Abstract
Background:
As a bioactive composite extracted from American cockroach, Xinmailong injection (XML) is used for the treatment of congestive heart failure (CHF) in China. Clinical data has provided evidence that XML has positive inotropic properties. The objective of this study was to assess the mechanisms involved in the therapeutical effect of XML on CHF.
Materials and Methods:
The effects of XML on the cardiac function in isolated rat heart were measured. A Ca2+ imaging technology was used in rat cardiomyocytes (H9c2 cells) to reveal the role of XML on Ca2+ channels. Meanwhile, the effects of XML on the activities of Na+/K+ ATPase and sodium/calcium exchanger were measured. In addition, the level of reactive oxygen species and the protein expressions for the superoxide dismutase and hemeoxygenase were determined in the cardiomyocytes.
Results:
The results showed that XML increased the electrical impulse-induced [Ca2+]i in H9c2 cells, which was dependant on extracellular Ca2+ and was abolished by ML218-HCl (a T-type Ca2+channels antagonist) but not nimodipine (a L-type Ca2+channels antagonist). Ouabain, a Na+/K+-ATPase inhibitor, increased the electrical impulse-induced [Ca2+]i, which was significantly inhibited by XML. Moreover, XML markedly inhibited the Na+/K+ ATPase activity in H9c2 cells. In addition, XML notably reduced the production of reactive oxygen species and enhanced the protein expressions of antioxidant enzymes including superoxide dismutase 1, superoxide dismutase 2 and hemeoxygenase 1 in H9c2 cell.
Conclusion:
Our findings pave the ways to the better understandings of the therapeutic effects of XML on cardiovascular system.
Keywords: Xinmailong Injection, American cockroach, Congestive heart failure, Calcium Channels, T-Type, Na+/K+-ATPase, Reactive oxygen species
Introduction
Clinically, congestive heart failure (CHF) is defined as a complex syndrome where the heart is incapable of maintaining the cardiac output that is adequate to meet metabolic requirements and accommodate venous return (Yancy et al., 2013). This structural or functional impairment of ventricular filling or ejection of blood always causes the fluid retention, such as the pulmonary and/or splanchnic congestion and/or peripheral edema (Yancy et al., 2013). Probably due to the increased aging population and the incidence of risk factors such as ischemic heart diseases and hypertension, the CHF prevalence has kept rising and is estimated to increase 46% from 2012 to 2030 (Bui et al., 2011; Khatibzadeh et al., 2013; Mozaffarian et al., 2015). Without doubt, CHF will continue to be a substantial burden on health-care systems and societies. Therefore, more effective medications with low side effects and low cost are required to improve the survival in patients with CHF.
In the present, the treatment of CHF is frequently based on the western medicine, which include β-receptor blockers, diuretics and renin-angiotensin-aldosteronesystem antagonists. In addition, some novel anti-CHF drugs such as lyophiluzed recombinant human brain natriuretic, levosimendan, ivabradine, tolvaptan and istaroxime are also used to treat CHF (Karl et al., 2015). Traditional Chinese Medicine (TCM) practices represent a healthy complement to practices. Since 2006, a number of TCMs were clinically tested on the CHF patients and exciting therapeutic effects were achieved (Chen et al., 2010; Miao et al., 2008). Cockroach has been described as an effective insect drug which can promote blood circulation and urination in Bencao Gangmu (Compendium of Materia Medica) in the Ming Dynasty by Li Shizhen, a well-known medical specialist and pharmacologist of ancient China. Xinmailong Injection (XML), a compound extracted from Periplaneta Americana (P. americana, also known as American cockroach), has been launched to market in China as a proprietary medication for treating patients with CHF (Ma et al., 2013). The major bioactive ingredients of P. americana include polypeptides, nucleosides and amino acids (He et al., 2008). Although it is highly effective, the mechanisms underlying the action of XML in the treatment of heart failure are rarely investigated. Therefore, the present study was designed to verify the mechanisms underlying the enhancive effects of XML on cardiac functions. Focus was put on studying the mechanism leading to the increase of [Ca2+] in cardiomyocytes. In addition, since oxidative stress is an important factor that deteriorates the functions of cardiomyocytes and is involved in the pathophysiology of CHF (Tsutsui et al., 2011), the antioxidant effect of XML on cardiomyocytes was also investigated.
Materials and Methods
Chemicals and drugs
XML (Lot.:120228) was provided by Tengyao Pharmaceutical Company Limited by Shares Yunnan. Ouabain, Bay K8644, Nimodipine, Thapsigargin and KBr7943 were purchased from Sigma Aldrich Inc. (St. Louis, MO, USA). ML218-HCl was obtained from Tocris Bioscience (Tocris, Bristol, UK). The other reagents were of analytical purity.
Animals
Male Sprague-Dawley rats (250-300 g) were supplied from the Laboratory Animal Unit of Kunming Medical University (Kunming, China). All experiments performed in this study were approved by the Committee on the Use of Live Animals in Teaching and Research of Yunnan Minzu University.
Perfusion of hearts
In vitro heart perfusion was performed as described (Mishra et al., 2014). Briefly, rats were injected intraperitoneally with sodium pentobarbital (50 mg/kg, i.p.) to induce anesthesia, and then sacrificed by cervical dislocation. Following excision and cleaning, hearts were mounted on a blunted 16-gauge needle of a Langendorff apparatus (GL-1, Chengdu TME Technology, Chengdu, China) and perfused retrogradely through the ascending aorta under constant pressure with oxygenated (95% O2/5% CO2) Krebs buffer maintained at 37°C. The coronary flow rate was monitored via a Doppler flow probe (0.5-mm id; Transonic Systems, Ithaca, NY, USA) and a Transonic T206 flow meter (Transonic Systems). Iso -volumetric left ventricular developed pressure was measured with a fluid-filled latex balloon inserted into the left ventricle via the left atrium and connected to a pressure transducer (BL-420F, Chengdu TME Technology, Chengdu, China). Accumulative concentrations of XML (0.1, 0.5, 1.0, 2.0 mg/mL) were added to the Krebs buffer to determine the effect of XML on the heart rates, the coronary flow rates, the left ventricular systolic pressure (LVSP), the left ventricular end-diastolic pressure (LVEDP) and the left ventricular pressure maximum rise/fall rate changes (±dP/dt max).
