Abstract
Background:
Jieyuanshen decoction (JYAS-D) - a traditional Chinese medicine was invented by Professor Nie based on classic formulas, chaihu jia longgu muli decoction has been proved as having favorable curative effects on depression in clinical practices. The aim of this study was to investigate the antidepressant effects and its molecular mechanism of JYAS-D.
Materials and Methods:
The model of depression was established by Chronic Unpredictable Stress. Different doses (8.2 g/kg, 16.3 g/kg, 32.7 g/kg) of JYAS-D was orally administered; Fluoxetine was orally administered with 10mg/kg. All treatments lasted for 28 days. Sucrose preference and open-field tests were adopted to observe the behavior of rats. OPA (ortho-phthalaldehyde) derivatization method was used to detect the contents of amino acid neurotransmitter. RIA (Radiation immunity analysis) method was used to measure the serum concentrations of CORT (Corticosterone), ACTH (Adrenocorticotropic hormone) and CRH (Corticotropin-releasing hormone). ELISA (Enzyme linked immunosorbent assay) method was adopted to examine the contents of Glucocorticoid receptor (GR) and Mineralocorticoid receptor (MR) in hippocampus.
Results:
Compared with the model group, sucrose preference was increased in all treatment groups. The concentration of serum CORT was reduced in the middle dose of JYAS-D and control groups; the concentration of serum ACTH was reduced in the low and high-dose of JYAS-D; the concentration of serum CRH was reduced in the middle and high-dose of JYAS-D. The content of hippocampus GR was increased in the middle and high-dose of JYAS-D; the content of hippocampus Glu (Glutamic acid) was reduced among the low, middle and high-dose of JYAS-D and fluoxetine group, the ratio of Glu/γ-GABA (y-aminobutyric acid was reduced in the low and high-dose of JYAS-D.
Conclusion:
JYAS-D had a significant antidepressant-like effect on rat model through regulating serum concentration of CORT, ACTH and CRH, increasing the content of hippocampus GR and regulating the equilibrium of amino acids neurotransmitter.
Keywords: Hypothalamic-pituitary-adrenal (HPA) axis, Glucocorticoid/Mineralocorticoid receptor (G/MR), Amino acid neurotransmitter, Chronic Unpredictable Stress (CUS), Jieyuanshen decoction(JYAS-D)
Introduction
Depression is one of the most prevalent public mental health problems (Wang et al., 2015) which will cause the highest burden in the middle and high income countries by the year 2030 according to WHO’s prediction. Antidepressant medications, including tricyclic antidepressants, selective serotonin reuptake inhibitors, serotonin and norepinephrine reuptake inhibitors, and dopamine reuptake inhibitors, have been used to treat depression for over half a century. Although these antidepressant medications represent the mainstay therapy for depression, almost one-quarter of the patients fail to respond to these therapeutics (Kupfer et al., 2012). Therefore, attempts are being made to seek alternative medication. The history of treating depression with Chinese traditional medicine is more than a thousand years. Chinese herb has been proved efficacious, stable with few side effects. Thus it draws more attention of scientists who are engaged in treating depression.
Nie Huimin, one of Beijing Physician Masters of Chinese Medicine, is a famous professor and doctor in Beijing university of Traditional Chinese Medicine who is good at treating depression with Chinese herbs. Jieyuanshen decoction (JYAS-D) which had favorable curative effects on depression in clinical practices was invented by Professor Nie based on classic formulas, chaihu jia longgu muli decoction. It is also one of formulas popularized by Ministry of Science and Technology of People’s Republic of China National Economic and Social Development 11th Five-Year Plan. The main characteristics of JYAS-D are that it can relieve the symptoms of depression by improving the quality of sleep and regulating the neuroendocrine system of patients in previous clinical observation.
In addition to monoamine neurotransmitters, hyperactivity of hypothalamic-pituitary-adrenal (HPA) axis and excitatory amino acids (EAAs) disorders are more recognized in pathogenesis of depression (Musazzi et al., 2013; Schule et al., 2009). Previous studies have proposed that hyperactivity of HPA is a result of the deficit negative feedback regulation of the axis which causally linked to the development of depressive symptoms and occurs 30% to 50% among the acutely depressed patients (Schule et al., 2009). Long-term chronic stress could cause hyper-function of HPA which led to excessive expression of glucocorticoid receptor (GR) (Pitchot et al., 2001). The prolonged overproduction of Corticosterone (CORT) caused by chronic stress could damage the hippocampus neurons and induce depressive symptoms such as depressed mood, feeling of worthlessness, insomnia, forgetfulness, sexual dysfunction, and other symptoms of depression (Iijima et al., 2010; Naert et al., 2007; Pilar-Cuellar et al., 2013). Meanwhile, excessive stress response, through the action of glucocorticoid (GC) could induce abnormal enhancement of glutamate release and excitatory transmission in selective brain areas, including amygdala, hippocampus, and prefrontal cortex (Musazzi et al., 2013). Our previous study had indicated that JYAS-D exerted a significant antidepression effect.
Investigations were required to identify pharmacodynamic material basis and explore its mechanism of anti-depression of JYAS-D.
Materials and Methods
Preparation of JYAS-D extracts
JYAS-D is comprised of seven commonly used Chinese herbs (Table 1) in the ratio of 1:1.5:0.5:1:1:3 on a dry weight basis, respectively. The crushed herbs of Radix Bupleuri (150 g), Radix Scutellariae (225 g), Semen Ziziphi spinosae (450 g), Radix et Rhizoma Glycyrrhizae (75 g), Bulbus Lilii (150 g), Rhizoma Pinelliae (150 g), and Concha Ostreae (450 g) purchased from the Beijing Tongrentang, a famous traditional herbal medicine store in China, were authenticated by Li Jia (Beijing, China). The Concha Ostreae was decocted with water (16.5 L) at boiling temperature for 2 h, and then the other herbal admixtures were added and kept on boiling for 2 h. After filtered, the residue was extracted with water (13.2 L) for 1.5 h and then filtered. The above filtrates were merged and evaporated under vacuum at 55 °C to obtain the concentrate (2 L). The concentrate was precipitated with 95% ethanol (4 L) and stored at -4 °C for 12 h. After centrifuged at 3000 rpm for 5 min, the supernatant was evaporated under vacuum at 55 °C to remove ethanol and obtained the aqueous extract of JYAS-D (500 mL). It was used for the following experiment and stored at -20 °C.
