2014;57(1):26–133. [Google Scholar]
- 14.Ercisli S. Chemical composition of fruits in some rose (rosa spp.) Species. Food chemistry. 2007;104(4):1379–1384. [Google Scholar]
- 15.Fattahi S, Jamei R, Sarghein SH. Antioxidant and antiradical activities of rosa canina and rosa pimpinellifolia fruits from west azerbaijan. Iranian journal ofplant physiology. 2012;2(4):523–530. [Google Scholar]
- 16.Fratianni F, Tucci M, De palma M, Pepe R, Nazzaro F. Polyphenolic composition in different parts of some cultivars of globe artichoke (cynara cardunculus l. Var. Scolymus (l.) Fiori) Food chemistry. 2007;104(3):1282–1286. [Google Scholar]
- 17.Ganeshpurkar A, diwedi V, bhardwaj Y. In vitro a -amylase and a -glucosidase inhibitory potential of trigonella foenum-graecum leaves extract materials and methods chemicals phytochemical screening phytoanalytical studies determination of total phenolic compounds enzyme inhibition studies por. Ayu. 2013;34(1):109–112. doi: 10.4103/0974-8520.115446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao X, Bjo L, Ttrajkovski V, Uggla M. Evaluation of antioxidant activities of rosehip ethanol extracts in different test systems. Journal of science and agriculture. 2000;80:2021–2027. [Google Scholar]
- 19.Ghazghazi H, Miguel MG, Hasnaoui B, Sebei H, Ksontini M, Barroso JG. Phenols, essential oils and carotenoids of rosa canin from tunisia and their antioxidant activities. Journal of biotechnology. 2010;9(18):2709–2716. [Google Scholar]
- 20.Golay A, Schneider H, Temler E, Felber JP. Effect of trestatin, an amylase inhibitor, incorporated into bread, on glycemic responses in normal and diabetic patients. Am j clin nutr. 1991;53(1):61–65. doi: 10.1093/ajcn/53.1.61. [DOI] [PubMed] [Google Scholar]
- 21.Gulati V, Harding I.H, Palombo EA. Enzyme inhibitory and antioxidant activities of traditional medicinal plants: potential application in the management of hyperglycemia. Bmc complementary and alternative medicine. 2012;12(77):1472–6882. doi: 10.1186/1472-6882-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hasenah A, Houghton P.J, Soumyanath A. a-amylase inhibitory activity of some malaysian plants used to treat diabetes;with particular reference to phyllanthus amarus. Journal of ethnopharmacology. 2006;107(3):449–455. doi: 10.1016/j.jep.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 23.Hashem Dabaghian FAM, Et Abdollahifard M, Khalighi Sigarudi F, Taghavi Shirazi M, Shojaee A, Sabet Z, Fallah Huseini H. Effects of rosa canina l. Fruit on glycemia and lipid profile in type 2 diabetic patients: a randomized, double-blind, placebo-controlled clinical. Journal of medicinal plants. 2015;14(55):95–104. [Google Scholar]
- 24.Joshi SR. E, Standl E, Tong N, Shah P, Kalra S, Rathod R. Therapeutic potential of a -glucosidase inhibitors in type 2 diabetes mellitus: an evidence-based review. Expert opinion on pharmacotherap. 2015;16(13):1959–1981. doi: 10.1517/14656566.2015.1070827. [DOI] [PubMed] [Google Scholar]
- 25.Khojasteh SS, El Basirat F, Sheikhzadeh SAO, Dehghan G, Hatami H, Farsad N. Effects of the ethanolic extract of rosa canina on some serum biochemical factors in diabetic adult male rats. Trakia journal of science. 2015;13(2):143–148. [Google Scholar]
- 26.Kuritzky L, Samraj G, Quillen DM. Improving management of type 2 diabetes mellitus: 2. Biguanides. Hospital practice (1999) 1999;34(10):43–46. doi: 10.1080/21548331.1999.11443912. [DOI] [PubMed] [Google Scholar]