Cell culture
H9c2, a cell line derived from embryonic BD1X rat heart tissue, was purchased from American Type Culture Collection (CRL-1446, ATCC, Manassas, VA). Cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) (Gibco, Invitrogen, Carlsbad, CA, USA) with 10% (v/v) fetal bovine serum (FBS) (Gibco, Invitrogen, Carlsbad, CA, USA), 100 units/ml penicillin and 100 μg/ml streptomycin (P/S) (Gibco, Invitrogen, Carlsbad, CA, USA). Cells were incubated in 37°C with 5% CO2/95% air (Napco, Thermo Fisher Scientific, MA, USA). Medium was changed every two days and cells were subjected to subculture when 95% of confluence was reached. Cells were sub-cultured to 6-well plates or 24-well plates.
Ca2+ imaging
H9c2 cells were seeded on round covered lip pre-coated with poly-D-lysine inside 24 well-plates. Prior to Ca2+ imaging study, culture medium was removed and the cells were rinsed twice with HEPES buffered Hanks’ balanced salt solution (HEPES-HBSS) containing CaCl2 (1.8 mM) and MgCl2 (0.8 mM), pH 7.4. Cells were then incubated with 2 μM Fura-2 AM (Invitrogen, Carlsbad, CA, USA) and 1% (v/v) bovine serum albumin for 60 min at room temperature. After dye loading, the covers lip was mounted in an open bath imaging chambers (RC-21B, Warner Instruments, Connecticut, USA) and visualized under a Nikon Eclipse Ti microscope (Nikon, Tokyo, Japan) using a 20* objective (Nikon CFIS Fluor Objective). Cells were then continuously superfused with HEPES-HBSS. Fura-2 AM was alternately excited at 340 and 380 nm, and the emitted fluorescence filtered at 510 nm was recorded by a SPOT RT3 CCD digital microscope camera (SPOT Imaging Solutions, Michigan, USA) every 5s controlled by Meta Fluor Imaging Software (Molecular Devices, CA, USA). The 340/380 ratio reflects the change of intracellular free Ca2+. An electrical stimulator (S48 Square Pulse Stimulator, GRASS, Technologies) was used to trigger the depolarization of cell membrane. Cells were stimulated with a stimulation rate of 1/s, pulse delay time of 0.01 ms, pulse duration of 40 ms and voltage of 150 V
For the experiment with electrical stimulation, H9c2 cells were incubated with XML (Tengyao, Yunnan, China), ouabain (Sigma, O3125, St. Louis, MO) or Bay K8644 (Sigma, B112, St. Louis, MO) for 30 min at 37°C before Ca2+ imaging. Nimodipine (Sigma, M3764, St. Louis, MO) or ML218-HCl (Tocris, 4507, Bristol, UK) was added to chamber just before the start of the Ca2+ imaging and the cells was incubated with these drugs for at least 5 min at room temperature. For the experiment without electrical stimulation, thapsigargin (Sigma, St. Louis, MO), ouabain (Sigma, St. Louis, MO) and KBr7943 (Sigma, St. Louis, MO) was added during Ca2+ imaging.
Protein Extraction and Western Blotting Analysis
H9c2 cells were incubated without (Control) or with XML for 24 h. Cells were then washed three times with ice-cold PBS and lysed in lysis buffer with protease inhibitor cocktail for 15 min. Cell lysates were homogenized and supernatant was collected by centrifugation at 18,000 g for 10 min at 4°C. Protein concentration was determined by Bio-Rad Bradford Assays using bovine serum albumin as standard. Protein samples were mixed with SDS-reducing buffer (final concentration of 2% SDS, 5% 2 -mercaptoethanol, 10% glycerol, 0.002% bromophenol blue, 0.0625 M Tris-HCl, pH 6.8) and denatured at 100°C for 10 min, and then subjected to electrophoresis (SDS-PAGE) with 12% SDS-polyacrylamide gel under 150 V for 2 h and transferred to PVDF membrane under 100 V for 2h. The membrane was blocked by 5% non-fat milk (in TBS containing 0.2% Tween-20, 150 mM NaCl, 50 mM Tris-HCl, pH 7.6, TBST) for 2 h at room temperature. The membrane was then incubated with anti-superoxide dismutase-1 (SOD-1) (Santa Cruz, CA, USA), anti-SOD2 (Santa Cruz, CA, USA) or anti-hemeoxygenase-1 (HO-1) (Santa Cruz, CA, USA) antibody at 4°C overnight. The concentration of primary antibody used was 1:500 (v/v). β-actin was used as internal control. After incubation of primary antibody, the membrane was washed three times with TBST for 10 min at room temperature. This is followed by immunoblotting with horseradish peroxidase-conjugated goat-anti-rabbit secondary antibody (BioRad, 170-6515, CA, USA) or with horseradish peroxidase (HRP)-conjugated goat-anti-mouse secondary antibodies (BioRad, 172-1011, CA, USA) at room temperature for 2 h. The concentration of secondary antibodies used was 1:10000 (v/v). The membrane was washed 3 times again with TBST for 10 min at room temperature, and followed by incubation with HRP substrate (Millipore, WBKLS0500, Billerica, USA) for 1 min at room temperature. The chemiluminescence emitted was captured by X-ray film (Fujifilm, Tokyo, Japan) with appropriate exposure time.