Table 1.
Components of JYAS-D
| Plant species (Latin) | Family | Plant part | Pinyin |
|---|---|---|---|
| Bupleurumchinense DC. | Umbelliferae | Root | Chaihu |
| ScutellariabaicalensisGeorgi | Lamiaceae | Root | Huangqin |
| Ziziphusjujuba Mill. var. spinosa (Bunge) Hu ex H.F. Chou | Rhamnaceae | Seed | Suanzaoren |
| GlycyrrhizauralensisFisch. | Leguminosae | Root and rhizoma | Gancao |
| Lilium brownie F.E. Brown var. viridulum Baker | Liliaceae | Bulbus | Baihe |
| Pinelliaternata (Thunb.) Breit. | Araceae Concha Ostreae | Rhizoma | Banxia Muli |
The combined effects of herbs in a multi-herbal formula may produce a powerful curative action, and the therapeutic effect is attributed to the synergistic effects of multiple constituents in the preparation. Pharmacological studies (Bondi et al., 2008; Liu et al., 2013; Papp, 2012; Pilar-Cuellar et al., 2013; Schweizer et al., 2009) showed that flavonoids from Radix Scutellariae and Radix et Rhizoma Glycyrrhizae Preparata Cum Melle have anti-viral, antioxidative, anti-microbial and anti-inflammatory activities. Alkaloids are representative chemicals of Rhizoma Coptidis (An et al., 2008). These chemicals have antibacterial, antispasmodic and antineoplastic activities (Christakis et al., 2012; Kakefuda et al., 2010; Mahar et al., 2014). Radix et Rhizoma Ginseng and Radix et Rhizoma Glycyrrhizae Preparata Cum Melle have remarkable pharmacological effects due to the bioactive saponins inside.
High performance liquid chromatography (HPLC) analysis
The authentic standards of spinosin, baicalin and wogonoside were purchased from Chengdu Must Bio-technology CO., LTD. (China) and stored at 4 °C. Acetonitrile and Formic acid (HPLC grade) were obtained from E. Merck (Darmstadt, Germany). The other reagents were analytical grade from Nanjing Chemical Factory (Nanjing, China). Water was purified using a Milli-Q water purification system (Millipore, USA).
The content of spinosin, baicalin and wogonoside in the extract of JYAS-D was determined with an Agilent 1260 liquid chromatography system (Agilent Technologies, USA), consisted of an auto sampler, a quaternary pump, a diode-array detector, and a column temperature controller. Agilent Chem Station Version B.04.03C software was used for data collection and analysis.
Before the chromatographic analysis, 0.5 mL sample was extracted with methanol (8 mL) by ultrasonic at 30 °C for 10 min, then diluted with methanol to 10 mL and filtrated through 0.45 μm membrane filter. The assay was performed on a Merck Hibar C18 column (4.6*250 mm, 5 μm) at a temperature of 30 °C, using a gradient mobile phase consisted of acetonitrile (solvent A) and 0.1% formic acid in water (solvent B). The linear gradient was: 0-15 min, 99-88% B; 15-20 min, 88-85% B; 20-30 min, 85-80% B; 30-40 min, 80-72% B; 40-60 min, 72-60% B; 60-80 min, 60-30%. The flow rate was 1.0 mL/min. The wavelength was set at 335 nm (for spinosin) and 277 nm (for baicalin and wogonosidein). The injection volume was 5 μL.
The contents of spinosin, baicalin and wogonosidein in the extract of JYAS-D were 0.364 mg/mL, 5.278 mg/mL, 2.692 mg/mL respectively (Figure 1, 2, 3).
Figure 1.
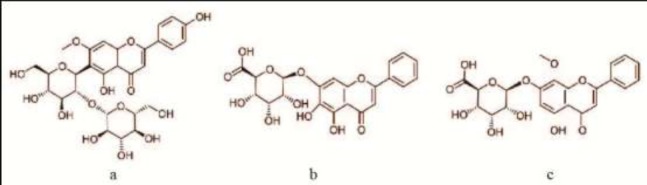
The chemical structures of spinosin (a), baicalin (b) and wogonosidein (c).
Figure 2.
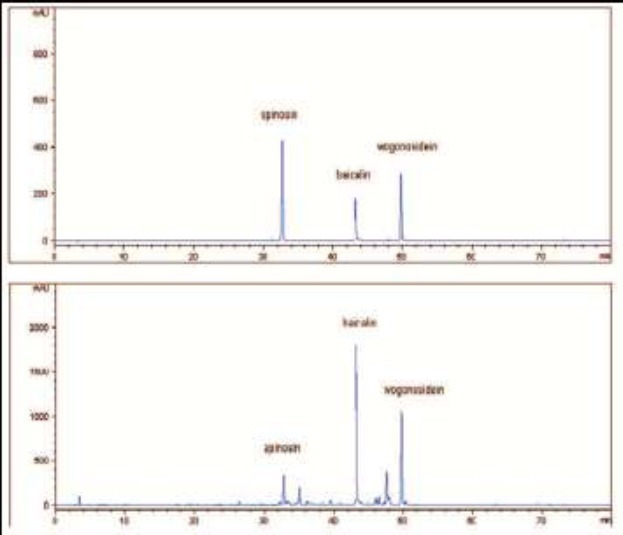
The HPLC chromatograms at 277 nm of the reference substance (upper) and the extract of JYAS-D (under).
