Abstract
Background:
Lysimachia vulgaris L. (Yellow loosestrife) is a medicinal plant in the family Myrsinaceae. It has been used in the treatment of fever, ulcer, diarrhea and wounds in folk medicine. It has also analgesic, expectorant, astringent and anti-inflammatory activities. Two different sources of the plant (field-grown and in vitro-grown) were used to evaluate the biological activities (antibacterial, antitumor and antioxidant) of L. vulgaris. In vitro-grown plant materials were collected from L. vulgaris plants that were previously regenerated in our laboratory.
Materials and Methods:
Plant materials were extracted with water, ethanol and acetone. For antibacterial test, disc diffusion method and 10 different pathogenic bacteria were used. Antioxidant activity was indicated by using DPPH method. The total phenol amount by using Folin-Ciocaltaeu method and the total flavonoid amount by using aluminum chloride (AlCl3) colorimetric method were determined.
Results:
Generally, yellow loosestrife extracts demonstrated antibacterial activity against Gram-positive bacteria (Staphylococcus aureus, S. epidermidis and Streptococcus pyogenes). Strong antitumor activity of yellow loosestrife was observed via potato disc diffusion bioassay. Nine different phenolics were also determined and compared by using High-Performance Liquid Chromatography (HPLC).
Conclusion:
Future investigations should be focused on fractionation of the extracts to identify active components for biological activity.
Keywords: Lysimachia vulgaris, antibacterial, antitumor, DPPH, Folin-Ciocalteau, Aluminum chloride colorimetric, HPLC
Introduction
Yellow loosestrife (Lysimachia vulgaris L.) is a rhizomatous perennial plant (Davis, 1978) that has been used in the treatment of diarrhea, dysentery, internal and external bleeding and to cleanse wounds (Chevallier, 1996). It has been known as expectorant, astringent, febrifuge, demulcent, analgesic, anti-inflammatory and vulnerary in traditional medicine (Grieve, 1982; Dobelis, 1990; Baytop, 1999). L. vulgaris is natural to Europe and can be found along roadsides and near water (Chevallier, 1996). It is also useful plant for phytopurification of wastewater (Borin, 2003). The genus was traditionally placed in the Primulaceae family, but upon review of molecular phylogenetic research, it has now been assigned in the much-enlarged family Myrsinaceae (Hao et al., 2004; Oh et al., 2008). Yellow loosestrife contains benzoquinones (embelin and rapanone), saponins, flavonoids, hydroxycinnamic acid, and tannins (Rzadkowskabodalska and Olechnowiczstepien, 1975; Yasukawa and Takido, 1988; Janik et al., 1994; Chevallier, 1996; Podolak et al., 1998; Podolak and Strzalka, 2008; Toth et al., 2012; 2014).
Antifungal and cytotoxic activities of underground parts (Podolak et al., 1998) and polyphenol and antioxidant activities of aerial parts (Toth et al., 2012, 2014) of L. vulgaris have been recorded. Numerous traditional usages of yellow loosestrife have been recorded but these usages have not been proved scientifically. Biological activities (antibacterial, antitumor and antioxidant) of aerial parts of L. vulgaris obtained from two different sources (field-grown and in vitro-grown plants) were revealed with this study. Phenolic constituents of field-grown and in vitro-grown leaves were also determined and compared by HPLC method for the first time.
Materials and Methods
Plant Material and Extraction
Field-grown plant parts (leaf, flower, stem and seed) were obtained from Abant Lake, Bolu, Turkey in August, 2013. Identification of the plant was performed by using “Flora of Turkey and the East Aegean Islands” (Davis, 1978) and voucher specimens (AUT-2008) were stored at the Department of Biology, Abant Izzet Baysal University (AIBU), Bolu, Turkey. Leaves and stems of in vitro cultured plants were collected from previously micropropagated yellow loosestrife plants in the laboratory (Turker and Guner, 2013). Plant materials were extracted with water, ethanol (EtOH) and acetone. Two different sources of the plant (field-grown and in vitro-grown) were used for extractions. All plant materials were dried in a room and then grounded.
Aqueous extraction-20 g of each plant sample was extracted with 200 mL of water in waterbath at 45°C for 12 hours and then strained. Frozen filtrate was lyophilized at -65 °C. Ethanol and acetone extractions-20 g of each plant sample were soxhlet extracted with 300 mL of ethanol at 75°C and with 300 mL of acetone at 50°C for 12 hours and then filtered. Filtrates were evaporated under low pressure at 40°C using a rotary evaporator. Plant materials, their designations and extraction yields are represented in Table 1.
Table 1.
Designation, plant material and yield of extracts.
| Extract | Designation | Part used | Yield* (%) |
|---|---|---|---|
| Water | NLW | Field-grown leaves | 12 |
| NFW | Field-grown flowers | 18 | |
| NAW | Field-grown aerial parts | 59 | |
| IVW | In vitro-grown leaves and stems | 22 | |
| SW | Field-grown seeds | 6 | |
| Ethanol | NLE | Field-grown leaves | 9 |
| NFE | Field-grown flowers | 12 | |
| NAE | Field-grown aerial parts | 7 | |
| IVE | In vitro-grown leaves and stems | 10 | |
| SE | Field-grown seeds | 7 | |
| Acetone | NLA | Field-grown leaves | 6 |
| NFA | Field-grown flowers | 12 | |
| NAA | Field-grown aerial parts | 7 | |
| IVA | In vitro-grown leaves and stems | 10 | |
| SA | Field-grown seeds | 7 |
Yield (%) = Weight of extract (g) / 20 gr of powdered plant sample * 100
Antibacterial activity
The disc diffusion method was used for antibacterial activity (Andrews, 2004). Three Gram-positive [Streptococcus pyogenes (ATCC® 19615), Staphylococcus aureus (ATCC® 25923) and Staphylococcus epidermidis (ATCC® 12228)] and seven Gram-negative [ Escherichia coli (ATCC® 25922), Pseudomonas aeruginosa (ATCC® 27853), Salmonella typhimurium (ATCC® 14028), Serratia marcescens (ATCC® 8100), Proteus vulgaris (ATCC® 13315), Enterobacter cloacae (ATCC® 23355) and Klebsiella pneumoniae (ATCC® 13883)] bacteria were tested. Agar cultures were prepared as described by Turker et al. (2012). Turbidity of each broth culture of bacteria was arranged with saline comparing with 0.5 McFarland standard and cotton swabs were used for inoculation on Mueller Hinton agar plates.
