Abstract
Background:
Holarrhena floribunda is a plant of wide usage in the Togolese folk medicine. A previous ethnobotanical survey on the latex plants of the Maritime region of the country revealed that this plant was included in several recipes curing malaria and microbial infections. Therefore, this study aimed to seek for the effectiveness of the ethanolic extract of the plant in the treatment of these diseases.
Methods:
The antimicrobial test was performed using the agar well-diffusion and the NCCLS broth microdilution methods, while the in vivo antimalarial activity was evaluated following the four-day suppressive test of Peters. The acute toxic effects of the extract were monitored after a single oral dose (5,000 mg/kg body weight) administration in NMRI mice.
Results:
The results indicated that the ethanolic extract of leaves of H. floribunda was active on Staphylococcus aureus ATCC 29213 and clinical strains of Staphylococcus aureus, Salmonella typhi and Klebsiella pneumoniae with MICs ranging from 0.62 to 1.25 mg/mL. The extract also showed significant parasitaemia suppression in a dose-dependent manner. In the acute toxicity assay, the oral administration of the extract to the mice did not affect the relative weight of vital organs, and there were no signs of toxicity or death during the study period. The LD50 of the tested extract was found to be greater than 5,000 mg/kg, indicating its safety.
Conclusion:
This study demonstrates the antibacterial and antimalarial activities of leaves of H. floribunda and then, supports its medicinal use in the treatment of microbial infections.
Keywords: Holarrhena floribunda, ethanolic extract, antibacterial, antimalarial, toxicity
Introduction
Holarrhena floribunda (G. Don) T. Durand and Schinz, is a plant belonging to the family of Apocynaceae. It grows as a shrub or a tree. The plant is widely distributed in West Africa, where several parts of the plant are used for medicinal purposes (Yemoa et al., 2015). The stem-bark and leaves are used to treat various diseases including malaria, fever, dysentery, amoebic diseases, diarrhoea, sterility, amenorrhea and diabetes (Bouquet & Debray, 1974; Kerharo & Adam, 1974; Arbonnier, 2000; Fotie et al., 2006; Bayala et al., 2006). The roots are boiled in milk and used to bathe boys attaining puberty in addition to cure snakebites and venereal diseases (Iwu, 2014). In our previous study on the latex plant used in the maritime region of Togo, we found that the decoction of the plant was administrated by oral route for the treatment of malaria and bacterial infections (Hoekou et al., 2016).
Some previous pharmacological screenings showed that, the stem bark of H. floribunda was febrifuge and could be a quinine substitute, since it showed remarkable inhibitory activity against drug-resistant strains of Plasmodium falciparum. The chemical screening of the plant revealed the presence of steroid alkaloids notably conessine, that is used for the destruction of amoeba without emetic effects (Berhaut, 1971; Fotie et al., 2006). The antioxidant, antimutagenic, and lipid peroxidation inhibition potentials were also reported (Badmus et al., 2013). The aqueous, ethanolic, and chloroformic extracts of the stem bark of H. floribunda have been found to exhibit significant activity in vitro against drug-resistant clones of Plasmodium falciparum W-2 and D-6 (Fotie et al., 2006). However, the pharmacological properties and toxic effects of the leaves have not been intensively studied. Our study aimed to evaluate the in vitro antibacterial and in vivo antimalarial activities of the ethanolic extract of the leaves. Further, the oral acute toxicity was assayed in order to validate its therapeutic potentials.
Materials and Methods
Plant materials
The leaves of H. floribunda were collected from Tsévié, located at 35 km north of Lomé (Togo). The plant materials were obtained in October 2014 and botanically authenticated at the Department of Plant Biology and Ecology, University of Lomé, where a voucher specimen (voucher number TG12749) was deposited.
Bacteria and parasite strains
The microorganisms used for the antibacterial tests were Gram-positive (Staphylococcus aureus ATCC 29213 and clinical strains of Staphylococcus aureus), and Gram-negative (Escherichia coli ATCC 25922, Pseudomonas aeruginosa ATCC 27853, and clinical strains of Salmonella typhi, Klebsiella pneumoniae). The ATCC strains were obtained from the American Type Culture Collection via National Institute of Public Health of Togo, whereas the clinical strains were from the laboratory of Navrongo Health Research Centre (Ghana). These strains are often involved in human pathology. Salmonella typhi strains were isolated from diarrhoeic stools. The clinical strains of S. aureus and K. pneumoniae were isolated from urine.