Figure 3.
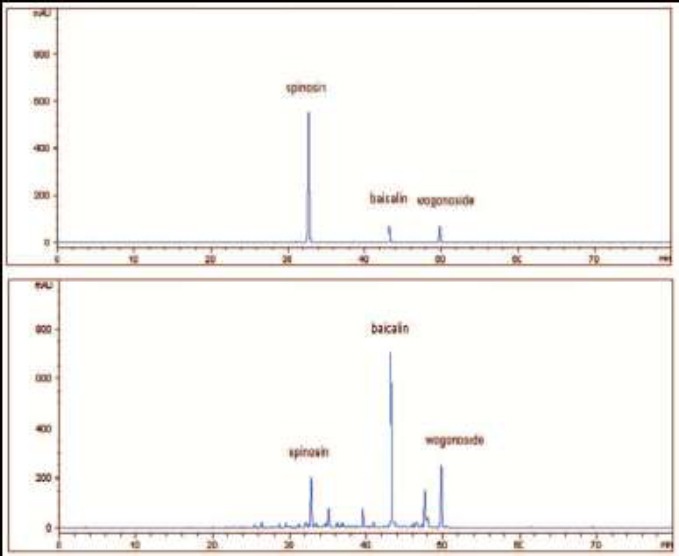
The HPLC chromatograms at 335 nm of the reference substance (upper) and the extract of JYAS-D (under).
Animals and grouping
Thirty-six adult healthy male Sprague-Dawley (SD) rats weighed 180-200 g (License No. SCXK (Jing) 2012-0001) were provided by the Vital River Laboratory Animal Technology Co., Ltd. The animals were kept on a 12 h/12 h light/dark cycle and given free access to food and water, and habituated to animal facilities for 1 week to adapt to the new environment before the experiments. All animal procedures conformed to the Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at Capital Medical University. Rats were assigned randomly into six groups (n=6 per group): normal, model, low-dose, middle-dose and high-dose of JYAS-D and control group (fluoxetine hydrochloride). Rats in normal and model groups were treated with saline.
Model of Depression by Chronic Unpredictable Stress (CUS) (Bondi et al., 2008; Liu et al., 2013; Papp, 2012; Schweizer et al., 2009).
Three rats per-cage were housed in the normal group without stimulus while one rat per-cage was housed in model group with all kinds of stress stimulation for 28 days. Stress stimulation included water deprivation(24 h), food deprivation (24 h), clamping the tail (1 -1.5 min), forced swim in ice water (4 °C, 5 min), Soiled cage (Pour 250 mL plain water into the corn straw-bedding, 24 h), converting their circadian rhythm, braking (2-3 h), water platform(4 °C, 5 min, Height of 20 cm). 1-2 kinds of stimulation were randomly assigned daily. Each stimulus was repeated 3-4 times. (Table 2 a)
Table 2.
Schedule of stressors used in 28 days of CUS procedure
| Stressor | Duration | Day | Stressor | Duration | Day |
|---|---|---|---|---|---|
| Clamping the tail | 1 min | 1 d | Physical restraint | 3 h | 15 d |
| Physical restraint | 2 h | 2 d | Clamping the tail | 1.5 min | 16 d |
| Water deprivation | 23 h | 3 d | Forced swimming at 4 °C | 5 min | 17 d |
| overnight illumination and noise | 12 h | 4 d | Water platform at 4 °C | 5 min | 18 d |
| Forced swimming at 4 °C | 5 min | 5 d | overnight illumination and noise | 12 h | 19 d |
| Water platform at 4 °C | 5 min | 6 d | Food deprivation | 40 h | 20-21 d |
| Food deprivation | 40 h | 7-8 d | Clamping the tail | 1.5 min | 22 d |
| Physical restraint | 2 h | 9 d | Forced swimming at 4 °C | 5 min | 23 d |
| Clamping the tail | 1 min | 10 d | overnight illumination and noise | 12 h | 24 d |
| Physical restraint Soiled cage | 3 h 24h | 11 d | Water deprivation | 23 h | 25 d |
| Forced swimming at 4 °C | 5 min | 12 d | Food deprivation | 24 h | 26 d |
| Water platform at 4 °C | 5 min | 13 d | Physical restraint Soiled cage | 3 h 24 h | 27 d |
| Water deprivation | 23 h | 14 d | Water deprivation | 23 h | 28 d |
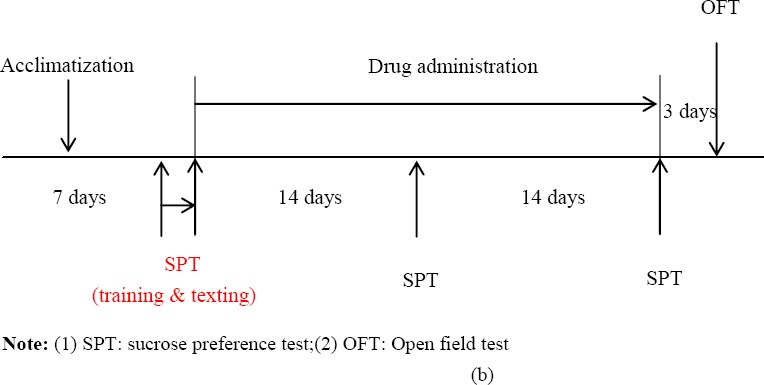
Table 2: Schematic drawing of experimental schedule Protocol 1 (a): after one week of acclimatization, the SD rats were randomly divided into 6 groups of 6 rats. All rats (except rats in the normal group) were subjected to CUS. Protocol 1 (b): the rats were given JYAS-D and fluoxetine by continuously intra-gastric administration for 28 days when the chronic unpredictable stress occurred. The sucrose preference was observed at 1 d,14 d and 28 dafter the model establishment. The behavior changes of rats were observed by the open field test.