All extracts were dissolved in distilled water for final concentration (100 mg/mL) and sterilized with 0.22 μm filter (Millex®). Sterile, 6 -mm diameter filter paper discs (Whatman®) containing 13 μl of extract and antibiotics [Erythromycin (15 μg), Ampicillin (10 μg), Carbenicillin (100μg), Tetracycline (30 μg) and Chloramphenicol (30 μg)] were put on Mueller Hinton agar. Water was used as a negative control. Inoculated plates with discs were stored in a 37°C incubator and after 24 hours, diameter (mm) of inhibition zone was reported. Three independent experiments were carried out.
Potato Disc Tumor Induction Assay
Antitumor bioassay was conducted with potato disc method as adapted by McLaughlin’s group (Ferrigini et al., 1982). Suspensions of Agrobacterium tumefaciens (ATCC® 23341) in phosphate buffer saline (PBS) were adjusted to 1.0 X 109 Colony Forming Units (CFU) with an absorbance value of 0.96 + 0.02 at 600 nm (Coker et al., 2003). Extracts were dissolved in water or DMSO (12.5%) (Ferrigini et al., 1982) and these solvents were used as negative controls. NLA, NFE, NFA, NAA, SE, SA and IVA extracts were dissolved in 12.5% DMSO to obtain 1000 mg/1 final concentration. NLW, NLE, NFW, NAW, NAE, SW, IVW, and IVE extracts were dissolved in water to a final concentration of 1000 mg/1 or 100.000 mg/1. All extracts and controls were sterilized with 0.22 μm filter (Millex®). Tumor suppressant Camptothecin (Sigma®) was used as a positive control. The test solutions included 600 μl extracts or controls, 150 μl water, and 600 μl adjusted bacterium suspension.
Potatoes (Solanum tuberosum L.) were surface sterilized by brushing under running water, and then dipped into 10% commercial bleach (Domestos®) for 20 min. The largest surface area of tubers was trimmed and then placed in 20% commercial bleach for 15 min. A sterile cork borer (10 mm) was used to obtain potato cylinders and cleaved into 0.5 cm thick after discarding each end of the cylinders. Discs were placed into 24-well culture plates including 15 g/L water-agar. Each disc was loaded with 50 μl of appropriate extracts/water/bacteria mix. Plates were kept at room temperature in the dark. After 2 weeks, the discs were dyed with Lugol’s Reagent and tumors on each disc were enumerated. Three independent experiments were conducted. Percent inhibition of tumors was determined “% inhibition= [(solvent control mean - tested extract mean) / solvent control mean] X 100” (McLaughlin et al., 1998; Coket et al., 2003).
Bacterial viability was controlled by incubating 1 ml of each plant extract with 1 ml of 1 x 103 CFU bacterial suspensions. After 30 min, 0.1 ml of inoculum (bacteria + extract) was inoculated on YEM media with spread plate technique. After 24-h incubation at 28°C, colonies were counted (Coker et al., 2003).
Data analysis
All data were interpreted by analysis of variance (ANOVA) and Duncan’s Multiple Range Tests (SPSS vers. 15 Inc., Chicago, IL, USA) was used for the comparison of mean values.
Antioxidant Assay
Free radical scavenging activity
Free radical scavenging activity of the aqueous and ethanolic extracts of L. vulgaris was evaluated spectrophotometrically by monitoring the disappearance of 2,2-diphenyl-1-picrylhydrazil (DPPH·, Sigma-Aldrich Chemie, Steinheim, Germany) at 517 nm, according to the method described by Brand-Williams et al. (1995). 1 ml of 0.15 mM solution of DPPH in ethanol was mixed with 3 mL of the extracts (NLW, NLE, NFW, NFE, NAW, NAE, IVW and IVE) at different concentrations (25, 50, 100 and 200 μg/ml), vortexed and then kept in the dark for 30 min. Decrease in the absorbance of these solutions was measured at 517 nm with Hitachi U-1900, UV-VIS Spectrophotometer 200V against blank samples. All analyses were made in triplicate. % inhibition was determined with a formula “DPPH· Scavenging Effect (% inhibition) = [(A0-A!/A0) x 100]” (Gülçin et al., 2004), where A0 is the absorbance of the control and A! is the absorbance of tested extracts.
Determination of total phenolics content
The phenolic contents of aqueous and ethanolic extracts of L. vulgaris were evaluated according to the method described by Slinkard and Singleton (1977) with slight modification. Calibration curve of standard phenolic compound (Gallic acid) was prepared (0, 50, 100, 150, 200, 250 and 500 mg/L). 1mg/ml plant extracts of L. vulgaris (NLW, NLE, NAW, NAE, NFW, NFE, IVW and IVE) was prepared with using distilled water. 20 μL from each calibration solution, sample, or blank was put into separate cuvettes. Then, 1.58 mL of water and 100 μL of Folin-Ciocalteu reagent (Sigma®) was transferred to each, and then mixed well. After 2 minutes, 300 μL of Na2CO3 solution was dropped and shaken very well. The solutions were stored at 20 °C for 2 hours and recorded the absorbance of each solutions at 765 nm against the blank (the “0 mL” solution) using the spectrophotometer. The amounts of total phenolic compounds in L. vulgaris extracts were calculated as micrograms of gallic acid equivalent, using an equation that was obtained from a gallic acid calibration curve (R2: 0.9944). All measurements were repeated three times.
Determination of total flavonoid
The amount of total flavonoids in the aqueous and ethanolic extracts was measured by aluminum chloride (AlCl3) colorimetric assay. Calibration curve of standard flavonoid compound (Catechol) was prepared (20, 40, 60, 80 and 100 mg/mL). 1250 mg/mL and 500 mg/mL concentrations of the extracts were prepared in ethanol. 500 μL of extract solution or standard solution of catechol was transferred to a 10-mL test tube containing 2 mL of distilled water. Then, 150 μL of 5% NaNO2 was put to the test tubes. After 5 min, 150 μL of 10 % AlCl3 was added. After 6 min, 1000 μL of 1M NaOH was incorporated into the mixture. Immediately, the reaction tube was diluted to volume of 5 mL with the addition of 1200 μL of distilled water and mixed. Absorbance of the mixture was measured at 510 nm versus a blank. Three replications were made for analysis (Marinova et al., 2005). The total flavonoid content of L. vulgaris samples was calculated as mg catechol equivalents (CE)/100g dried weight of plant.