The in vivo antimalarial testing in mice was done using a chloroquine sensitive strain of Plasmodium berghei (ANKA strain) obtained from MR4. On weekly basis, the parasites were maintained by serial passage of blood from infected mice having a parasitemia level of 10-15% to non-infected ones.
Experimental animals
Female NMRI mice (6-8 weeks and 22.92 ± 0.23 g weight) were used for the in vivo antiplasmodial test and female and male NMRI mice (8-10 weeks of age and 29.3 ± 1.64 g of weight) were used for acute oral toxicity assay. The mice were housed five per cage for in vivo antimalarial test and three per cage for toxicity assay. All the mice were purchased from the “Centre International de Recherche Développement sur l’Elevage en Zone Subhumide” (CIRDES), Bobo-Dioulasso, Burkina Faso, and acclimatised for a week prior to the start of the study. Animals were maintained in the same environmental conditions (temperature 24 ± 3°C and 12hours photoperiod) throughout the experiments, and fed with standard food provided by “Service Regional d’élevage de Bobo Dioulasso, Burkina Faso”. Water and food were left ad libitum. Experimental animal rearing and handling were approved by the Office of Laboratory Animal Welfare (A5926-01) and were conducted in full adherence with NIH guidelines for care and use of Laboratory animals (Pub. No. 85-23, Revised 1985).
Extractions
The leaves of H. floribunda were washed thoroughly and dried in air at room temperature for two weeks. Afterwards, the plant materials were powdered using a grinding machine. The ethanolic extraction was performed by maceration of 100 g powder in 1000 mL of 70% (v/v) ethanol, under continuous stirring for 48 hours at room temperature. The extract was filtered through Whatman No.1 filter paper and evaporated at 45°C using a rotary evaporator to dryness under reduced pressure. The yield of the extract was 16.45%. The extract was preserved at 4°C in refrigerator till used.
In vitro antibacterial activity
The agar well-diffusion method was used to investigate the antimicrobial properties of the extracts as described by the National Committee for Clinical Laboratory Standards (NCCLS, 2003). The bacterial strains grown on nutrient agar at 37°C for 18 to 24hours were suspended in a saline solution (0.9%, w/v) to a turbidity of 0.5 Mac Farland standards (108 CFU/mL). The suspension was used to inoculate Mueller Hinton agar, 90 mm diameter Petri dishes with a sterile cotton swab on a wooden applicator. A sterilized steel borer of an internal diameter of about 6 mm was used to bore holes in the Mueller Hinton plates. The dried ethanolic extract was dissolved in 1% dimethylsulfoxide at the desired concentration to fill the wells punched in the plates. Then the plates were incubated at 37°C for 24 hours. The antibacterial susceptibility was assessed by measuring the inhibition zone diameters around the wells (CASFM, 2014). Ciprofloxacin (5 μg/mL) and DMSO 1% in sterilized distilled water were used as positive and negative controls respectively. The Minimum Inhibitory Concentrations (MIC) and Minimal Bactericidal Concentrations (MBC) were determined using the Muller Hinton broth microdilution in 96 well-plates according to National Committee for Clinical Laboratory (NCCLS, 2003). The 0.5 Mac Farland suspensions were diluted with the broth to inoculate 96 well-plates containing 2-fold serial dilutions of extracts. The extract concentrations were ranged from 20 to 0.039 mg/mL, and the ones of standard drug, from 5 to 0.0025 μg/mL. The final volume in wells was 200 μL and the bacterial load was approximately 105CFU/well hours. The plates were incubated at 37°C for 24 hours. The MIC was recorded as the lowest extract concentration demonstrating no visible growth in the broth, while the MBC was recorded as the lowest extract concentration killing 99.9% of the bacterial inoculums. The MBC values were determined by removing 100 μL of the bacterial suspension from subculture demonstrating no visible growth and inoculating the nutrient agar plates that were incubated at 37°C for 24 hours. In addition to MIC, the total activity of the extract was calculated as the total mass in mg extracted from 1g of plant material divided by the MIC value (mg/mL). The totalactivity in mL/g indicates the volume to which the extract derived from g of plant material can be diluted and still inhibits the growth of the microorganism (Eloff, 2000).