Reagents and drugs administration
Prozac (fluoxetine hydrochloride dispersible tablets, pantheons France company, France, Lot: 2090A, 20 mg/tablet), was used in concentration to 2 mg/mL liquid by distilled water. The model group was normally fed after 28-day stress stimulation without any treatment. JYAS-D groups and fluoxetine group were subjected to a model of chronic unpredictable stress and fed isolation. JYAS-D was orally administered 8.2 g/kg, 16.3 g/kg 32.7 g/kg respectively once per day through gastric perfusion. Fluoxetine was dissolved into 2 mL normal saline and was orally administered 10mg/kg through gastric perfusion. The treatment lasted for 28 days. All rats were included in the final analysis. (Table 2 b)
Sucrose preference test
Sucrose preference test (An et al., 2008; Mahar et al., 2014) was employed herein to determine anhedonia, one of the core symptoms of major depression in human. The procedure was composed of training and testing courses. After 1 week of acclimatization, the rats were trained to drink 1% (w/v) sucrose solution before the start of the CUS protocol. In the training course, the rats were deprived of food and water for 48 h and only exposed to1% (w/v) sucrose solution. Three days later, after 23 h food and water deprivation, 1 h baseline test was performed, in which rats could select between two pre-weighted bottles, one with 1% (w/v) sucrose solution and the other with tap water. Then the preference percentage of sucrose was calculated according to the following formula:
Preference percentage= (sucrose intake (g) / (sucrose intake (g) +water intake (g)) ><100%
Finally, on 14th day and the end of the experiment, the sucrose preference test was performed to evaluate the CUS model and drugs action.
Behavior observation
An open-field (Christakis et al., 2012; Liang et al., 2013; Kakefuda et al., 2010) method was used to conduct behavior test with a software monitoring animal spontaneous activity (JLBehv-LG, made in Shanghai) in all group rats. The open-field device was made of opaque materials with 40 cm* 40 cm* 45 cm cube. The rat was put in the central square. The spontaneous activity of the rat was recorded in 10 minutes. The field was cleaned thoroughly and sprayed with 75% alcohol at intervals.
Radiation immunity analysis (RIA) method
3 mL blood was taken from rats and then centrifugated (4 °C, 10000 RPM, 10 min). Serum of rats were separated and stored at -20 °C. RIA was applied to examine the serum level of corticosterone CORT, ACTH and CRH.
Enzyme linked immunosorbent assay (ELISA) method
Rats from each group were sacrificed after anesthesia, the whole brain was then removed. Hippocampus of rats was divided and rinsed in ice-cold PBS (0.02mol/L, pH 7.0-7.2) to remove excess blood thoroughly and weighed before homogenization. Samples were stored at -20 °C. The contents of GR and MR in hippocampus were measured by using commercially available ELISA kits according to the manufacturer’s instructions. Determined the Optical Density (OD.) values at 450 nm using a kind of micro-plate reader.
OPA (Ortho-phthalaldehyde) derivatization method
OPA derivatization method was used to detect the contents of amino acid neurotransmitter (Glu, γ-GABA) in hippocampus. Rat hippocampus were weighed and homogenized in 5% ethanol (1:9). Chromatographic column: Agilent C18 chromatographic column (5 μm, 4.6*25 cm); Mobile phase: A phase; PH 6.5 potassium dihydrogen phosphate buffer solution (0.025 mol/L), phase B: methanol; Flow rate: 1.0 mL/min; Column temperature: 35°C; Sample quantity: 10-μL; Fluorescence detection conditions: the excitation wavelength of 357 nm, emission wavelength of 455 nm.
Statistical Analysis
All results are calculated with means ± SD. The variance analysis was used to compare the differences among groups. The change weight was calculated on the repeated measures analysis of variance. If P value is less than 0.05, the difference was considered statistically significant. We use test for homogeneity to examine the data: if the data is homogeneous, we conduct analysis of variance (one-way ANOVA) directly on the data. Between the two groups, we used the LSD method to compare any difference. All statistical analyses were carried out by using SPSS for Windows (SPSS 15).
Results
Body weight
Before model establishment at 1 d, there were no significant differences of weights in rats among all of the groups (P>0.05). In the period of model establishment, the weights of rats were examined at 6 d, 11 d, 16 d, 19 d, 22 d, 25 d and 28 d. The weights of rats were decreased in model group compared with those of the normal group (P<0.05). JYAS-D and fluoxetine could not alleviate the CUS-induced weight reduction. (Figure 4 a)
Figure 4.
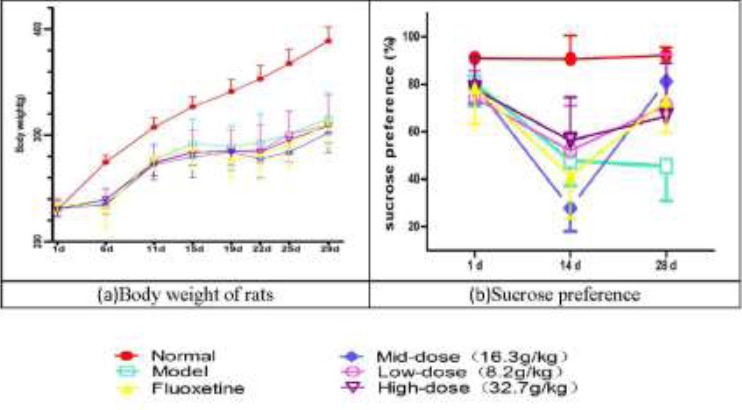
Effects of JYAS-D on body weight (a) and Sucrose preference of rats with CUS (b).