Extraction and HPLC Analysis
Preparation of plant extracts for HPLC
L. vulgaris leaves obtained from field-grown and in vitro-grown plants were soxhlet extracted with methanol for 24 hours. Methanol was evaporated under vacuum at 40°C and residue was dissolved in sterile distilled water and lyophilized at - 65 °C. Each extract was dissolved in HPLC grade acetonitrile (ACN) to obtain a final concentration (10 mg/mL) prior to HPLC analyses. Coumarin, gallic acid monohydrate, apigenin, caffeic acid, kaempferol, rutin hydrate, quercetin, luteolin-7-O-β-D glucoside and myricetin were used as phenol standards (Sigma®). Different concentrations (1, 5, 10, 20, 40, 60, 80, 100 and 200 mg/L) of standards were prepared for obtaining standard curve. Hydrophilic polypropylene (GHP) Acrodiscs (0.2-mm-25 mm) (Pall Corporation®) were used for filtration of all extracts and standards into 2-ml HPLC vials. Three different vials were prepared for each sample.
HPLC analysis of phenolic compounds
Methanolic extracts (10 mg/ml) were analyzed using a HPLC system (VWR-Hitachi LaChrom Elite®) equipped with a Hitachi L-2455 Diode-Array Detector (DAD), Hitachi L-2130 Pump, Hitachi L-2200 Autosampler. Chromatographic separation was performed using Hitachi column oven L-2300 and Venusil XBP C18 column (Bonna-Agela Technologies, particle size 5 μm 4.6 x 250 mm). Injection volume was 20 μL with 1mL/min flow rate and 25 °C oven. HPLC grade (Merck®) solvents were used and the mobile phase was consisted of solvent (A) Acetonitrile (ACN) and solvent (B) 0.1% Acetic acid. The gradient program was conducted with 10% of A and 90% of B at 0 min and adjusted to 20%, 40%, 60%, 80%, 10% A at 5, 10, 15, 20 and 20.1 min, respectively. Hydrophilic polypropylene membrane filters (0.45 μm-47 mm) (Pall Corporation®) were used for mobile phases and ultrapure water prior to HPLC injection. Spectra data were taken from to 200 to 400 nm during the entire run. The chromatograms were recorded at 280 nm.
Results and Discussion
Antibacterial activities
In this study, fifteen different extracts (EtOH, acetone and aqueous extracts of field-grown and in vitro-grown plants) of L. vulgaris were used to show their antibacterial activities (Table 2).
Table 2.
Antibacterial activities of L. vulgaris extracts. Data presented as zone of inhibition of bacterial growth in mm. Means with the same letter within columns are not significantly different at P>0.05
| Mean diameter of inhibitory zones (mm ± SE) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Treatments | S. aureus | S. epidermidis | S. pyogenes | S. marcescens | S. typhimurium | P. aeruginosa | P. vulgaris | K. pneumonia | E. cloacae | E. coli |
| NLW | 7.4 ± 0.2fg | - | - | - | - | - | - | 7.0 ± 0.0e | - | - |
| NLE | 7.5 ± 0.2fg | 10.7 ± 0.5fg | 9.3 ± 0.2hij | - | - | - 7.9 ± 0.2f | 7.0 ± 0.0e | - | - | |
| NLA | - | 11.4 ± 0.14f | 12.3 ± 0.8g | 7.6 ± 0.3f | - | - | - | - | - | - |
| NFW | 8.4 ± 0.2ef | 7.7 ± 0.1hi | 7.4 ± 0.1j | - | - | - | - | - | - | - |
| NFE | 17.7 ± 0.3d | 19.1 ± 0.4e | 10.9 ± 0.2ghi | 7.6 ± 0.3f | - | - | 9.8 ± 0.4e | 7.4 ± 0.1e | 8.0 ± 0.2e | 8.6 ± 2.0f |
| NFA | - | 8.2 ± 0.1hi | 11.3 ± 0.7g | - | - | - | - | - | - | - |
| NAW | 7.9 ± 0.3fg | 8.8 ± 0.2ghi | 7.8 ± 0.3j | - | - | 7.3 ± 0.1e | - | 8.8 ± 0.1d | - | - |
| NAE | 7.9 ± 0.3fg | 10.0 ± 0.4fgh | 16.1 ± 0.5f | - | - | 7.1 ± 1.0e | - | 9.3 ± 0.1d | - | - |
| NAA | 7.0 ± 0.7g | 7.0 ± 0.0i | 11.1 ± 0.3gh | - | - | - | - | - | - | - |
| IVW | - | - | - | - | - | - | - | - | - | - |
| IVE | - | - | - | - | - | - | - | - | - | - |
| IVA | 9.2 ± 0.12e | 8.6 ± 0.3ghi | 9.1 ± 0.2ij | - | - | - | 7.3 ± 0.1f | - | - | - |
| SW | - | - | - | - | - | - | - | - | - | - |
| SE | 7.0 ± 0.0g | 8.1 ± 0.3hi | 12.8 ± 0.7g | - | - | - | - | - | - | - |
| SA | - | 9.3 ± 0.3%K | 12.4 ± 0.7g | - | - | - | - | - | - | - |
| Ampicillin (10 mg) | 39.2 ± 1.9a | 23.2 ± 4.3d | 48.0 ± 2.6a | 13.4 ± 1.3d | 26.2 ± 0.6b | - | 23.2 ± 2.9c | - | 28.0 ± 1.3d | 21.6 ± 0.9d |
| Carbenicillin (100mg) | 39.6 ± 0.9a | 28.0 ± 4.3c | 44.4 ± 2.5b | 25.2 ± 0.4b | 26.4 ± 2.6b | 21.2 ± 2.8a | 34.4 ± 2.9a | - | 33.0 ± 1.4a | 23.2 ± 0.9c |
| Chloramphenicol (30 mg) | 27.0 ± 1.1c | 32.4 ± 2.8b | 34.0 ± 1.1e | 29.2 ± 1.5a | 28.0 ± 1.1a | 8.6 ± 2.4d | 21.8 ± 1.6d | 28.8 ± 0.8a | 30.8 ± 0.6b | 27.8 ± 0.9b |
| Erythromycin (15 mg) | 26.6 ± 1.4c | 35.0 ± 3.1a | 38.6 ± 1.3d | 9.4 ± 1.6e | 10.8 ± 0.7c | 10.4 ± 1.4c | 10.4 ± 0.8e | 11.8 ± 1.2c | - | 14.0 ± 2.1e |
| Tetracycline (30 mg) | 32.6 ± 1.1b | 9.4 ± 0.3fgh | 42.2 ± 2.4c | 23.6 ± 0.9c | 26.0 ± 1.1b | 17.8 ± 1.2b | 32.6 ± 2. db | 27.4 ± 1.1b | 29.6 ± 1.2c | 29.2 ± 0.6a |
| DMSO | - | - | - | - | - | - | - | - | - | - |
| Water | - | - | - | - | - | - | - | - | - | - |
Generally, tested Gram-positive bacteria (Streptococcus pyogenes, Staphylococcus aureus and Staphylococcus epidermidis) were more susceptible to the inhibitory effects of the L. vulgaris extracts than the Gram-negative bacteria. Generally, antibacterial activity was observed with positive controls (antibiotics) to our test organisms. Distilled water and DMSO were used as negative controls. Because, final concentrations of all extracts were arranged with them and no inhibition was observed with negative controls (Table 2). Generally, field-grown plant extracts exhibited better antibacterial activities than in vitro-grown plant extracts. Among the field-grown plant extracts, ethanol and acetone extracts demonstrated better antibacterial activity than aqueous extracts.