In vivo antiplasmodial activity
The experiment was performed in mice based on the 4-day suppressive test (Peters & Robinson, 1992; Fidock et al., 2004; Tarkang et al., 2014). Five groups of five mice were used. At day 0, the mice were inoculated by intra peritoneal injection with 107 red blood cells of donor mouse parasitized with Plasmodium berghei ANKA strain. Two hours later, 200 μL of plant extract at 100, 250, and 500 mg kg-1 body weight were administered to the animals by oral route once a day, from day 0 to day 3. The negative control group received the vehicle (mixture 90% distilled sterile water - 7% Tween 80 - 3% Ethanol) and the positive control group received chloroquine at the dose of 25 mg/kg/day. On the day 4, thin blood Giemsa-stained smears from each animal were examined microscopically. The parasitaemia level was determined by counting the number of parasitized erythrocytes out of 100 erythrocytes in random fields of the microscope. The parasitaemia for each group of mice was recorded and the percentage suppression of parasitaemia calculated as follow:
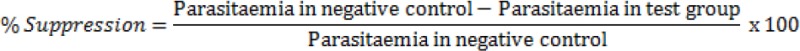
The body weights of the mice were taken to observe whether the test extract prevented the weight loss in infected mice. The weights were measured at D0 and D4.
Determination of the mean survival time
The mortality was monitored daily and the number of days from the time of inoculation of the parasite up to death was recorded for each mouse in the treatment and control groups. The mean survival time in days for each mouse was recorded after the treatment periods in comparison to untreated and standard drug treated groups.
Acute oral toxicity study
The acute oral toxicity of ethanolic extract was evaluated according to the procedures outlined by the Organization for Economic Co-operation and Development (OECD, 2001). The mice were assigned randomly to the control and treatment groups. In each group, there were 6 mice out of which 3 males and 3 females. The treated groups were given oral administration using an intubation needle of H. floribunda leaves ethanolic extract at a single dose of 5,000 mg/kg body weight suspended in a mixture of distilled sterile water - Tween 80 - ethanol (90-7-3) %, while the control group received the vehicle. Food and water were provided to the mice approximately an hour after treatment. The animals were observed 30 min after administration, followed by hourly observation for 8 hours and once a day for the next 13 days. Observations were systematically recorded for each animal. The mice were observed for toxic signs (mortality, behavioural pattern, changes in physical appearance, injury, pain and signs of illness) and the body weight was recorded at day 0, day 7, and day 14 throughout the 14-day study period. The animals were sacrificed on day-15 by an overdose of ether diethyl and were dissected to obtain the organs such as liver, heart, lungs, spleen, and kidneys for macroscopic examination and each organ’s weight (absolute organ weight in g) was recorded. Using the following formula, the relative organ weight (ROW) of each organ was calculated:
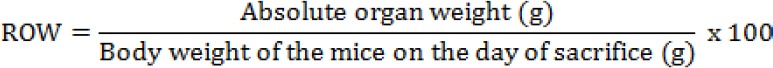
Data analysis
The data were analyzed using the computer software SPSS, version 22. The Results were expressed as a mean ± standard deviation. The statistical significance was determined by one way analysis of variance (ANOVA) with multiple comparison tests of Tukey. Two tailed paired t-test was used to compare the mean body weight before and after treatment. The statistical significance was set at P < 0.05.
Results
Antibacterial activities
Table 1 summarizes the results of the antimicrobial assays. The agar diffusion assay with the extract yielded inhibition diameters ranging from 0 mm to 19.5 ± 0.7 mm. The highest inhibition was recorded with Staphylococcus species both the clinical strains and the reference one. This was followed by K. pneumoniae and S. typhi which recorded inhibition zone of 17.5 ± 0.7 mm and 14 ± 1.4 mm, respectively. E. coli ATCC 25922 and P. aeruginosa ATCC 27853 resisted to the extract by displaying no inhibition zone around the wells. This result on the agar was confirmed by the broth microdilution. Thus, the MIC and the MBC with these strains were greater than 10 mg/mL. For the rest, the extract inhibited the growth of Staphylococcus species, S. typhi and K. pneumoniae with MIC values ranging from 0.62 to 1.25 mg/mL. The calculated total activity of the extract was 265.3 mL/g for S. aureus ATCC 29213, and 131.6 mL/g for the clinical strains (S. aureus, K. pneumoniae and S. typhi). The reference antibiotic ciprofloxacin used also displayed varying diameters. It was more active on E. coli ATCC 25922 (MIC = 0.01 μg/mL) and less active on P. aeruginosa ATCC 27853 (MIC = 0.62 μg/mL).
Table 1.