The weights of rats were decreased in model group compared with those of the normal group at 6 d, 11 d, 16 d, 19 d, 22 d, 25 d and 28 d; The sucrose preference was decreased in model group (P<0.05) at 14 d of model establishment compared with the normal group. At 28 d of model establishment, the sucrose preference was significantly increased among the low, mid, high-dose JYAS-D group and fluoxetine group (P<0.05) at 28 d.
Sucrose Preference
The sucrose preference was measured at 1 d, 14 d and 28 d. There was no significant difference before treatment in all of groups (P>0.05). At 14 d of model establishment, compared with the normal group, the sucrose preference was decreased in model group (P<0.05). At 28 d of model establishment, the sucrose preference was decreased in model group. The sucrose preference was significantly increased among the low, mid, high-dose JYAS-D group and fluoxetine group (P<0.05) at 28 d. (Figure 4 b)
Open-Field Test (OFT)
There was a significant difference in the open-field test among these groups. Compared with the normal group, there were significant increases of the proportion of central active distance and the central time in model group(P<0.05). The proportion of edge active distance and edge time were significantly decreased in model group(P<0.05). Compared with the model group, the proportion of central active distance was decreased and edge active distance was increased among the low, mid, high-dose JYAS-D and fluoxetine group(P<0.05); the central active time was decreased and the edge active time was increased among the low, high-dose JYAS-D and fluoxetine group (P<0.05) (Figure 5).
Figure 5.
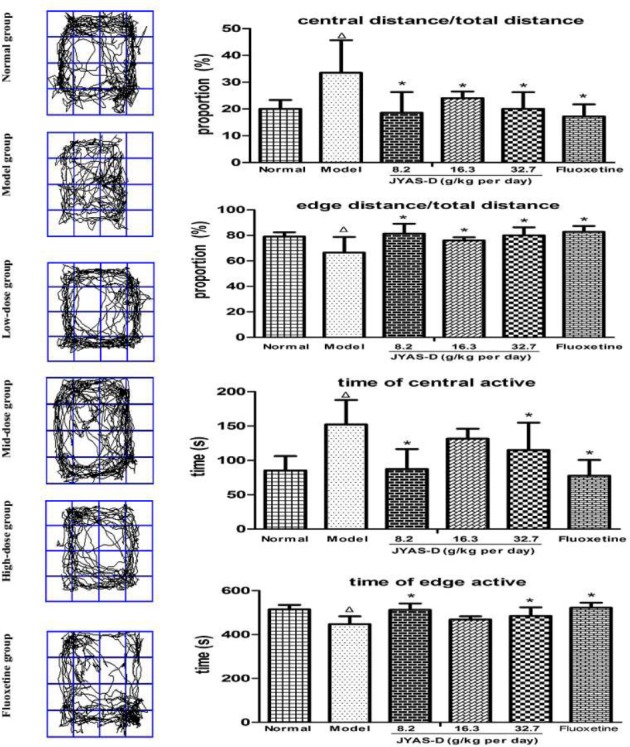
Effects of JYAS-D on open-field test of rats with CUS. A series of the behavior changes in rats were observed by the open field test and the following parameters were measured: central distance / total distance, edge distance / total distance, central active time and edge active time. Data are expressed as mean ±SD, n = 6 per group. Δp< 0.05 versus normal, *p< 0.05versus model.
Serum concentrations of CORT, ACTH and CRH
The serum concentration of CRH, ACTH and CORT were significantly increased in model group. Compared with the normal group, the contents of CORT, ACTH and CRH in serum were increased in model group (P<0.05). Compared with the model group, the concentration of CORT in serum was significantly reduced in the middle dose JYAS-D group and fluoxetine group (P<0.05). The concentration of ACTH in serum was significantly reduced in the low and high-dose JYAS-D group in comparison with the model group (P<0.05). The concentration of CRH in serum reduced significantly in the middle and high-dose JYAS-D group in comparison with the model group (P<0.05) (Figure 6a, b, c).
Figure 6.
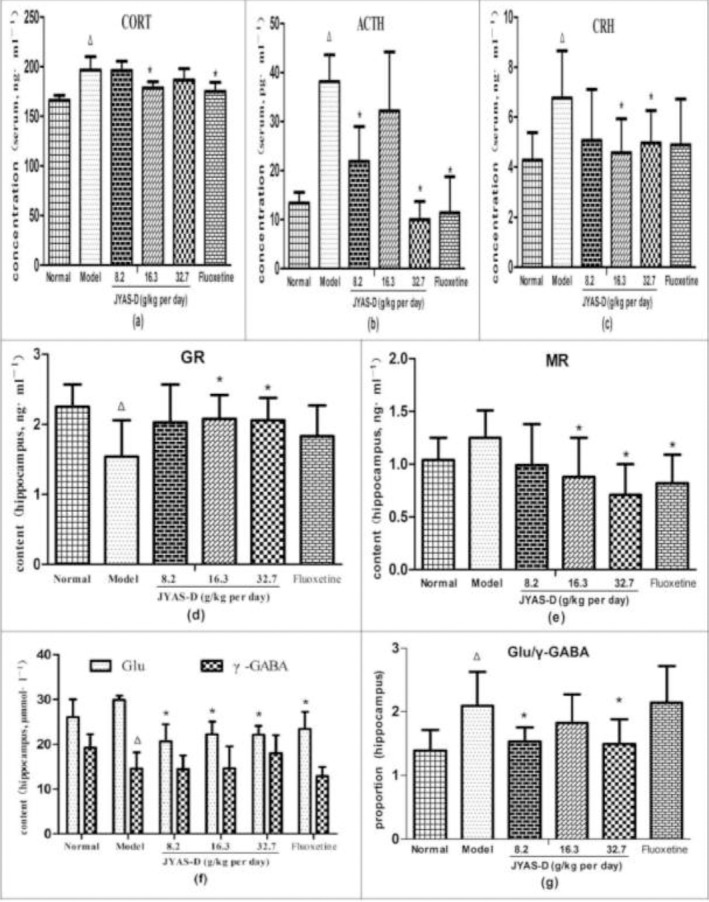
Effects of JYAS-D on serum CRH, ACTH, CORT and hippocampus GR,MR,Glu,γ-GABA,Glu/γ-GABA in depressive rats. Data are expressed as mean ± SD, n= 6 per group. Δp< 0.05 versus normal, *p< 0.05 versus model. ACTH: adrenocorticotropic hormone; CORT: corticosterone; CRH: corticotropin-releasing hormone; GR: Glucocorticoid receptor; MR: Mineralocorticoid receptor; Glu: Glutamic acid; γ-GABA: γ-aminobutyricacid.