Best antibacterial activity was obtained with ethanolic extract of field-grown flowers (NFE) against S. aureus (17.7 mm) and S. epidermidis (19.1 mm), and ethanolic extract of field-grown aerial parts (NAE) against S. pyogenes (16.1 mm) (Table 2). NFE showed greater antibacterial activity than reference antibiotic tetracycline against S. epidermidis (Table 2). Generally, ethanol extracts of field-grown plant materials (leaves, flowers and aerial parts) exhibited a broad spectrum of activity against both gram-positive bacteria. S. typhimurium did not show sensitivity against to the extracts used. In addition, only ethanol extract of field-grown flowers (NFE) inhibited E. coli and E. cloacae bacteria. Ethanol and water extract of in vitro-grown plant materials (IVW and IVE) did not show any inhibitory activity against used bacteria. Only the acetone extract of in vitro-grown plant materials (IVA) exhibited moderate antibacterial activity against S. epidermidis, S. aureus, and S. pyogenes (Table 2). Ethanol and acetone extracts of field-grown seeds displayed moderate inhibition against only S. pyogenes (12.8 and 12.4 mm, respectively) and S. epidermidis (8.1 and 9.3 mm, respectively) (Table 2).
Generally, the gram-positive bacteria are much more susceptible to the plant extracts than the gram-negative bacteria. Although multi-layered and complex structure of gram-negative cell wall may cause endurance, cell wall structure of gram-positive bacteria consisting of a single layer may lead to susceptibility to tested extracts (Essawi and Srour, 2000).
Generally, antibacterial activities of field-grown plants were better than in-vitro grown plants against used bacteria. The reason may be the level of the detected secondary products in shoot cultures that is lower than the donor plants (Stafford, 1991). For example, production of steroids obtained from shoot tip cultures of Digitalis species was much lower than those found in the donor plant (Seidel and Reinhard, 1987). If the secondary product synthesis is low, there are some procedures for enhancing the productivity. Optimization of hormone regime is often effective. The type and concentration of phytohormones available to cultured cells is probably the most important factor influencing their potential for secondary product synthesis. Alterations in other environmental factors such as nutrient levels, light regime and temperature may also be effective in increasing productivity and reduced phosphate levels often stimulate product accumulation (Parr, 1989).
In the present study, alcoholic extracts of L. vulgaris exhibited better activity than aqueous extracts. Ethanol extracts of plant materials may contain active components such as tannins, polyphenols, polyacetylenes, flavonols, terpenoids, alkaloids, sterols and propolis. Also, acetone extracts of plant materials may contain flavonols (Cowan, 1999). Recio et al. (1989) showed that the phenolics were the predominant active chemicals in plants, with gram positive bacteria being the most sensible germs (Rios and Recio, 2005). It can be conceivable that antibacterial activity may come from benzoquinone, triterpene saponaside and tannin constituents of L. vulgaris (Rzadkowskabodalska and Olechnowiczstepien, 1975; Janik et al., 1994; Chevallier, 1996; Podolak et al., 1998). Flavone, quercetin and morine were found to be active against S. epidermidis (Nishino et al., 1987). The growth of S. aureus was inhibited very effectively by flavone, flavonoids, flavonones, flavonols and naringenin (Mori et al., 1987; Rauha et al., 2000).
Strong antibacterial activity of L. vulgaris extracts against S. epidermidis, S. aureus, and S. pyogenes may explain why yellow loosestrife is used in folk medicine as anti-diarrheal, anti-pyretic, anti-inflammatory and vulnerary (Grieve, 1982; Dobelis, 1990; Chevallier, 1996; Baytop, 1999).
Antitumor activities
Antitumor activity of L. vulgaris extracts was tested with the potato disc method (Ferrigini et al., 1982). For preparation of the final concentration of extracts, they were dissolved in water or DMSO (12.5%). Final concentration of extracts (NLA, NFE, NFA, NAA, SE, SA and IVA) dissolved in DMSO were 1000 mg/l. Other extracts (NLW, NLE, NFW, NAW, NAE, SW, IVW, and IVE) were dissolved in water and final concentrations of them were adjusted to 100.000 mg/l or 1000 mg/l (Table 3-5).
Table 3.