Antibacterial activities of ethanolic extract of leaves of H. floribunda
| Bacteria | Extract | Ciprofloxacin | ||||
|---|---|---|---|---|---|---|
| IZ (mm) | MIC (mg/mL) | MBC (mg/mL) | Total activity (mL/g) | IZ (mm) | MIC (μg/mL) | |
| E. coli ATCC 25922 | 0 | > 10 | > 10 | < 16.45 | 29.5 ± 0.7 | 0.01 |
| P. aeruginosa ATCC 27853 | 0 | > 10 | > 10 | < 16.45 | 25 ± 0 | 0.62 |
| S. aureus ATCC 29213 | 19 ± 0 | 0.62 | 0.62 | 265.3 | 32.5 ± 2.1 | 0.31 |
| S. aureus | 19.5 ± 0.7 | 1.25 | 1.25 | 131.6 | 28 ± 1.4 | 0.31 |
| S. typhi | 14 ± 1.4 | 1.25 | 2.5 | 131.6 | 29 ± 1.4 | 0.16 |
| K. pneumoniae | 17.5 ± 0.7 | 1.25 | 2.5 | 131.6 | 24 ± 2.8 | 0.16 |
IZ: inhibition zones; MIC: Minimum Inhibitory Concentration; MBC: Minimal Bactericidal Concentration.
Antimalarial activity
The effects of the extract on the parasitaemia and the mean survival time of mice are presented in table 2. The extract reduced the parasitaemia for 6.96%, 29.06 and 37.71% respectively at 100, 250 and 500 mg/kg. The results indicated that the extract exerted a dose dependent chemosuppressive effect in mice infected with P. berghei parasite. The mice treated with chloroquine 25 mg/kg were completely free from the parasites after four days. The extract significantly suppressed (P < 0.05) the parasitaemia at all dose levels compared to the negative control group. P. berghei infected mice treated with extract showed a shorter survival time as compared to chloroquine treated infected mice. The groups of mice treated at 500 mg/kg dose of extract, and chloroquine survived significantly (P < 0.05) compared to negative control group.
Table 2.
Suppressive effect of ethanolic extract of leaves of H. floribunda and mean survival time
| Treatment / Extract | Doses (mg/kg) | Percentage parasitaemia | Percentage inhibition | Mean survival time (days) |
|---|---|---|---|---|
| Negative Control (W-Tween 80-E) | 0.2 ml | 23.4 ± 2.07 | 0 | 7.64 ± 0.41 |
| Chloroquine | 25 | 0 ± 0.00 | 100 ± 0.00* | 16.15 ± 0.51* |
| Ethanolic extract | 100 | 21.8 ± 2.58 | 6.96 ± 2.64 | 7.57 ± 0.53 |
| 250 | 16.6 ± 2.07 | 29.06 ± 5.49* | 8.39 ± 0.61 | |
| 500 | 14.6 ± 1.82 | 37.71 ± 3.05* | 8.81 ± 0.44* |
W-Tween 80-E = 90% of distilled sterile water + 7% Tween 80 + 3% Ethanol; n = 5;
= - values are significantly different as compared to the negative control.
The body weight of the infected mice changed significantly (p < 0.05) after 4 days for the control groups and group treated at 250 mg/kg of extract. No significant (p > 0.05) change was observed in the body weight of groups treated at 100 mg/kg and 500 mg/kg of extract. The body weight measured on day 4 indicated that the tested extract of H. floribunda prevented significantly (P < 0.05) the body weight loss at dose levels of 250 mg/kg, and 500 mg/kg, due to parasitaemia. In the negative control, there was significant (P < 0.05) reduction in body weight on day 4 (Table 3).
Table 3.
Weight (g) of P. berghei infected mice before and after treatment in the suppressive test.
| Treatment / Extract | Doses (mg/kg) | Day 0 | Day 4 | P-value |
|---|---|---|---|---|
| Negative Control (W-Tw 80-E) | 0.2 ml | 22.70 ± 0.97 | 21.89 ± 1.10 | 0.010 |
| Chloroquine | 25 | 22.64 ± 0.64 | 24.77 ± 1.01 | 0.001 |
| Ethanolic extract | 100 | 23.03 ± 1.65 | 22.62 ± 1.43 | 0.482 |
| 250 | 23.09 ± 0.98 | 24.08 ± 1.11 | 0.030 | |
| 500 | 23.12 ± 0.84 | 23.76 ± 1.47 | 0.367 |
Acute oral toxicity
To assess the acute toxic effects of the extract, 5,000 mg/kg body weight dose was orally given to male and female mice. There were no adverse effects throughout the 14-day study period. All the mice survived. None of the mice showed any signs of toxicity, such as behavioural patterns, diarrhoea, and changes on skin, falling of the fur, trembling, sleep or coma. All mice in the control and treatment groups showed an increase in the body weight on day-7 and day-14 compared to day-0 (Table 4); however, the increase in body weight was not statistically significant. The estimated medium lethal dose (LD50) was above 5,000 mg/kg body weight for the tested extract.