Measurement of Na+/K+ ATPase Activity
The effect of XML on Na+/K+-ATPase activity was measured using the previous protocol (Fuller et al., 2003). H9c2 cells were homogenized by buffer A (20 mM HEPES, 250 mM sucrose, 2 mM EDTA, 1 mM MgCI2, pH 7.4) and centrifuged at 20,000 g for 30 min. The supernatant was discarded and the pellet was resuspended in buffer A. The amount of protein was pre-determined by Bio-Rad Bradford Assays (Bio-Rad, 500-0001, CA, USA). Two 50 μl aliquots of homogenate were taken. One aliquote was mixed with 50 μl of reaction buffer 1 (200 mM Tris-HCl, 30 mM MgCl2, 200 mM NaCl, 60 mM KCl, 10 mM EGTA, pH 7.5). Another one was mixed with reaction buffer 2 (buffer 1 + 1 mM ouabain). Each reaction contained 100 μg/mL PMSF, 2 μg/mL apronitin and 2 μg/mL pepstatin A to prevent protein degradation. The reaction was started by the addition of 1 mM of ATP. Reactions proceeded for 10 min at 37°C and 10 μL of 100% (w/v) trichloroacetic acid were added to stop the reaction. Samples were left on ice for 1 h and then centrifuged at 20,000 g for 30 min to pellet the precipitated protein. Free phosphate in supernatant was assayed using the ammonium molybdate spectrophotometry assay kit (Sigma, MAK030, St. Louis, MO). The absorbance at 650 nm was measured.
Measurement of Intracellular Reactive Oxygen Species
H9c2 cells were cultured in black 96 well-plates without (as Control) or with XML for 4 h at 37°C. OxiSelect™ intracellular ROS assay kit (green fluorescence) (Cell Biolabs, STA342, San Diego, CA, USA) was used to determine the ROS in the samples by using DCFH-DA as a probe. DCF standard curve was constructed according to the manufacturer’s instruction. Before the experiments, the sample was washed once with HEPES-HBSS, followed by incubation with 1 mM DCFH-DA at 37°C for 1h. Afterwards, the sample was washed by HEPES-HBSS once again to remove the unloaded DCFH-DA. The plate was then placed in a microplate reader to measure the fluorescence (emission at 480 nm and excitation at 530nm).
Statistical Analyses
All data were expressed as means ± S.E.M. Statistical analyses were performed using SPSS 22.0. Comparison between two groups was analyzed using Student’s t-test. Comparison among three or more groups was analyzed using one-way ANOVA, p<0.05 was considered statistically significant.
Results
Effect of XML on Cardiac Function
As demonstrated in Fig 1A-C, XML significantly increased the coronary blood flow, LVSP, LVEDP and ±dp/dt max of the hearts in a dose-dependent manner. In contrast, the heart rates were mildly decreased (10.57±2.10%) after treatment with XML (Fig 1D).
Figure 1.
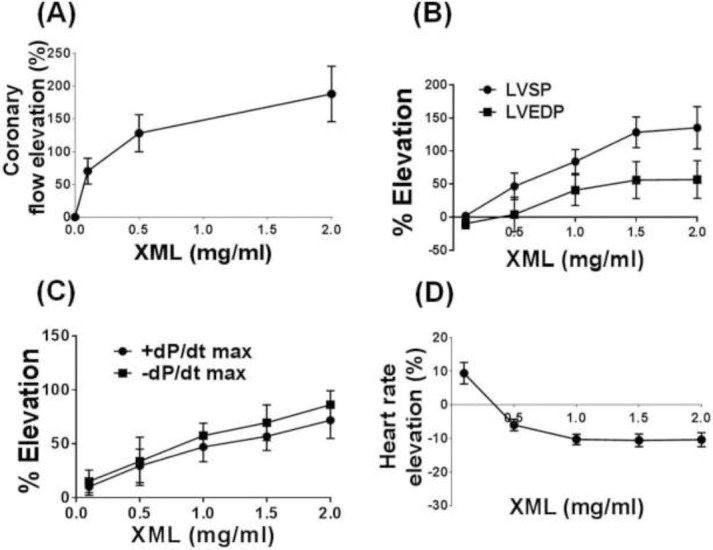
Effects of XML on the cardiac functions in rats. In vitro heart perfusion was performed by using a Langendorff apparatus. Accumulative concentrations of XML (0.1, 0.5, 1.0, 2.0 mg/ml) were added to the perfusion buffer. (A) Effect of XML on the coronary flow rates. (B) Effect of XML on the left ventricular systolic pressure (LVSP) and the left ventricular end-diastolic pressure (LVEDP). (C) Effect of XML on the left ventricular pressure maximum rise/fall rate changes (±dP/dt max). (D) Effect of XML on the heart rates. Values are means ± S.E.M. (n = 5).
Effect of XML on [Ca2+]i of H9c2 under electrical stimulation
XML elevated the electrical pulse-induced [Ca2+] in H9c2 cells (Fig 2A). The effect of XML was concentration-dependent with an EC50 value of about 0.5 mg/ml. The threshold concentration of XML was 0.01 mg/ml and the effect of XML was leveled off at 10mg/ml (Fig 2B).
Figure 2.