Mean number of tumors observed with L. vulgaris extracts that dissolves in water at 100.000 mg/l and controls (water and camptothecin). Means with the same letter are not significantly different at P>0.05.
| Treatments | Me an Numbe r of Tumors (± SE) | Tumor Inhibition (%) |
|---|---|---|
| Water (negative control) | 77.1 ± 5.3d | _ |
| Camptothecin (positive control) | 0 | 100 |
| NFW | 0.3 ± 0.1a | 99.6 |
| IVW | 9.4 ± 2.1b | 87.8 |
| IVE | 10.5 ± 1.3b | 86.4 |
| NAW | 11.1 ± 1.6b | 85.6 |
| NLW | 13.3 ± 1.4b | 82.7 |
| NAE | 20.4 ± 2.2c | 73.5 |
| SW | 22.8 ± 1.8c | 70.4 |
| NLE | 26.7 ± 2.3c | 65.4 |
Table 4.
Mean number of tumors observed with L. vulgaris extracts that dissolves in water at 1000 mg/l and controls (water and camptothecin). Means with the same letter are not significantly different at P>0.05.
| Treatments | Mean Number of Tumors (± SE) | Tumor Inhibition (%) |
|---|---|---|
| Water (negative control) | 119.9 ± 6.1e | _ |
| Camptothecin (positive control) | 0 | 100 |
| IVE | 50.0 ± 3.4b | 58.3 |
| IVW | 54.3 ± 4.6bc | 54.7 |
| NAE | 65.3 ± 5.1bcd | 45.5 |
| NFW | 67.4 ± 6.0bcd | 43.8 |
| SW | 72.7 ± 7.8cd | 39.4 |
| NLE | 78.6 ± 4.6d | 34.4 |
| NAW | 84.8 ± 6.8d | 29.3 |
| NLW | 131.8 ± 10.3e | - |
Table 5.
Mean number of tumors observed with L. vulgaris extracts that dissolved in DMSO at 1000 mg/l and controls (DMSO and camptothecin). Means with the same letter are not significantly different at P>0.05.
| % Inhibition of DPPH Concentrations | ||||
|---|---|---|---|---|
| Treatments | 25 μg/ml | 50 μg/ml | 100 μg/ml | 200 μg/ml |
| Ascorbic acid | 62.35 | 97.01 | 97.21 | 96.91 |
| NLW | 96.3 | 97.2 | 97.6 | 97.7 |
| NLE | 94.7 | 95.6 | 96.7 | 98.1 |
| NFW | 91.3 | 94.6 | 94.9 | 95.9 |
| NFE | 95.3 | 96.5 | 97.1 | 98.5 |
| NAW | 94.0 | 94.4 | 94.8 | 95.1 |
| NAE | 94.4 | 94.9 | 95.2 | 95.8 |
| IVW | 80.5 | 84.7 | 85.2 | 91.3 |
| IVE | 62.8 | 63.7 | 65.7 | 61.7 |
All extracts in high concentration (100.000 mg/l) in water showed strong antitumor activity (Table 3). Best antitumor activity was observed with water extract of field-grown flowers (NFW) (99.6% inhibition) at 100.000 mg/l. The extracts in low concentration (1000 mg/l) in water also showed moderate antitumor activity except water extract of field-grown leaves (Table 4). Among these extracts in water, best antitumor activity was observed with ethanol extract of in vitro-grown leaves and flowers (IVE) at 1000 mg/l (Table 4). On the other hand, although water extract of field-grown leaves (NLW) showed 82.7% tumor inhibition at 100.000 mg/l, the tumor inhibition of NLW was not observed at 1000 mg/l (Table 3 and 4). Extracts in DMSO showed moderate antitumor activity (Table 5). Best antitumor activity was observed with acetone extract of field-grown leaves (NLA) and aerial parts (NAA) (Table 5).
Generally, when compared with control (water or DMSO), the percentage inhibition of all extracts was more than 29% except in ethanol extract of field-grown flowers (NFE) and water extract of field-grown leaves (NLW) in three separate experiments (Table 3-5). No tumor formation was observed with camptothecin (100% inhibition) (Table 3-5).
Inhibition of crown gall formation on potato discs is caused by two effects: by anti-tumorogenesis or decreasing the viability of the A. tumefaciens. Viability tests were conducted with extracts to separate between these possibilities. Bacterial viability was evaluated by incubating L. vulgaris extracts with 1x109 colony-forming units (CFU) of A. tumefaciens bacterial suspension. L. vulgaris extracts had no effect on the viability of bacterium. Therefore, we concluded that the tested L. vulgaris extracts affected on the formation of tumors and not on the viability of bacterium.
Final concentrations of the extracts were adjusted with water or DMSO (12.5%). Because, there was dissolution problem of some extracts (NLA, NFE, NFA, NAA, SE, SA and IVA). They were dissolved in 12.5% DMSO (Ferrigini, 1982) that did not affect the viability of bacteria. This concentration of DMSO was safe for using as a dissolving solvent in antitumor assays. Concentration of extracts (NLA, NFE, NFA, NAA, SE, SA and IVA) dissolved in DMSO were 1000 mg/l. On the other hand, other extracts (NLW, NLE, NFW, NAW, NAE, SW, IVW, and IVE) were dissolved in water easily and final concentrations of them were adjusted to 1000 mg/l or 100.000 mg/l.
In the present study, alcoholic extracts, especially ethanol extracts, showed higher antitumor activity than aqueous extracts. Ethanol and acetone extracts of plant materials may contain active components such as tannins, polyphenols, polyacetylenes, flavonol, terpenoids, sterols, alkaloids and propolis groups (Kaul et al., 1985; Scalbert, 1991; De Pasquale et al., 1995). These components may cause strong antitumor activity against A. tumefaciens.
Cytotoxic activity of underground parts of L. vulgaris was reported by Podolak et al. (1998). Antitumor activity of aerial parts of L. vulgaris was observed for the first time with this study.
Antioxidant activities
DPPH assay has been widely used to evaluate the free radical scavenging effectiveness of various antioxidant substances. So, DPPH radical is often used as a substrate to evaluate the scavenging activity of antioxidant compounds. The reduction capability on the DPPH radical is determined by the decrease in its absorbance at 517 nm induced by antioxidants. The decrease in absorbance of DPPH radical caused by antioxidants is due to the reaction between antioxidant molecules and radical.