Table 4.
Summary of body weights recorded during the acute toxicity study of H. floribunda’s leaves extract.
| Groups | Body weight (g) | |||||
|---|---|---|---|---|---|---|
| Day 0 | Day 7 | Day 14 | ||||
| Male | Female | Male | Female | Male | Female | |
| Control | 31.92±0.40 | 29±0.37 | 35.57±0.54 | 29.77±1.32 | 37.23±0.52 | 30.51±1.26 |
| Treatment | 28.42±0.71 | 27.85±0.12 | 32.21±1.3 | 29.44±0.08 | 34.74±1.54 | 30.43±0.04 |
The macroscopic examination did not show any significant changes in the vital organs of treated mice compared to the control group, indicating that the tested extract was not toxic to mice. The relative organ weight of the livers, hearts, lungs, spleens, and kidneys of the mice in the treatment group showed no significant changes as compared to the control group (Table 5). These data collectively indicated that the ethanolic extract of H. floribunda did not affect the vital organs of the mice.
Table 5.
Summary of relative organ weights (per 100 g body weight) at the end of toxicity study.
| Organs | Control | Treatment | ||
|---|---|---|---|---|
| Male | Female | Male | Female | |
| Liver | 7.61 ± 0.50 | 4.84 ± 0.53 | 8.23 ± 1.46 | 5.35 ± 0.72 |
| Heart | 0.69 ± 0.03 | 0.56 ± 0.11 | 0.61 ± 0.15 | 0.53 ± 0.13 |
| Lung | 1.09 ± 0.12 | 0.85 ± 0.13 | 1.43 ± 0.13 | 0.85 ± 0.10 |
| Spleen | 0.70 ± 0.14 | 0.65 ± 0.01 | 0.68 ± 0.21 | 0.67 ± 0.04 |
| Kidney | 2.05 ± 0.04 | 1.23 ± 0.01 | 2.28 ± 0.20 | 1.28 ± 0.12 |
Values are expressed as mean ± SD. n = 3. There is no statistically significant difference compared to control group.
Discussion
Holarrhena floribunda leaves are an important source of drugs used in traditional medicine to cure different diseases, including diabetes, malaria, cancer, and oxidant damage related diseases (Fotie et al., 2006; Badmus et al., 2013). This study was focused on antibacterial, antimalarial activities, and acute toxicity of ethanolic extract of leaves of H. floribunda in order to validate its therapeutic potentials.
For the antibacterial activity, the ethanolic extract of leaves displayed various inhibitory activities against tested bacteria. Equal values of MIC and MBC were found for the Staphylococcus species suggesting a bactericidal effect of the extract on these microorganisms. For S. typhi and K. pneumoniae the MBC was twofold the value of the MIC. In other words, the extract had a bacteriostatic activity against S. typhi and K. pneumoniae. The antibacterial activity of leaves of H. floribunda has not been well established but some previous studies showed that alkaloids from its stem bark inhibited the growth of E. coli with MIC equal to 0.625 mg/mL and 1.25 mg/mL according to two geographical zones of Burkina Faso (Kabore & Millogo, 1997). The same MICs were found in this study for the leaves extract against S. aureus, K. pneumoniae, and S. typhi. The crude extract and four alkaloid fractions from aerial parts of H. floribunda were found active against M. ulcerans (Yemoa et al., 2015). Bogne et al. (2007) found that the crude extracts and the alkaloids from stem bark inhibited the growth of species of the genus Bacillus, namely B. cereus, B. subtilis, B. megaterium and B. stearothermophilus. As MICs, the total activity of extract against Gram-positive bacteria (265.3 mL/g) was better than those against Gram-negative bacteria. This means that the extract from 1 g of raw materials could be diluted to 265.3 mL and still retains activity against the Gram-positive bacteria tested in this study.