- 27.Lee WK, Wong LL, Loo YY, Kasapis S, Huang D. Evaluation of different teas against starch digestibility by mammalian glycosidases. Journal of agricultural and food chemistry. 2010;58(1):148–154. doi: 10.1021/jf903011g. [DOI] [PubMed] [Google Scholar]
- 28.Montazeri N, Baher E, Mirzajani F, Barami Z, Yousefian S. phytochemical contents and biological activities of rosa canina fruit from iran. Journal of medicinal plants research. 2011;5(18):4584–4589. [Google Scholar]
- 29.Nadpal JD, Lesjak M M, Sibul F.S, Anac Kov GT, Cetojevic-Simin DD, Mimica-Dukic N.M, Beara IN. Comparative study of biological activities and phytochemical composition of two rose hips and their preserves: rosa canina l. And rosa arvensis huds. Food chemistry. 2016;192:907–914. doi: 10.1016/j.foodchem.2015.07.089. [DOI] [PubMed] [Google Scholar]
- 30.Nojavan S, Khalilian F, Kiaie FM, Rahimi A, Arabanian A, Chalavi S. Extraction and quantitative determination of ascorbic acid during different maturity stages of rosa canina l. Fruit. Journal of food composition and analysis. 2008;21(4):300–305. [Google Scholar]
- 31.Orhan DD, Hartevio Glu A, Kupeli E, Yesilada E. In vivo anti-inflammatory and antinociceptive activity of the crude extract and fractions from rosa canina l. Fruits Journal of ethnopharmacology. 2007;112:394–400. doi: 10.1016/j.jep.2007.03.029. [DOI] [PubMed] [Google Scholar]
- 32.Puntel RL, Nogueira C.W, Rocha JBT. Krebs cycle intermediates modulate thiobarbituric acid reactive species (tbars) production in rat brain in vitro. Neurochemical research. 2005;30(2):225–235. doi: 10.1007/s11064-004-2445-7. [DOI] [PubMed] [Google Scholar]
- 33.Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved abts radical cation decolorization assay. Free radical biology and medicine. 1999;26(9-10):1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 34.Roglic G, Unwin N. Mortality attributable to diabetes: estimates for the year 2010. Diabetes research and clinical practice. 2010;87(1):15–19. doi: 10.1016/j.diabres.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 35.Sabu M.C, Kuttan R. Anti-diabetic activity of medicinal plants and its relationship with their antioxidant property. Journal of ethnopharmacology. 2002;81:155–160. doi: 10.1016/s0378-8741(02)00034-x. [DOI] [PubMed] [Google Scholar]
- 36.Sanchez-Moreno C. Review: methods used to evaluate the free radical scavenging activity in foods and biological systems. Food science and technology international. 2002;8(3):121–137. [Google Scholar]
- 37.Singleton V.L, Slinkard K. Total phenol analysis: automation and comparison with manual methods. Am. J. Enol. Vitic. 1977;28(1):49–55. [Google Scholar]
- 38.Van De Laar F.A, Lucassen P. L, Akkermans R. P, Van De Lisdonk E. H, Rutten G.E, Vanweel C. α-glucosidase inhibitors for patients results from a cochrane systematic review and meta-analysis. Diabetes care. 2005;28(1):166–175. doi: 10.2337/diacare.28.1.154. [DOI] [PubMed] [Google Scholar]
- 39.Wenzig EM, Widowitz U, Kunert O, Chrubasik S.F, Bucar E Knauder, Bauer R. Phytochemical composition and in vitro pharmacological activity of two rose hip (rosa canina l.) Preparations. Phytomedicine. 2008;15(10):826–835. doi: 10.1016/j.phymed.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 40.WHO. Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia. Vol. 53. World health organisation geneva; 2006. p. 41. [Google Scholar]