The results indicated that at minimum concentration (25 μg/ml), extracts showed higher DPPH scavenging activity than ascorbic acid that was used as control antioxidant. After this concentration (25 μg/ml), there was no significant increase in DPPH scavenging activity except in IVW extract. The extracts other than IVW and IVE extracts demonstrated similar DPPH scavenging activity at 50 μg/ml and over as in the ascorbic acid. IVE extract had little tendency to scavenge DPPH radicals than the other extracts (Table 6).
Table 6.
% inhibition of DPPH by L. vulgaris extracts.
| % inhibition of DPPH Concentrations | ||||
|---|---|---|---|---|
| Treatments | 25 μg/ml | 50 μg/ml | 100 μg/ml | 200 μg/ml |
| Ascorbic acid | 62.35 | 97.01 | 97.21 | 96.91 |
| NLW | 96.3 | 97.2 | 97.6 | 97.7 |
| NLE | 94.7 | 95.6 | 96.7 | 98.1 |
| NFW | 91.3 | 94.6 | 94.9 | 95.9 |
| NFE | 95.3 | 96.5 | 97.1 | 98.5 |
| NAW | 94.0 | 94.4 | 94.8 | 95.1 |
| NAE | 94.4 | 94.9 | 95.2 | 95.8 |
| IVW | 80.5 | 84.7 | 85.2 | 91.3 |
| IVE | 62.8 | 63.7 | 65.7 | 61.7 |
Phenols are very important plant constituents because of their radical scavenging ability due to their hydroxyl groups. In water and ethanol extracts of L. vulgaris (NLW, NLE, NFW, NFE, NAW, NAE and IVW), 280.3, 585.9, 188.4, 260.9, 266.2, 483.8 and 40.9 mg/g gallic acid equivalent of phenols were detected. Among the extracts, NLE extract had the highest phenolic content. When extracts were compared with respect to their phenol contents, ethanolic extracts contained more phenolics than water extracts (Table 7).
Table 7.
Total phenolic and flavonoid content of L. vulgaris extracts.
| Treatments | Total Phenolics in mg GA/g dry extract | Total Flavonoids in mg CE/g dry extract |
|---|---|---|
| NLW | 93.55 ± 0.000 | 96.54 ± 0.00 |
| NLE | 195.36 ± 0.000 | 99.37 ± 0.00 |
| NFW | 62.94 ± 0.00 | 47.14 ± 0.00 |
| NFE | 86.88 ± 0.00 | 64.08 ± 0.00 |
| NAW | 88.69 ± 0.00 | 72.55 ± 0.00 |
| NAE | 161.42 ± 0.00 | 95.13 ± 0.00 |
| IVW | 13.55 ± 0.00 | 27.38 ± 0.00 |
The results for total flavonoid content in the studied extracts indicated that the methanolic extracts contained the highest amount of flavonoids than water extracts. The result was obtained such in an order of NLE>NLW>NAE>NAW>NFE>NFW>IVW (99.37, 96.54, 95.13, 72.55, 64.08, 47.14 and 23.38 mg/g catechol equivalent, respectively) (Table 7). There was a correlation between total phenol and flavonoid contents of the tested extracts except NAE. Although NAE extract contained more phenol than NLE extract, the total flavonoid content of NLE extract was more than in NAE extract.
Toth et al. (2012; 2014) compared polyphenol composition and antioxidant capacity of three Lysimachia species (L. nummularia L., L. punctate L. and L. vulgaris). L. vulgaris extract had lower scavenger capacity than the other studied Lysimachia species. Similarly, they detected caffeic acid, myricetin, quercetin and kaempferol in L. vulgaris. However, the gallic acid, rutin hydrate, luteolin-7-O-β-D glucoside, coumarin and apigenin in L. vulgaris were demonstrated for the first time with this study. Among the extracts, the leaf extracts (NLE and NLW) had higher total flavonoid content than aerial and flower extracts of L. vulgaris in our study. Similarly, Toth et al. (2012) reported that the methanolic extract of L. vulgaris leaf contained higher amount of flavonoid, hydroxycinnamic acid and tannin than stem and herba.
On the basis of our results, it is evident that extracts of L. vulgaris have powerful in vitro antioxidant activity and they are rich in terms of phenolics and flavonoids. They can be used as a reachable source of natural antioxidants, as a possible food supplement or in pharmaceutical industry. The antioxidant mechanisms of the phenols or flavonoids may be based on strong hydrogen donating ability of them. Phenolic compounds seem to be responsible for the antioxidant activity of extracts of yellow loosestrife. The reason of high phenolic content of them may be interrelated with stress conditions in the natural environment. Exposing of the plants to different types of stress conditions such as drought, heat, ultraviolet light, air pollution, and pathogen attack, might induce the synthesis of some phenolic compounds to adapt these stresses (Rout et al., 2000). Gülçin et al. (2004) pointed out that antioxidants activated the analgesic responses. Traditional usage of yellow loosestrife as analgesic may be related with their antioxidant activities.
HPLC analysis of phenolic compounds
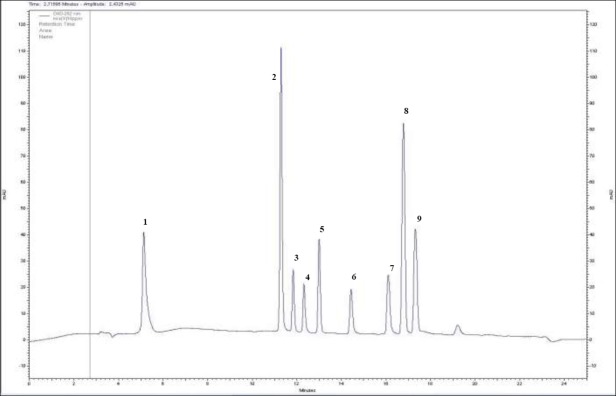
HPLC analysis was carried out with methanol extracts of field-grown (NLM) and in vitro-grown L. vulgaris (ILM). A mixture of nine standard phenolic compounds (apigenin, caffeic acid, coumarin, gallic acid monohydrate, kaempferol 3-β-Ο-glucopyranoside, luteolin-7-O-β-D glucoside, myricetin, quercetin, rutin hydrate) was eluted using HPLC and a chromatogram for the standards was obtained after elution with suitable solvent (Figure 1). Then, the phenolic contents of methanol extracts were compared with the standard chromatogram and the amounts (mg/1g extract) of each phenolic compound in the extracts were determined (Table 8).