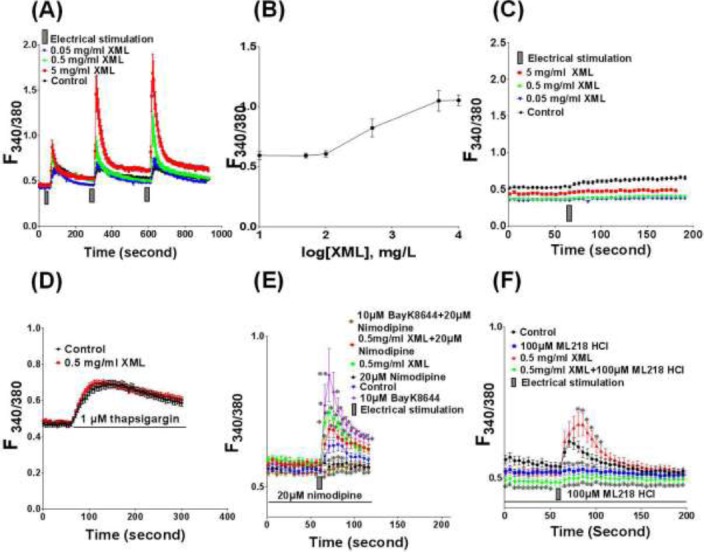
Effect of XML on the [Ca2+]i. Ca2+ was measured by Ca2+ imaging with the use of Fura-2 AM. (A) Effect of XML on the electrical impulse-induced [Ca2+]i of H9c2 cells. Cells were incubated with different concentration XML (from 0 to 5 mg/ml) for 30 mins before stimulation with electrical impulse. (B) Concentration-response relationship of XML on the electrical impulse-induced Ca2+ in H9c2 cells. (C) Effect of XML on the electrical impulse-induced [Ca2+]i in H9c2 cells measured under extracellular Ca2+-free condition. (D) Effect of XML on the thapsigargin-induced [Ca2+]i in H9c2 cells. Thapsigargin is an apotent SERCA inhibitor which depletes Ca2+ stores in sacroplasmic reticulum and endoplasmic reticulum by blocking Ca2+ uptake via SERCA and allows Ca2+ to passively leak out. (E) Effect of XML on the L-type Ca2+ channels in H9c2 cells. Nimodipine (20 μM), an L-type Ca2+ channels antagonist. BayK8644 (10 μM), an L-type Ca2+ channels agonist. (F) Effect of XML on the T-type Ca2+channels in H9c2 cells. ML218-HCl (100 μM), a T-type Ca2+ channels antagonist. Values are expressed as mean ± S.E.M. (n = 5). *P< 0.05, **P< 0.01, compared with Control.
Effect of XML on Ca2+ influx and intracellular store of Ca2+
To study the effect of XML on Ca2+ influx, Ca2+ was measured with the use of Ca2+ free solution as bathing solution. When Ca2+ was absent in the bathing solution, the electrical stimulation could not induce any elevation of [Ca2+]i regardless any concentration of XML (Fig 2C).
To study the effect of XML on Ca2+ release from intracellular store, thapsigargin (TG, 1 μM), an apotent sacroplasmic reticulum Ca2+ ATPase (SERCA) inhibitor which depletes Ca2+ stores in sacroplasmic reticulum and endoplasmic reticulum by blocking Ca2+ uptake and allows Ca2+ to passively leak out, was added to H9c2 cells. TG induced a transient increase in [Ca2+]P However, there was no significant difference between the Control and XML-treated cells (Fig 2D).
Effect of XML on T-type Ca2+channels
Nimodipine (20 μM), an L-type Ca2+channels antagonist, abolished the electrical impulse-induced [Ca2+] increase (Fig 2E). BayK8644 (10 μM), a L-type Ca2+ channels agonist, potentiated the electrical impulse-induced [Ca2+] increase which was completely inhibited by nimodipine (20 μM). The effect of XML on electrical impulse-induced [Ca2+] increase was not affected by nimodipine, but was abolished by ML218-HCl (100 μM), a T-type Ca2+channels antagonist (Fig 2F).
Effect of XML on sodium/calcium exchanger (NCX) and Na+/K+-ATPase
To investigate the effects of XML on NCX, KB-r7943 (10 pM) was used. As an inhibitor of NCX KB-r7943 reverses the mode of exchanger so that Ca + is transported into cells while Na+ is transported out of cell. KB-r7943 induced an increase in Ca2+ in H9c2 cells even without electrical stimulation (Fig 3A). Unlike KB-r7943, XML (0.05 mg/ml and 0.5 mg/ml) could not increase the Ca2+ in H9c2 cells if no electrical stimulation was used.
Figure 3.