The present evaluation of antimalarial effect of leaves extract of H. floribunda is an additional proof of its medicinal values. The 4-day test using the P. berghei infected mice model is widely used as a test for the in vivo antiplasmodial activity of potential antimalarial agents, as it provides a preclinical indication of potential bioactivity of the test sample (Peters et al., 1975). According to Munoz et al. (2000), an active compound should lead at least 30% reduction of the parasitaemia. On this basis, the ethanolic extract of leaves of H. floribunda is active at 500 mg/kg. Mean survival time is another parameter evaluates the antimalarial activity of plant extracts. Accordingly, a plant material that can prolong the survival time of infected experimental animals compared to the negative control is considered as active agent against malaria (Oliveira et al., 2009). In this study, statistically, the mice treated with 500 mg/kg of extract had significantly lived longer than negative control and this could be attributed to parasitemia suppression effect of the extract. The survival times of mice treated with the extract were shorter as compared to positive control. This may show that half-life of the active compounds in plasma metabolism is shorter (Franssen et al., 1997). The mice treated with chloroquine were completely free from parasites after 4 days, but survived only for 16 days. This could be due to the toxicity of chloroquine (Phipps et al., 2011), or to any other unknown mechanisms. These findings on mean survival time were in agreement with similar studies (Abdulelah & Zainal-Abidin, 2007; Mengiste et al., 2012; Mohammed et al., 2014; Muluye et al., 2015). Previous studies showed that extracts and fractions from stem bark of H. floribunda had a good in vitro antiplasmodial activity against the chloroquine-resistant strain FCR-3 isolated from Gambia and the chloroquine-sensitive standard strain 3D7 (Fotie et al., 2006). This activity could be related to the plant alkaloids contents (Janot et al., 1959; Yemoa et al., 2015). According to Saxena et al. (2003) and Ramazani et al. (2010) several classes of secondary metabolites are responsible for the antimalarial activity, but the most important and diverse potential bioactivity has been observed with alkaloids. Alkaloids are one of the major antimalarial natural products and various classes have been reported to exhibit promising activities.
One of the oldest and most important antimalarial drugs, quinine, belongs to this class of compounds (Mojab, 2012).
For the toxicity, the evidence of safety of H. floribunda reported in the literature is limited. According to Garner et al. (1977), any compound or drug with an oral LD50 estimate greater than 1,000 mg/kg could be considered low toxic and safe. Arising from this, the ethanolic extract of H. floribunda at an oral dose of 5,000 mg/kg can be considered relatively safe on acute exposure. Previous studies on the toxicity of H. floribunda focused on its stem bark extracts. Thus, Bogne et al. (2012) in Cameroon showed that the methanolic extract of stem bark of H. floribunda is non-toxic with an LD50 in the range of 6,500 mg/kg and 7,000 mg/kg. These results are close to those obtained in this study for the leaves ethanolic extract, and confirm the non-toxicity of alcoholic extracts of H. floribunda. Therefore, in order to find the equivalent safe dose of ethanolic extract of leaves of H. floribunda for human consumption, the human equivalent dose (HED) was calculated using the following formula; which is developed based on the body surface area (BSA) normalization method; reported in the literature (Reagan-Shaw et al., 2007).
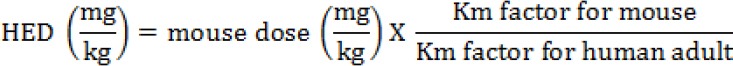
Where Km factor for mouse is 3, and Km factor for human adult is 37 (Reagan-Shaw et al., 2007).
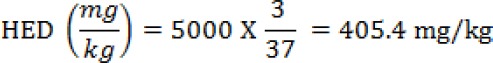
From the above calculations, the leaves ethanolic extract of H. floribunda may be safe until a dose of 405.4 mg/kg bw for human consumption.
Conclusion
The present study provided evidence that the ethanolic extract of H. floribunda is safe, and demonstrated its antibacterial and antiplasmodial effects suggesting its pharmacological usefulness. These findings support the traditional usage of the plant to combat microbial infections in Togolese folk medicine. Further investigations of this plant are needed to isolate, identify and characterize the active compounds responsible for the observed biological activity of the plant.
Acknowledgements
We would like to acknowledge the West African Health Research Network (WAHRNET) for financial support of this study. The authors are also grateful to the staff of laboratories of Navrongo Health Research Centre (Ghana) and Regional Research Institute for Health Sciences (Burkina Faso).