Hippocampus contents of GR and MR
Compared with the normal group, the hippocampus content of GR decreased significantly in model group (P<0.05). Compared with the model group, the content of GR in hippocampus increased significantly in the middle and high dose JYAS-D group (P<0.05);the content of MR reduced significantly in the mid, high-dose JYAS-D group and fluoxetine group (P<0.05) (Figure 6d, e).
Hippocampus contents of Glu and γ-GABA
Compared with the normal group, the contents of γ-GABA in hippocampus was significantly reduced in model group (P<0.05). The proportion of Glu/γ-GABA was increased in model group (P<0.05). Compared with the model group, the content of Glu in hippocampus was decreased among the low, middle, high dose JYAS-D group and fluoxetine group (P<0.05). The proportion of Glu/γ-GABA was reduced in the low and high-dose JYAS-D group (P<0.05) (Figure 6 f, g).
Discussion
Chronic Unpredictable Stress is widely used in scientific research of depression animal model simulating core symptoms of depression -reward reactivity decreased (anhedonia) (Dai et al., 2010; Luo et al., 2008; Mao et al., 2010; Willner, 1986). This study found that the weight of model group was significantly lower than that of normal group (P<0.05). Anhedonia occurred in 95% of the depression patients (Keller et al., 1995). Sucrose preference test can be used to evaluate anhedonia of CUS model rats (Grippo et al., 2004; Gronli et al., 2005). Sucrose preference in CUS model rats were decreased obviously after chronic stress (Shimamoto et al., 2011; Zurita et al., 2000). This study also found sucrose preference in model group was significantly lower than that of normal group after 14 d and 28 d (P<0.05). Sucrose preference was significantly restored by the administration of JYAS-D. OFT is one of the most popular assessment methods in behavioral research and can be used to evaluate the environmental manipulations applied on the emotions of rodents (Liang et al., 2013; Ma et al., 2011). The results of our experiment found that JYAS-D could alleviate the spontaneous activity.
Studies have suggested that increased serum levels of GC (CORT in rodents), ACTH and CRH are associated with an increased risk of depression (Paredes et al., 2007; Viinamaki et al., 2012). Chronic unpredictable stress can lead to dysfunction of HPA axis. CORT, ACTH and CRH in rats increased significantly through chronic stress exposure. A large secretion of CORT is one of he most important feature of stress response. Chinese herbs could significantly reduce the serum levels of CRH, CORT and ACTH in the depressed mouse model (Chen et al., 2013). In our experiment, serum levels CORT, ACTH and CRH were increased in CUS model rats, while JYAS-D could significantly reduce serum levels of CORT, ACTH and CRH.
Hippocampus, known to contain high density of GR and MR (Rybnikova et al., 2007), is the key structure in the inhibition of stress-induced HPA axis activation, and is also more sensitive to glucocorticoid mediated impairment. Hippocampus can affect HPA mainly by regulating concentrations of MR and GR. MR is high affinity with endogenous GC. MR can be combined with the GC of physiological level to regulate HPA axis. The GR is low affinity with GC, but GR can combine excessive of GC and make the hyper-function HPA axis back to normal. Therefore, GR played an important role to regulate high concentrations of GC. Prolonged overproduction of CORT could damage hippocampus neurons leading to atrophy and apoptosis of hippocampal neurons (Urbach et al., 2008; Xiu et al., 2008).
Excessive CORT could induce the decline of GR in the hippocampus which in turn could reduce the negative feedback regulation in HPA axis. On the other hand, excessive CORT could promote the sustaining hyper-secretion of glucocorticoids (this process is also known as the glucocorticoid cascade hypothesis) (Pariante and Lightman, 2008). The weakened effects of the negative feedback regulation in HPA axis would worsen the symptoms of depression patients (Schule et al., 2009). In animals, chronic stress paradigms have been evidenced to down-regulate hippocampus GR number (An et al., 2011). The content of hippocampus GR decreases and then a large secretion of CORT occurs which together manifest the hyperactivity. Feedback inhibition of HPA axis is often impaired in chronic stress rats. In the present study, we have found that the content of hippocampus GR in rats was significantly reduced by CUS procedure. JYAS-D could increase the content of GR in hippocampus significantly. In our experiment, no significant difference has been found in the content of hippocampus MR between normal group and model group.