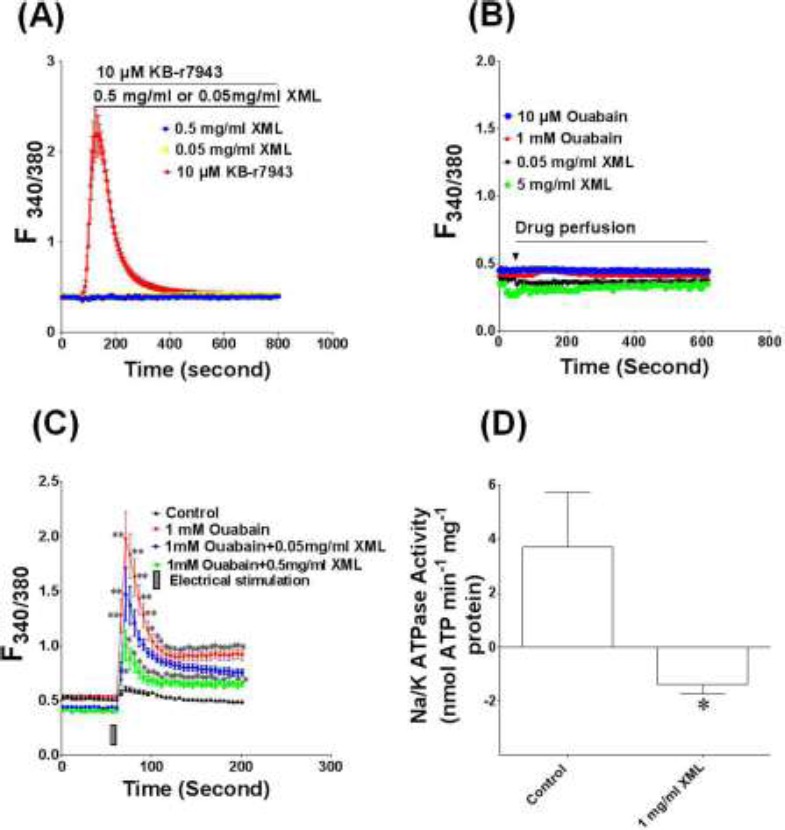
Effect of XML on sodium/calcium exchanger and Na+/K+-ATPase. (A) Effect of XML on the sodium/calcium exchanger in H9c2 cells. KB-r7943 (10 μM), an inhibitor of sodium/calcium exchanger. (B) Effect of oubain and XML on the [Ca2+]i of H9c2 cells without electrical stimulation. Ouabain (10 μM), a potent Na+/K+ ATPase inhibitor. (C) Effect of XML on the ouabain-induced [Ca2+]i increase in H9c2 cells. (D) Effect of XML on the Na+/ K+-ATPase activity in H9c2 cells. Effect of ouabain and XML on the [Ca2+]i of H9c2 cells without electrical stimulation. Values are means ± S.E.M. (n = 5). *P< 0.05, **P< 0.01, compared with Control.
Ouabain (10 μM), a potent Na+/K+ ATPase inhibitor, was used to study any involvement of Na+/K+ATPase in the effect of XML. Ouabain did not affect the [Ca2+] in H9c2 cells without electrical stimulation (Fig 3B). Under the electrical stimulation ouabain (1 mM, the concentration that produced maximum effect) had no additive effect on XML-induced [Ca2+] i increase but XML significantly inhibited the [Ca2+] increase induced by ouabain (Fig 3C), suggesting the overlapping of the action site of ouabain and XML. Further study was carried out to investigate the effect of XML on the activity of Na+/K+ ATPase. Na+/K+ ATPase activity was also markedly inhibited after cells were treated with XML (Fig 3D).
Antioxidant effect of XML
H9c2 cells were incubated without (Control) or with 1 mg/ml of XML for 4 h at 37 °C. Western blotting was performed to detect the changes in the protein expression levels of SOD-1 (Fig 4A and B), SOD-2 (Fig 4C and D) and HO-1 (Fig 4E and F). The results showed that the protein expressions of these antioxidant enzymes in H9c2 cells were elevated after treatment with XML (1 mg/mL). H2O2 increased the DCF fluorescent signal and the fluorescence was significantly decreased by 0.5 mg/mL of XML (Fig 4G).
Figure 4.
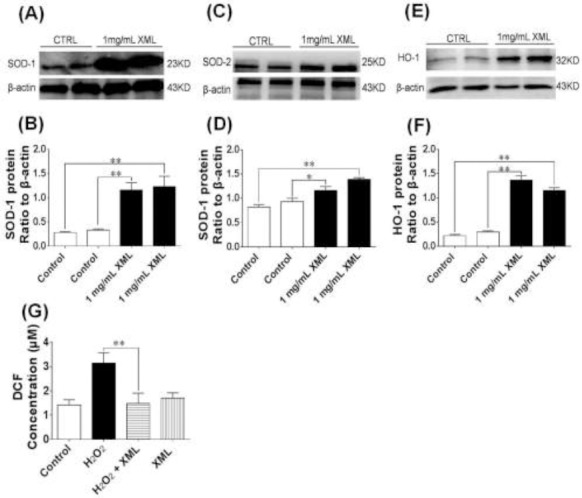
Antioxidant effect of XML. (A) Effect of XML on the protein expression of superoxide dismutase-1 (SOD-1) in H9c2 cells. (B) Averaged densitometric quantification of SOD-1 protein levels. β-actin served as a loading control. (C) Effect of XML on protein expression of SOD-2 in H9c2 cells. (D) Averaged densitometric quantification of SOD-2 protein levels. β-actin served as a loading control. (E) Effect of XML on protein expression of hemeoxygenase-1 (HO-1) in H9c2 cells. (F) Averaged densitometric quantification of HO-1 protein levels. β-actin served as a loading control. (G) Effect of XML on the intracellular reactive oxygen species (ROS) production. ROS was determined by using DCFH-DA as a probe. Data was expressed as means ± S.E.M. (n = 6). *P< 0.05, **P< 0.01, compared with Control.
Discussion
XML, as a TCM used for the treatment of CHF, shows a lot of therapeutic properties similar to those in western medicine. Clinically, XML is able to relieve the symptoms of CHF after a completed treatment for 15 d (Ma et al., 2013). The plasma angiotensin II level in patient with CHF is lowered after the XML treatment (Ma et al., 2013). Animal study also shows the enhancive effect of XML on the concentration of plasma prostacyclin (Yang et al., 2015). Since the elevation of PGI2 level is crucial in the management of pulmonary arterial hypertension (Buckley et al., 2013; Montani et al., 2014), which increases the afterload of the right ventricle and often induces the right-heart failure (Bogaard et al., 2009; Ryan et al., 2014), XML might exert its therapeutical effect not only on the left heart failure but also on the right heart failure. Meanwhile, these findings may also provide interpretations why XML also increases the flow of the coronary artery.