References
- 1.Abdulelah HAA, Zainal-Abidin BAH. In vivo antimalarial tests of Nigella sativa (Black Seed) different extracts. Am. J. Pharm. & Toxicol. 2007;2:46–50. [Google Scholar]
- 2.Arbonnier M. Arbres, arbustes et lianes des zones sèches d’Afrique de l’Ouest. 2emeéd. CIRAD-MNHN. 2000:541. [Google Scholar]
- 3.Badmus JA, Odunola OA, Yekeen TA, Gbadegesin AM, Fatoki JO, Godo MO, Oyebanjo KS, Hiss DC. Evaluation of antioxidant, antimutagenic, and lipid peroxidation inhibitory activities of selected fractions of Holarrhenafloribunda (G. Don) leaves. Acta Biochim. Pol. 2013;60(3):435–442. [PubMed] [Google Scholar]
- 4.Bayala B, Tamboura HH, Pellicer MTR, Zongon D, Traoré A, Ouédraogo L, Malpaux B, Sawadogo L. Effets oestrogéniques du macéréaqueux des feuilles de Holarrhena floribunda (G. Don). Dur & Schinz chez la rate ovariectomisée. Biotechn. Agr. Soc. Env. 2006;10:173–180. [Google Scholar]
- 5.Berhaut J. Dicotylédones. Volume 1. Acanthacées àAvicenniacées. Gouvernement du Sénégal, Ministère du Développement Rural et de l’Hydraulique, Direction des Eaux et Forets. Dakar, Sénégal: 1971. Flore illustrée du Sénégal; p. 626. [Google Scholar]
- 6.Bogne KP, Penlap BV, Mbofung CM, Etoa F-X. Acute and subacute toxicity of the methanol extract from Holarrhena floribunda G. Don (Apocynaceae) Eur. J. Exp. Biol. 2012;2(4):1284–1288. [Google Scholar]
- 7.Bogne KP, Penlap BV, Lontsi D, Etoa F-X. Antibacterial activities of the extracts and conessine from Holarrhena floribunda G. Don. (Apocynaceae). AJTCAM. 2007;4(3):352–356. doi: 10.4314/ajtcam.v4i3.31229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bouquet A, Debray M. Travaux et Documents de l’ORSTOM n°32 ORSTOM. Paris, France: 1974. Plantes médicinales de Côte d’Ivoire; p. 231. [Google Scholar]
- 9.CASFM. Recommandations 2014, SociétéFrançaise de Microbiologie, France. 2014:59. [Google Scholar]
- 10.Eloff JN. On expressing the antibacterial activity of plant extracts - A small first step in applying scientific knowledge to rural primary health care. S. Afr. J. Sci. 2000;96(3):116–118. [Google Scholar]
- 11.Fidock DA, Rosenthal PJ, Croft SL, Brun R, Nwaka S. Antimalarial drug discovery: efficacy models for compound screening. Nat. Rev. 2004;3:509–520. doi: 10.1038/nrd1416. [DOI] [PubMed] [Google Scholar]
- 12.Fotie J, Bohle DS, Leimanis ML, Georges E, Rukunga G, Nkengfack AE. Lupeol long-chain fatty acid esters with antimalarial activity from Holarrhena floribunda J. Nat. Prod. 2006;69(1):62–67. doi: 10.1021/np050315y. [DOI] [PubMed] [Google Scholar]
- 13.Franssen FJ, Smeijsters LW, Berger I, Aldana BM. In vitro and in vivo antiplasmodial activities of some plants traditionally used in Guatemala against malaria. Antimicrob. Agents and Chemother. 1997;41:1500–1503. doi: 10.1128/aac.41.7.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garner R.J, Clarke EGC, Clarke ML. Lander’s veterinary toxicology. London: Harcourt Publishers; 1977. pp. 268–277. [Google Scholar]
- 15.Hoekou YP, Tchacondo T, Karou SD, Koudouvo K, Atakpama W, Pissang P, Gbogbo AK, Woegan AY, Batawila K, Akpagana K, Gbeassor M. Ethnobotanical Study of Latex Plants in the Maritime Region of Togo. Phcog. Res. 2016;8(2):128–134. doi: 10.4103/0974-8490.175613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iwu MM. Handbook of African Medicinal Plants. 2nd ed. CRC Press; Boca Raton, Fla USA: 2014. p. 506. [Google Scholar]
- 17.Janot MM, Cave A, Goutarel R. Sterol alkaloids. III. Togholamine, holaphyllamine, and holaphylline, new alkaloids from the leaves of Holarrhena floribunda. Bull. Soc. Chim. de France. 1959:896–900. [Google Scholar]
- 18.Kabore ZI, Millogo KH. Etude antibactérienne in vitro d’extraits alcaloidiques de Holarrhena floribunda vis-à-vis ďEscherichia coli entéropathogène, sérotype 0127. Pharm. Méd. Trad. Afr. 1997;9:17–23. [Google Scholar]