CORT could promote the hyper-secretion of excitatory amino acids, and release to specific areas of the brain, such as amygdala, hippocampus and prefrontal cortex (Musazzi et al., 2013). Glu is one of the excitatory amino acid. Excessive Glu could produce excitatory neurotoxicity and damage to hippocampus neurons. γ-GABA is generally considered as an important inhibitory amino acid in brain of mammals which is closely associated with mental activity (Lee et al., 2010). The increase of γ-GABA could inhibit the excitatory neurons, attenuate the excessive synthesis of Glu and block the excitatory neurotoxicity of Glu. γ-GABA could protect hippocampal neurons and have the effect of antidepressant. Chronic stress might cause excessive CORT, and consequently, the Glu would aggregate outside hippocampal neurons, which leads to the death of hippocampal neuron (Lee et al., 2007). Previous researches have shown that the level of hippocampus Glu in depression rats significantly increases (Reagan et al., 2012) and γ-GABA decreases significantly (Catenadell’Osso et al., 2013; Walker et al., 2013). The content of γ-GABA in hippocampus has been reduced and the proportion of Glu/γ-GABA in hippocampus has increased in model group in our experiment. JYAS-D could reduce excitatory neurotoxicity of excessive Glu and regulate the equilibrium of ratio of amino acids neurotransmitter.
Collectively, this study has demonstrated that JYAS-D could produce a significant antidepressant effect in CUS model rats through down-regulating serum concentration of CORT, ACTH and CRH levels, increasing content of hippocampus GR and regulating the equilibrium of proportion of amino acids neurotransmitter. Therefore, JYAS-D might serve as a novel antidepressant in future clinical practices.
Ethical Approval
This study received permission from the Animal Ethics Committee of Capital Medical Science, China.
Competing interest
The authors declare that there is no conflict of interests.
Acknowledgment
This study was supported by the National Natural Science Foundation of China (NSFC) Grant No.81373526 and the Beijing Municipal fund for Technology and Science No.KM201310025013. This work was also supported by and program for changcheng scholars of the importation and development of Higher-caliber Talents project of Beijing Municipal Institution (CTT&TCD 20140329).
References
- 1.An L, Zhang YZ, Liu XM, Yu NJ, Chen HX, Zhao N, Yuan L, Li YF. Total flavonoids extracted from xiaobuxin-tang on the hyperactivity of hypothalamic-pituitary-adrenal axis in chronically stressed rats. Evid Based Complement Alternat Med, eCAM 2011. 2011:367619. doi: 10.1093/ecam/nep218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.An L, Zhang YZ, Yu NJ, Liu XM, Zhao N, Yuan L, Li YF. Role for serotonin in the antidepressant-like effect of a flavonoid extract of Xiaobuxin-Tang. Pharmacol Biochem Behav. 2008;89:572–580. doi: 10.1016/j.pbb.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 3.Bondi CO, Rodriguez G, Gould GG, Frazer A, Morilak DA. Chronic unpredictable stress induces a cognitive deficit and anxiety-like behavior in rats that is prevented by chronic antidepressant drug treatment. Neuropsychopharmacology. 2008;33(2):320–31. doi: 10.1038/sj.npp.1301410. [DOI] [PubMed] [Google Scholar]
- 4.Catenadell’Osso M, Fagiolini A, Rotella F, Baroni S, Marazziti D. Glutamate system as target for development of novel antidepressants. CNS Spectr. 2013;18(4):188–198. doi: 10.1017/S1092852912000971. [DOI] [PubMed] [Google Scholar]
- 5.Chen Y, Liu J, Wu X, Nice EC. Xiangshao Granule Exerts Antidepressive Effects in a Depression Mouse Model by Ameliorating Deficits in Hippocampal BDNF and TrkB. Evid Based Complement Alternat Med, eCAM 2013. 2013:309262. doi: 10.1155/2013/309262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grippo AJ, Na ES, Johnson RF, Beltz TG, Johnson AK. Sucrose ingestion elicits reduced Fos expression in the nucleus accumbens of anhedonic rats. Brain Res. 2004;1019:259–264. doi: 10.1016/j.brainres.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 7.Gronli J, Murison R, Fiske E, Bjorvatn B, Sorensen E, Portas CM, Ursin R. Effects of chronic mild stress on sexual behavior, locomotor activity and consumption of sucrose and saccharine solutions. Physiol Behav. 2005;84:571–577. doi: 10.1016/j.physbeh.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 8.Iijima M, Ito A, Kurosu S, Chaki S. Pharmacological characterization of repeated corticosterone injection-induced depression model in rats. Brain Res. 2010;1359:75–80. doi: 10.1016/j.brainres.2010.08.078. [DOI] [PubMed] [Google Scholar]
- 9.Kakefuda K, Oyagi A, Ishisaka M, Tsuruma K, Shimazawa M, Yokota K, et al. Diacylglycerol kinase P knockout mice exhibit lithium-sensitive behavioral abnormalities. PLoS One. 2010;5(10):e13447. doi: 10.1371/journal.pone.0013447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keller MB, Klein DN, Hirschfeld RM, Kocsis JH, McCullough JP, Miller I, First MB, Holzer CP, 3rd, Keitner GI, Marin DB, et al. Results of the DSM-IV mood disorders field trial. Am J Psychiatry. 1995;152:843–849. doi: 10.1176/ajp.152.6.843. [DOI] [PubMed] [Google Scholar]
- 11.Kupfer DJ, Frank E, Phillips ML. Major depressive disorder: new clinical, neurobiological, and treatment perspectives. Lancet. 2012;379:1045–1055. doi: 10.1016/S0140-6736(11)60602-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee BJ, Kim JS, Kang YM, Lim JH, Kim YM, Lee MS, et al. Antioxidant activity and y-aminobutyric acid (gaba) content in sea tangle fermented by lactobacillus brevis, bj20 isolated from traditional fermented foods. Food Chem. 2010;122(1):271–276. [Google Scholar]