XML also significantly enhances LVSP and LVEDP which is similar to cardiac glycosides and other positive inotropic drugs. However, most of the positive inotropic drugs are used to treat CHF target on L-type Ca2+ channels. β-adrenergic receptor agonists are good examples (Woo et al., 2012). β-adrenoceptors are coupled to Gs-proteins and then activate adenylyl cyclase. The resulting increase in intracellular cyclic adenosine monophosphate (cAMP) activates protein kinase A (PKA) and then phosphorylates the L-type Ca2+ channel. The activation of L-type Ca2+ channels allow more Ca2+ entering the cardiomyocytes. Another example is bipyridine such as milrinone. It acts by inhibiting phosphodiesterase IV, thereby increasing intracellular cAMP and then PKA which activates L-type Ca2+ channels (Nagy et al., 2014). However, until now, there are no drugs targeting on the T-type Ca2+ channels. T-type Ca2+ channels are important in a wide variety of physiological functions such as hormone secretion, smooth muscle contraction, neuronal firing, cell proliferation of cardiac tissue and myoblast fusion (Vassort et al., 2006; Perez-Reyes et al., 2003). In the cardiac tissues, T-type Ca2+ channels are highly abundant in sinoatrial pacemaker cells and Purkinje fibers. They can maintain the pacemaker activity by setting the frequency of firing (Perez-Reyes et al., 2003). However, it was also found that T-type Ca2+ channels are re-expressed in hypertrophied and failing ventricles. Increasing evidence has shown that T-type Ca2+ channel may be related to the progression of CHF (Yasui et al., 2005; Chiang et al., 2009). Our data showed the effect of XML on potentiating the electrical impulse-induced increase in [Ca2+] which is not dependent on intracellular Ca2+ but dependent on extracellular Ca2+ pool. This effect of XML was inhibited by T-type Ca2+ channel blockers but not L-type Ca2+ channel blockers. Our data indicates that XML may facilitate the T-type-dependent Ca2+ influx and enhance cardiac muscle contraction in CHF patients. More importantly, to the best of our knowledge, XML may be the first drug that targets on T-type Ca2+ channels.
In addition to the T-type Ca2+ channels, our data also suggests that XML may also exert its positive inotropic effect through the inhibition of Na+/K+ATPase. This mechanism of action is similar to cardiac glycoside such as digoxin, which promotes Na+-Ca2+ exchange via NCX, resulting in the Ca2+ influx and increased myocardial contraction. Digoxin is the oldest drug used in clinical practice for treating CHF. However, there are a lot of limitations of using digoxin due to its complicated pharmacokinetic properties, narrow therapeutic index and high toxicity. Moreover, digoxin has parasympathomimetic action such the slowdown of the atrioventricular-nodal conduction (Gheorghiade et al., 2006). Although the mechanism of action of XML involves the inhibition of Na+/K+-ATPase, which is similar to digoxin, XML has a potential to replace digoxin in clinical practice because several clinical studies have demonstrated the low toxicity and high therapeutic index of XML compared to the western drugs. Further investigations are required to study if XML may have significant interaction with other drugs.
Free radicals and reactive oxygen species (ROS), such as hydrogen peroxide, superoxide anion, lipid peroxides (Blankenberg et al., 2003) may be the important factors leading to CHF because they deteriorate the decompensating myocardium (Keith et al., 1998; Prasad et al., 1996; Dieterich et al., 2000). Excess production of these free radicals and ROS can result in oxidative stress. The degree of oxidative stress directly correlates to the severity of subsequent myocardial damage (Shiomi et al., 2004). An increased ROS production results in apoptosis of cardiomyocytes, interstitial fibrosis, myocyte hypertrophy and myocardial ischemia and cardiac cell death. (Shiomi et al., 2004; Giordano 2005; Qiao et al., 2016). It has also been suggested that heart failure subsequent to myocardial infarction may be associated with antioxidant deficit as well as increased myocardial oxidative stress (Khalid et al., 2014). It is worth mentioning that XML showed significant antioxidant effect in our study. XML significantly reduced ROS induced by H2O2 in H9c2 cells, and increase the expression of SOD-1, SOD-2, OH-1. Therefore, the antioxidant effect of XML might also play an important role in its therapeutic actions on CHF. However, since oxidative stress is a complex process, further investigations are also required to study the signaling pathways involved in the antioxidant effect of XML.
Conclusions
Our study has provided evidence that XML increase intracellular calcium level by activating T-type calcium channels and inhibiting Na+/K+-ATPase. The antioxidant effect of XML may also contribute to the cardioprotective effect of XML but further investigations are required. The antioxidant effect of XML may also contribute to the cardioprotective effect of XML but further investigations are required (Fig. 5).
Figure 5.