- 19.Kerharo J, Adam JG. Plantes Médicinales et Toxiques. Vigot Frères; Paris, France: 1974. Pharmacopée Sénégalaise Traditionnelle; p. 1011. [Google Scholar]
- 20.Mengiste B, Makonnen E, Urga K. In vivo antimalarial activity of Dodonaea angustifolia seed extracts against Plasmodium berghei in mice model. MEJS. 2012;4:47–63. [Google Scholar]
- 21.Mohammed T, Erko B, Giday M. Evaluation of antimalarial activity of leaves of Acokanthera schimperi and Croton macrostachyus against Plasmodium berghei in Swiss albino mice. BMC Compl. Alt. Med. 2014;14:314. doi: 10.1186/1472-6882-14-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mojab F. Antimalarial Natural Products: A Review. Avic. J. Phytomed. 2012;2:52–62. [PMC free article] [PubMed] [Google Scholar]
- 23.Muluye AB, Melese E, Adinew GM. Antimalarial activity of 80 % methanolic extract of Brassica nigra (L.) Koch. (Brassicaceae) seeds against Plasmodium berghei infection in mice. BMC Compl. Alt. Med. 2015;15:367. doi: 10.1186/s12906-015-0893-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Munoz V, Souvain M, Bourdy G. The search for natural bioactive compounds through a multidisciplinary approach in Bolivia: Part II. Antimalarial activity of some plants used by Mosetena Indians. J. Ethnopharmacol. 2000;69:139–155. doi: 10.1016/s0378-8741(99)00096-3. [DOI] [PubMed] [Google Scholar]
- 25.NCCLS. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard. Sixth ed. Wayne, PA: NCCLS. NCCLS document no. M07-A6; 2003. p. 32. [Google Scholar]
- 26.OECD. Guidance Document on Oral Toxicity Testing 423 and 425, Environment, Series on testing and Assessment N°24. Paris, France: Health and Safety Publications; 2001. p. 24. [Google Scholar]
- 27.Oliveira AB, Dolabela MF, Braga FC, Jácome RLR.P, Varotti FP, Póvoa MM. Plant-derived antimalarial agents: New leads and efficient phytomedicines. Part I. Alkaloids. Ann. Acad. Bras. Cienc. 2009;81:715–740. doi: 10.1590/s0001-37652009000400011. [DOI] [PubMed] [Google Scholar]
- 28.Peters W, Robinson BL. The chemotherapy of rodent malaria. XLVII. Studies on pyronaridine and other Mannich base antimalarials. Ann. Trop. Med. Parasitol. 1992;86:455–465. doi: 10.1080/00034983.1992.11812694. [DOI] [PubMed] [Google Scholar]
- 29.Peters W, Portus H, Robinson L. The four-day suppressive in vivo antimalarial test. Ann. Trop. Med. Parasitol. 1975;69:155–171. [Google Scholar]
- 30.Phipps C, Chan K, Teo F, Ponampalam R. Fatal Chloroquine Poisoning: A Rare Cause of Sudden Cardiac Arrest. Ann. Acad. Med. 2011;40(6):296–297. [PubMed] [Google Scholar]
- 31.Ramazani A, Zakeri S, Sardari S, Khodakarim N, Djadid N. In vitro and In Vivo Antimalarial Activity of Boerhavia elegans and Solanum surattense. Malaria J. 2010:124. doi: 10.1186/1475-2875-9-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2007;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 33.Saxena SN, Pant D, Jain C, Bhakuni RS. Antimalarial Agents from Plant Sources. Curr. Sci. 2003;85(9):1314–1329. [Google Scholar]
- 34.Tarkang AP, Okalebo FA, Ayong LS, Agbor GA, Guantai AN. Anti-malarial activity of a polyherbal product (Nefang) during early and established Plasmodium infection in rodent models. Malaria J. 2014;13:456. doi: 10.1186/1475-2875-13-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yemoa A, Gbenou J, Affolabi D, Moudachirou M, Bigot A, Anagonou S, Portaels F, Martin A, Quetin-Leclercq J. Beninese medicinal plants as a source of antimycobacterial agents: bioguided fractionation and in vitro activity of alkaloids isolated from Holarrhena floribunda used in traditional treatment of Buruli Ulcer. BioMed Res. Int. 2015:5. doi: 10.1155/2015/835767. Article ID 835767. doi:10.1155/2015/835767. [DOI] [PMC free article] [PubMed] [Google Scholar]