Figure 1.
Chromatogram of the selected standards. 1. Gallic acid, 2. Caffeic acid, 3. Rutin hydrate, 4. Luteolin-7-O-β-D glucoside, 5. Kaempferol, 6. Myricetin, 7. Quercetin, 8. Coumarin, 9. Apigenin.
Table 8.
Identified phenolic compounds and their amounts in the methanolic extracts of field-grown and in vitro-grown L. vulgaris.
| STANDART COMPOUNDS | PLANT EXTRACTS (mg/g dry extract) | |||
|---|---|---|---|---|
| Name | Peak number | RT (min) | NLM | ILM |
| Gallic acid monohydrate | 1 | 5.15 | 0.1416 | 0.00562 |
| Caffeic acid | 2 | 11.25 | 0.0747 | 0.19345 |
| Rutin hydrate | 3 | 11.82 | 29.0241 | 0.56243 |
| Luteolin-7-O-β-D glucoside | 4 | 12.3 | 8.9261 | 1.80619 |
| Kaempferol-3β-D-glucopyranoside | 5 | 12.98 | 0.4370 | 0.30864 |
| Myricetin | 6 | 14.4 | 0.4953 | 0.30491 |
| Quercetin | 7 | 16.07 | 1.6796 | 0.04125 |
| Coumarin | 8 | 16.76 | 0.0189 | 0.00478 |
| Apigenin | 9 | 17.29 | 0.4531 | 0.04957 |
| Total phenolics | 41.2474 | 3.27685 | ||
According to the results of HPLC analyses, the amount of studied phenolic compounds except caffeic acid in field-grown plant extract was higher than those in in vitro-grown plant extracts. Luteolin-7-O-β-D glucoside and rutin hydrate was dominant compound in ILM extract (1.80619 mg/g and 0.56243 mg/g, respectively). Also, in NLM extract, rutin hydrate and luteolin-7-O-β-D glucoside (29.0241 mg/g and 8.9261 mg/g, respectively) were dominant compounds. Along with the rutin hydrate and luteolin-7-O-β-D glucoside, the quercetin was found in high concentrations (1.6796 mg/g) in NLM extract. Moreover, kaempferol 3-β-D-glucopyranoside and myricetin were also present in high amounts in ILM extract. These two flavonols have been found in similar concentrations (0.30864 mg/g and 0.30491 mg/g, respectively) in ILM extract. And these two flavonols and apigenin have been found in similar concentrations (0.437 mg/g, 0.4953 mg/g and 0.4531 mg/g, respectively) in NLM extract.
The amounts of studied phenolic compounds except kaempferol 3-β-D-glucopyranoside, myricetin and caffeic acid were found quite higher in NLM extract than those found in ILM extract. Coumarin was found the lowest in two extracts but its amount in NLM extract was four times higher than ILM extract. Among the studied phenolic compounds, only the amount of caffeic acid was found higher in ILM extract (0.19345 mg/g) than found in NLM extract (0.0747 mg/g).
Conclusion
Herein, the results confirmed the antibacterial, antitumor and antioxidant activities of yellow loosestrife extracts. Definitive clinical studies are needed to fully understand different medicinal uses of yellow loosestrife. Future studies should focus on fractionation of the extracts in hopes of identifying other active components. This plant may be a suitable source for drugs that could improve the treatment of infections caused by gram-positive bacteria and also it can be a source of food additives as antioxidant due to preventing the liposomal oxidation and prevention the cancer in diet.
Acknowledgements
The authors are grateful to The Scientific and Technological Research Council of Turkey (TUBITAK) for financial support (TBAG-HD-109T506). We are grateful to Dr. Hakan Turker for his technical support.
References
- 1.Andrews JM. BSAC standardized disc susceptibility testing method. J. Antimicrob. Chemother. 2004;53:713–28. doi: 10.1093/jac/dkh113. [DOI] [PubMed] [Google Scholar]
- 2.Baytop T. Türkiye’de bitkiler ile tedavi. Nobel Tıp Kitabevleri; Istanbul: 1999. [Google Scholar]
- 3.Borin M. Phytopurification solutions for treatment of wastewater with plants. Edagricole Press; Italy: 2003. [Google Scholar]
- 4.Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. Food Science and Technology-Lebensmittel-Wissenschaft & Technologie. U.-Technol. 1995;28(1):25–30. [Google Scholar]
- 5.Chevallier A. The Encyclopedia of Medicinal Plants. Darling Kindersley Limited; London: 1996. [Google Scholar]
- 6.Coker PS, Rodecke J, Guy C, Camper ND. Potato disc tumor induction assay: A multiple mode of drug action assay. Phytomedicine. 2003;10:133–138. doi: 10.1078/094471103321659834. [DOI] [PubMed] [Google Scholar]
- 7.Cowan MM. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999;12(4):564–582. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis PH. Vol. 6. Edinburgh University Press; England: 1978. Flora of Turkey and the East Aegean Islands. [Google Scholar]
- 9.De Pasquale R, Germano MP, Keita A, Sanogo R, Iauk L. Antiulcer activity of Pteleopsis suberosa. J. Ethnopharmacol. 1995;47:55–58. doi: 10.1016/0378-8741(95)01256-d. [DOI] [PubMed] [Google Scholar]
- 10.Dobelis IN. Magic and Medicine of Plants. The Reader’s Digest Association; New York: 1990. [Google Scholar]
- 11.Essawi T, Srour M. Screening of some Palestinian medicinal plants for antibacterial activity. J. Ethnopharmacol. 2000;70:343–349. doi: 10.1016/s0378-8741(99)00187-7. [DOI] [PubMed] [Google Scholar]
- 12.Ferrigini NR, Putnam JE, Anderson B, Jacobsen LB, Nichols DE, Moore DS, McLaughlin JL. Modification and evaluation of the potato disc assay and antitumor screening of Euphorbia seeds. J. Nat. Prod. 1982;45:679–686. doi: 10.1021/np50024a005. [DOI] [PubMed] [Google Scholar]