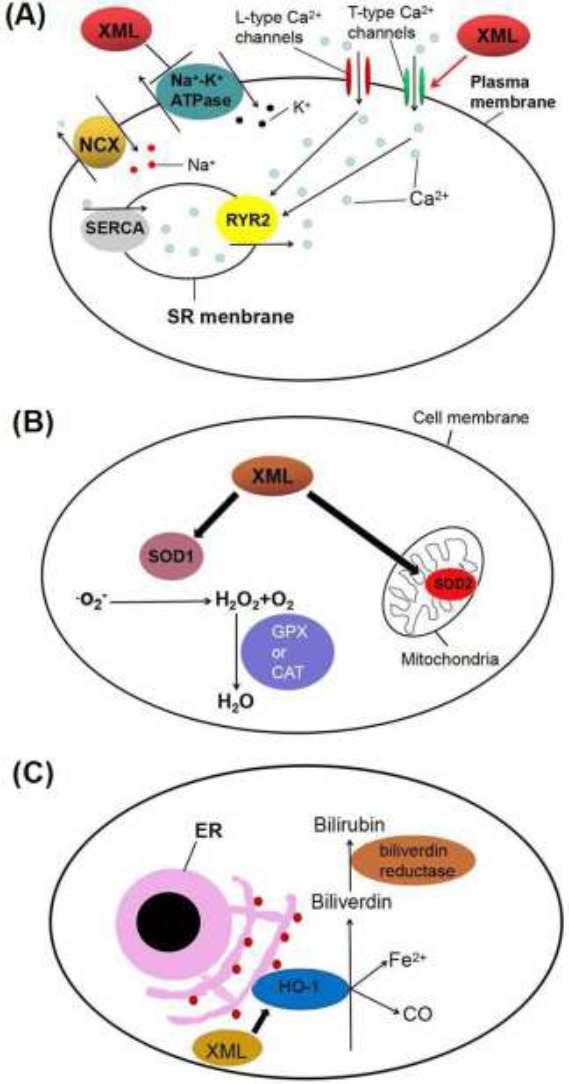
Schematic diagram of the mechanisms underlying the action of XML in the treatment of heart failure. (A) XML may activate the T-type Ca2+ channels which cause Ca2+ influx. In addition, its inhibitory effect on Na+-K+ ATPase causes an increase of [Na+]i, which would reverse the activity of sodium/calcium exchanger so that it pumps out Na+ and pumps in Ca2+ instead. As a result, [Ca2+]i is increased, which make the contraction force of the myocardium stronger and increase cardiac output to relieve the symptoms of CHF. (B) and (C) XML may also up-regulate the expressions of three antioxidant enzymes SOD-1, SOD-2 (B) and HO-1 (C). These pathways are supposed to be involved in ROS scavenging and confer the cardioprotective properties of XML. XML: Xinmailong Injection; NCX: sodium/calcium exchanger; SERCA: sarcoplasmic reticulum Ca2+ ATPase; RYR2: ryanodine receptors type 2; SR: sarcoplasmic reticulum; SOD: superoxide dismutase; GPx: glutathione peroxidase; CAT: catalase; ER: endoplasmic reticulum; HO-1: hemeoxygenase-1.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (No. 81460553), and supported by Key Natural Scientific Fund of Yunnan Province (No. 2014FA036).
References
- 1.Bogaard HJ, Abe K, Vonk Noordegraaf A, Voelkel NF. The right ventricle under pressure: cellular and molecular mechanisms of right-heart failure in pulmonary hypertension. Chest. 2009;135:794–804. doi: 10.1378/chest.08-0492. [DOI] [PubMed] [Google Scholar]
- 2.Buckley MS, Staib RL, Wicks LM. Combination therapy in the management of pulmonary arterial hypertension. Int J Clin Pract Suppl. 2013:13–23. doi: 10.1111/ijcp.12136. [DOI] [PubMed] [Google Scholar]
- 3.Blankenberg S, Rupprecht HJ, Bickel C, Torzewski M, Hafner G, Tiret L, Smieja M, Cambien F, Meyer J, Lackner KJ Athero Gene Investigators. Glutathione peroxidase 1 activity and cardiovascular events in patients with coronary artery disease. New England Journal of Medicine. 2003;349:1605–1613. doi: 10.1056/NEJMoa030535. [DOI] [PubMed] [Google Scholar]
- 4.Bui AL, Horwich TB, Fonarow GC. Epidemiology and risk profile of heart failure. Nat Rev Cardiol. 2011;8:30–41. doi: 10.1038/nrcardio.2010.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiang CS, Huang CH, Chieng H, Chang YT, Chang D, Chen JJ, Chen YC, Chen YH, Shin HS, Campbell KP, Chen CC. The Ca(v)3.2 T-type Ca(2+) channel is required for pressure overload-induced cardiac hypertrophy in mice. Circ Res. 2009;104:522–530. [Google Scholar]
- 6.Chen CX, Gao JP, Wu Q, Guo J, Gu WL. [Progress in treatment of chronic heart failure in Western medicine and treatment strategies in traditional Chinese medicine] Zhong Xi Yi Jie He Xue Bao. 2010;8:7–14. doi: 10.3736/jcim20100102. [DOI] [PubMed] [Google Scholar]
- 7.Dieterich S, Bieligk U, Beulich K, Hasenfuss G, Prestle J. Gene expression of antioxidative enzymes in the human heart increased expression of catalase in the end-stage failing heart. Circulation. 2000;101:33–39. doi: 10.1161/01.cir.101.1.33. [DOI] [PubMed] [Google Scholar]
- 8.Fuller W, Parmar V, Eaton P, Bell JR, Shattock MJ. Cardiac ischemia causes inhibition of the Na/K ATPase by a labile cytosolic compound whose production is linked to oxidant stress. Cardiovasc Res. 2003;57:1044–1051. doi: 10.1016/s0008-6363(02)00810-6. [DOI] [PubMed] [Google Scholar]
- 9.Giordano FJ. Oxygen, oxidative stress, hypoxia, and heart failure. The Journal of clinical investigation. 2005;115:500–508. doi: 10.1172/JCI200524408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gheorghiade M, van Veldhuisen DJ, Colucci WS. Contemporary use of digoxin in the management of cardiovascular disorders. Circulation. 2006;113:2556–2564. doi: 10.1161/CIRCULATIONAHA.105.560110. [DOI] [PubMed] [Google Scholar]
- 11.He ZC, Peng F, Song LY, Wang XY, Hu MH, Zhao Y, Liu GM. [Review on investigations related to chemical constituents and biological activities of Periplaneta americana] Zhongguo Zhong Yao Za Zhi. 2007;32:2326–2331. [PubMed] [Google Scholar]
- 12.Keith M, Geranmayegan A, Sole MJ, Kurian R, Robinson A, Omran AS, Jeejeebhoy KN. Increased oxidative stress in patients with congestive heart failure. Journal of the American College of Cardiology. 1998;31:1352–1356. doi: 10.1016/s0735-1097(98)00101-6. [DOI] [PubMed] [Google Scholar]