- 13.Lee Y, Gaskins D, Anand A, Shekhar A. Glia mechanisms in mood regulation: a novel model of mood disorders. Psychopharmacology (Berl) 2007;191:55–65. doi: 10.1007/s00213-006-0652-4. [DOI] [PubMed] [Google Scholar]
- 14.Liang Y, Guo XL, Chen JX, Yue GX. Effects of the chinese traditional prescription xiaoyaosan decoction on chronic immobilization stress-induced changes in behavior and ultrastructure in rat hippocampus. Evid Based Complement Alternat Med, eCAM 2013. 2013:984797. doi: 10.1155/2013/984797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma XC, Jiang D, Jiang WH, Wang F, Jia M, Wu J, Hashimoto K, Dang YH, Gao CG. Social isolation-induced aggression potentiates anxiety and depressive-like behavior in male mice subjected to unpredictable chronic mild stress. PLoS One. 2011:e20955. doi: 10.1371/journal.pone.0020955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahar I, Bambico FR, Mechawar N, Nobrega JN. Stress, serotonin, and hippocampal neurogenesis in relation to depression and antidepressant effects. Neurosci Biobehav Rev. 2014;38:173–192. doi: 10.1016/j.neubiorev.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 17.Musazzi L, Treccani G, Mallei A, Popoli M. The action of antidepressants on the glutamate system: regulation of glutamate release and glutamate receptors. Biol Psychiatry. 2013;73:1180–1188. doi: 10.1016/j.biopsych.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 18.Naert G, Maurice T, Tapia-Arancibia L, Givalois L. Neuroactive steroids modulate HPA axis activity and cerebral brain-derived neurotrophic factor (BDNF) protein levels in adult male rats. Psychoneuroendocrinology. 2007;32:1062–1078. doi: 10.1016/j.psyneuen.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 19.Paredes D, Granholm AC, Bickford PC. Effects of NGF and BDNF on baseline glutamate and dopamine release in the hippocampal formation of the adult rat. Brain Res. 2007;1141:56–64. doi: 10.1016/j.brainres.2007.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pariante CM, Lightman SL. The HPA axis in major depression: classical theories and new developments. Trends Neurosci. 2008;31:464–468. doi: 10.1016/j.tins.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 21.Pilar-Cuellar F, Vidal R, Diaz A, Castro E, dos Anjos S, Pascual-Brazo J, Linge R, Vargas V, Blanco H, Martinez-Villayandre B, Pazos A, Valdizan EM. Neural plasticity and proliferation in the generation of antidepressant effects: hippocampal implication. Neural Plast. 2013;2013:537265. doi: 10.1155/2013/537265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pitchot W, Herrera C, Ansseau M. HPA axis dysfunction in major depression: relationship to 5-HT(1A) receptor activity. Neuropsychobiology. 2001;44:74–77. doi: 10.1159/000054919. [DOI] [PubMed] [Google Scholar]
- 23.Reagan LP, Reznikov LR, Evans AN, Gabriel C, Mocaer E, Fadel JR. The antidepressant agomelatine inhibits stress-mediated changes in amino acid efflux in the rat hippocampus and amygdala. Brain Res. 2012;1466:91–98. doi: 10.1016/j.brainres.2012.05.039. [DOI] [PubMed] [Google Scholar]
- 24.Rybnikova E, Mironova V, Pivina S, Tulkova E, Ordyan N, Nalivaeva N, Turner A, Samoilov M. Involvement of the hypothalamic-pituitary-adrenal axis in the antidepressant-like effects of mild hypoxic preconditioning in rats. Psychoneuroendocrinology. 2007;32:813–823. doi: 10.1016/j.psyneuen.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 25.Schule C, Baghai TC, Eser D, Rupprecht R. Hypothalamic-pituitary-adrenocortical system dysregulation and new treatment strategies in depression. Expert Rev Neurother. 2009;9:1005–1019. doi: 10.1586/ern.09.52. [DOI] [PubMed] [Google Scholar]
- 26.Schweizer MC, Henniger MSH, Sillaber I. Chronic mild stress (cms) in mice: of anhedonia, ‘anomalous anxiolysis’ and activity. PLoS One. 2009;4(1):e4326–e4326. doi: 10.1371/journal.pone.0004326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shimamoto A, Debold JF, Holly EN, Miczek KA. Blunted accumbal dopamine response to cocaine following chronic social stress in female rats: exploring a link between depression and drug abuse. Psychopharmacology. 2011;218(1):271–9. doi: 10.1007/s00213-011-2364-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Urbach A, Redecker C, Witte OW. Induction of neurogenesis in the adult dentate gyrus by cortical spreading depression. Stroke. 2008;39:3064–3072. doi: 10.1161/STROKEAHA.108.518076. [DOI] [PubMed] [Google Scholar]
- 29.Viinamaki H, Lehto SM, Palvimo JJ, Harvima I, Valkonen-Korhonen M, Koivumaa-Honkanen H, Hintikka J, Kirsi H, Niskanen L. Glucocorticoids and aetiopathogenesis of depression. Duodecim. 2012;128:1022–1029. [PubMed] [Google Scholar]
- 30.Wang W, Lu Y, Xue Z, Li C, Wang C, Zhao X, Zhang J, Wei X, Chen X, Cui W, Wang Q, Zhou W. Rapid-acting antidepressant-like effects of acetyl-l-carnitine mediated by PI3K/AKT/BDNF/VGF signaling pathway in mice. Neuroscience. 2015;285:281–291. doi: 10.1016/j.neuroscience.2014.11.025. [DOI] [PubMed] [Google Scholar]
- 31.Willner P. Validation criteria for animal models of human mental disorders: learned helplessness as a paradigm case. Prog Neuropsychopharmacol Biol Psychiatry. 1986;10(6):677–690. doi: 10.1016/0278-5846(86)90051-5. [DOI] [PubMed] [Google Scholar]
- 32.Xiu LJ, Yang YX, Yu S, Sun DZ, Wei PK. Research of depression in traditional Chinese medicine: review and prospect. Zhong xi yi jie he xue bao. 2008;6:416–421. doi: 10.3736/jcim20080418. [DOI] [PubMed] [Google Scholar]