- 13.Grieve M. A modern herbal. Vol. 2. Dover Publications; New York: 1982. [Google Scholar]
- 14.Gülçin I, Küfrevioglu I.Ö, Oktay M, Büyükokuroğlu ME. Antioxidant, antimicrobial, antiulcer and analgesic activities of nettle (Urtica dioica L.) J. Ethnopharmacol. 2004;90:205–215. doi: 10.1016/j.jep.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 15.Hao G, Yuan YM, Hu CM, Ge XJ, Zhao NX. Molecular phylogeny of Lysimachia (Myrsinaceae) based on chloroplast trnL-F and nuclear ribosomal ITS sequences. Mol. Phylogenet. E. 2004;31(1):323–339. doi: 10.1016/S1055-7903(03)00286-0. [DOI] [PubMed] [Google Scholar]
- 16.Janik I, Janeczko Z, Sendra J, Burczyk J. Benzoquinone pigment from Lysimachia vulgaris. Fitoterapia. 1994;65(5):476. [Google Scholar]
- 17.Kaul TN, Middletown JE, Ogra PL. Antiviral effect of flavonoids on human viruses. J. Med. Virol. 1985;15:71–79. doi: 10.1002/jmv.1890150110. [DOI] [PubMed] [Google Scholar]
- 18.Marinova D, Ribarova F, Atanassova M. Total phenolics and total flavonoids in Bulgarian fruits and vegetables. J. Univ. Chem. Technol. Metallurgy. 2005;40:255–260. [Google Scholar]
- 19.McLaughlin JL, Rogers LL, Anderson JE. The use of biological assays to evaluate botanicals. Drug Inf. J. 1998;32:513–524. [Google Scholar]
- 20.Mori A, Nishino C, Enoki N, Tawata S. Antibacterial activity and mode of action of plant flavonoids against P. vulgaris and S. aureus. Phytochemistry. 1987;8:2231–2234. [Google Scholar]
- 21.Nishino C, Enoki N, Tawata S, Morim A, Kobayashi K, Fukushima M. Antibacterial activity of flavonoids against S.epidermidis a skin bacterium. Agric. Biol. Chem. 1987;1:139–143. [Google Scholar]
- 22.Oh IC, Anderberg AL, Schonenberg J, Anderberg AA. Comparative seed morphology and character evolution in the genus Lysimachia (Myrsinaceae) and related taxa. Plant Syst. E. 2008;271(3-4):177–197. [Google Scholar]
- 23.Parr AJ. The production of secondary metabolites by plant cell cultures. J. Biotechnol. 1989;10:1–26. [Google Scholar]
- 24.Podolak I, Elas M, Cieszka K. In vitro antifungal and cytotoxic activity of triterpene saponosides and quinoid pigments from Lysimachia vulgaris L. Phytother. Res. 1998;12:70–73. [Google Scholar]
- 25.Podolak I, Strzalka M. Qualitative and quantitative LC profile of embelin and rapanone in selected Lysimachia species. Chromatographia. 2008;67(5-6):471–475. [Google Scholar]
- 26.Rahua JP, Remes S, Heinonen M, Hopia A, Kahkonen M, Kujala T, Pihlaja K, Vuorela H, Vuorela P. Antimicrobial effects of Finnish plant extracts containing flavonoids and other phenolic compounds. Int. J. Food Microbiol. 2000;56:3–12. doi: 10.1016/s0168-1605(00)00218-x. [DOI] [PubMed] [Google Scholar]
- 27.Recio MC, Ríos JL, Villar A. A review of some antimicrobial compounds isolated from medicinal plants reported in the literature 1978-88. Phytother. Res. 1989;3:117–125. [Google Scholar]
- 28.Rios JL, Recio MC. Medicinal plants and antimicrobial activity. J. Ethnopharmacol. 2005;100:80–84. doi: 10.1016/j.jep.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 29.Rout GR, Samantaray S, Das P. In vitro manipulation and propagationof medicinal plants. Biotechnol. Adv. 2000;18:91–120. doi: 10.1016/s0734-9750(99)00026-9. [DOI] [PubMed] [Google Scholar]
- 30.Rzadkowskabodalska H, Olechnowiczstepien W. Flavonoids in herb of yellow loosestrife (Lysimachia vulgaris L.) Pol. J. Pharmacol. Pharm. 1975;27(3):345–348. [PubMed] [Google Scholar]
- 31.Scalberti A. Antimicrobial properties of tannins. Phytochemistry. 1991;30:3875–3883. [Google Scholar]
- 32.Seidel S, Reinhard E. Major cardenolide glycosides in embryogenic suspension cultures of Digitalis lanata. Planta Med. 1987;15:308–309. doi: 10.1055/s-2006-962722. [DOI] [PubMed] [Google Scholar]
- 33.Slinkard K, Singleton VL. Total phenol analyses: Automation and comparison with manual methods. Am. J. Enol. Viticult. 1977;28:49–55. [Google Scholar]
- 34.Stafford A. Natural products and metabolite from plants and plant tissue cultures. In: Stafford A, Warren G, editors. Plant Cell and Tissue Culture. Open University Press; Buckingham: 1991. pp. 125–162. [Google Scholar]
- 35.Toth A, Riethmüller E, Alberti A, Végh K, Kery A. Comparative phytochemical screening of phenoloids in Lysimachia species. Eur. Chem. Bull. 2012;1(1-2):27–30. [Google Scholar]
- 36.Toth A, Toth G, Kery A. Polyphenol composition and antioxidant capacity of three Lysimachia species. Nat. Prod. Commun. 2014;9:1473–1478. [PubMed] [Google Scholar]
- 37.Turker AU, Guner B. Efficient plant regeneration of Yellow Loosestrife (Lysimachia vulgaris L.), a medicinal plant. Acta Biol. Hung. 2013;64(2):218–230. doi: 10.1556/ABiol.64.2013.2.8. [DOI] [PubMed] [Google Scholar]
- 38.Turker AU, Yildirim AB, Karakas FP. Antibacterial and antitumor activities of some wild fruits grown in Turkey. Biotechnol. Biotech. Eq. 2012;26(1):2765–2772. [Google Scholar]
- 39.Yasukawa K, Takido M. Studies of the constituents of genus Lysimachia3.Quercetin 3-Rhamnosyl (1-2) galactoside from Lysimachia vulgaris var. davurica. Phytochemistry. 1988;27(9):3017–3018. [Google Scholar]