- 13.Khatibzadeh S, Farzadfar F, Oliver J, Ezzati M, Moran A. Worldwide risk factors for heart failure: a systematic review and pooled analysis. Int J Cardiol. 2013;168:1186–1194. doi: 10.1016/j.ijcard.2012.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Numair KS, Chandramohan G, Alsaif MA, Veeramani C, El Newehy AS. Morin, Aflavonoid, on lipid peroxidation and antioxidant Status in experimental myocardial ischemic rats. African Journal of Traditional, Complementary and Alternative Medicines. 2014;11:14–20. doi: 10.4314/ajtcam.v11i3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dietz R, Rauch B. Guidelines for diagnosis and treatment of chronic heart failure: executive summary. European Heart Journal. 2015;26:1115–1140. doi: 10.1093/eurheartj/ehi204. [DOI] [PubMed] [Google Scholar]
- 16.Miao Y, Zhao WJ, Jing L. [Retrospective analysis on integrative medicinal treatment of chronic heart failure] Zhongguo Zhong Xi Yi Jie He Za Zhi. 2008;28:406–409. [PubMed] [Google Scholar]
- 17.Qilin Ma, Youxi Luo, Pi Guo, Gan Gao, Meixue Yang, Gaurav Sablok, Yanchun Zhang, Fengfeng Zhou. Clinical effects of Xinmailong therapy in patients with chronic heart failure. Int J Med Sci. 2013;10:624–633. doi: 10.7150/ijms.5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mishra RC, Wulff H, Cole WC, Braun AP. A pharmacologic activator of endothelial KCa channels enhances coronary flow in the hearts of type 2 diabetic rats. J Mol Cell Cardiol. 2014;72:364–373. doi: 10.1016/j.yjmcc.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 19.Montani D, Chaumais MC, Guignabert C, Günther S, Girerd B, Jaîs X, Algalarrondo V, Price LC, Savale L, Sitbon O, Simonneau G, Humbert M. Targeted therapies in pulmonary arterial hypertension. Pharmacol Ther. 2014;141:172–191. doi: 10.1016/j.pharmthera.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 20.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jiménez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation. 2015 doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 21.Nagy L, Pollesello P, Papp Z. Inotropes and inodilators for acute heart failure: sarcomere active drugs in focus. J Cardiovasc Pharmacol. 2014;64:199–208. doi: 10.1097/FJC.0000000000000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prasad K, Gupta JB, Kalra J, Lee P, Mantha SV, Bharadwaj B. Oxidative stress as a mechanism of cardiac failure in chronic volume overload in canine model. Journal of molecular and cellular cardiology. 1996;28:375–385. doi: 10.1006/jmcc.1996.0035. [DOI] [PubMed] [Google Scholar]
- 23.Perez-Reyes E. Molecular physiology of low-voltage-activated t-type calcium channels. Physiol Rev. 2003;83:117–161. doi: 10.1152/physrev.00018.2002. [DOI] [PubMed] [Google Scholar]
- 24.Qiao Z, Xu Y. Myocardium protective function of Salvianolic acid B in ischemia reperfusion rats. African Journal of Traditional, Complementary and Alternative Medicines. 2016;13:157–161. doi: 10.21010/ajtcam.v13i4.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ryan JJ, Archer SL. The right ventricle in pulmonary arterial hypertension: disorders of metabolism, angiogenesis and adrenergic signaling in right ventricular failure. Circ Res. 2014;115:176–188. doi: 10.1161/CIRCRESAHA.113.301129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shiomi T, Tsutsui H, Matsusaka H, Murakami K, Hayashidani S, Ikeuchi M, Wen J, Kubota T, Utsumi H, Takeshita A. Overexpression of glutathione peroxidase prevents left ventricular remodeling and failure after myocardial infarction in mice. Circulation. 2004;109:544–549. doi: 10.1161/01.CIR.0000109701.77059.E9. [DOI] [PubMed] [Google Scholar]
- 27.Tsutsui H, Kinugawa S, Matsushima S. Oxidative stress and heart failure. Am J Physiol Heart Circ Physiol. 2011;301:H2181–2190. doi: 10.1152/ajpheart.00554.2011. [DOI] [PubMed] [Google Scholar]
- 28.Vassort G, Talavera K, Alvarez JL. Role of T-type Ca2+channels in the heart. Cell Calcium. 2006;40:205–220. doi: 10.1016/j.ceca.2006.04.025. [DOI] [PubMed] [Google Scholar]
- 29.Woo AY, Xiao RP. beta-Adrenergic receptor subtype signaling in heart: from bench to bedside. Acta Pharmacol Sin. 2012;33:335–341. doi: 10.1038/aps.2011.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yasui K, Niwa N, Takemura H, Opthof T, Muto T, Horiba M, Shimizu A, Lee JK, Honjo H, Kamiya K, Kodama I. Pathophysiological significance of T-type Ca2+channels: expression of T-type Ca2+channels in fetal and diseased heart. J Pharmacol Sci. 2005;99:205–210. doi: 10.1254/jphs.fmj05002x3. [DOI] [PubMed] [Google Scholar]
- 31.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:1810–1852. doi: 10.1161/CIR.0b013e31829e8807. [DOI] [PubMed] [Google Scholar]
- 32.Yang C, Zhang L, Dai R, Ji S, Li F, Shao W, Que Y, Hu L, Lin Q. Vasoconstrictive effect of Xinmailong injection in rat aorta. African Journal of Traditional, Complementary and Alternative Medicines. 2015;12:46–52. [Google Scholar]


